Abstract
Contemporary childbearing is associated with greater gestational weight gain and post-partum weight retention than in previous decades, potentially leading to a more pronounced effect of childbearing on women’s long-term obesity risk. Previous work on the association of childbearing with women’s long-term obesity risk mostly examined births in the 1970s and 1980s and produced mixed results.
OBJECTIVE
We estimated the association of childbearing and obesity incidence in a diverse, contemporary sample of 2,731 U.S. women.
DESIGN AND METHODS
Propensity-score (PS) matching was used for confounding control when estimating the effect of incident parity (1996 to 2001) on 7-year incident obesity (BMI≥30 kg/m2) (2001 to 2008).
RESULTS
In the sample, 19.3% of parous women became obese while 16.1% of unmatched nulliparous women did. After PS matching without and with replacement, the differences in obesity incidence were, respectively, 0.0 percentage points (ppts) (95% CI: −4.7 to 4.7) and 0.9 ppts (95% CI: −4.9 to 6.7). Results were similar in analyses of prevalent parity and obesity in 2008 (n=6601) conducted to explore possible selection bias.
CONCLUSIONS
These results imply that, in contemporary U.S. parous women in their late 20s and early 30s, childbearing may not increase obesity incidence.
Keywords: obesity, parity, propensity score, matching, confounding
INTRODUCTION
Between the late 1970s and 2010, age-adjusted prevalence of obesity in U.S. women more than doubled, increasing from 15% to 36% (1, 2). It is believed that childbearing may contribute to increased obesity prevalence in U.S. women (3). If so, the unique risk posed by childbearing may constrain the effectiveness of population-based strategies to reduce obesity prevalence in women of childbearing age.
Despite the widespread belief that childbearing increases women’s obesity risk, the evidence is mixed. There are few long-term, population-based studies in diverse samples that attempt to estimate the association between childbearing and weight gain or obesity. Many studies investigate related issues, such as the effect of gestational weight gain on weight retention among parous women (4, 5). However, these types of studies do not control for aging and secular trends, nor account for what a woman’s weight gain would have been had she not had a child (3). Of the studies that have investigated the association between childbearing on long-term weight gain or obesity risk, some find an association (6–9) while others find inconsequential (10, 11) or inverse associations (12).
Previous studies may have been limited by inadequate confounding control. Uncontrolled confounding is a source of substantial bias in this literature: both the timing of childbearing and excess weight gain are strongly associated with demographic and socioeconomic factors, such as family education, immigration status, race, ethnicity, and residential characteristics (7). In any given study, adjustment for available covariates may have been inadequate to produce conditional exchangeability between parous and nulliparous women.
Further, the effect of childbearing on women’s obesity risk may be dynamic. Previous studies examined births in the 1970s, 1980s, and 1990s among mostly White or Black women. However, the literature on gestational weight gain provides circumstantial evidence that the association between childbearing and obesity varies by ethnicity and may have changed in more recent maternal cohorts (13). For instance, levels of gestational weight gain and post-partum weight retention, risk factors for higher BMI and obesity, appear to be increasing over time and are greater in U.S. Blacks versus Whites (4). In a more obesogenic and ethnically diverse population of women, the association between childbearing and obesity may be stronger than seen in previous studies. Alternatively, with higher obesity rates among contemporary young women, childbearing may be a less salient risk factor for incident obesity than in previous maternal cohorts.
Using data from an ethnically diverse, contemporary population of women, this analysis seeks to estimate the effect of childbearing among contemporary women who first gave birth in their late teens and early 20s.
METHODS AND PROCEDURES
Population
Data were from female respondents to the National Longitudinal Study of Adolescent Health (Add Health). The baseline wave of Add Health (1994–1995) was a nationally representative survey of U.S. public and private school students enrolled in grades 7 through 12 (14). The original Add Health survey focused on adolescent risk behaviors and collected a wealth of behavioral data. The survey was cluster-sampled by school and also over-sampled subgroups including Chinese students, black students with a parent who had completed college or attained a professional degree, and disabled students.
At baseline, detailed questionnaires were administered to each student and to the student’s primary in-residence caregiver, preferentially female. Over the next 13 years, three more waves of data were collected. For instance, in 1995–1996 (wave 2), a year after the baseline survey, all students except those in 12th grade at baseline were re-interviewed. In 2001–2002 (wave 3), seven years after baseline, all baseline study respondents were re-interviewed. Finally, in 2008 (wave 4), baseline study respondents were interviewed again.
Exposure: incidence model
The main exposure was incident parity between waves 2 and 3. We examined parity as a dichotomous variable (yes/no) because previous studies suggest that the first birth is uniquely important for obesity risk (3, 6, 8). We defined parity as having delivered a live birth or completed a full-term pregnancy, including a full-term pregnancy that may have ended in stillbirth. Data on pregnancy histories were collected at wave 3 from questionnaires administered via computer-assisted devices. The respondent detailed all her pregnancies, including how and when they ended. Pregnancy data were not collected at wave 2 (1995–96). Therefore, to exclude girls who were already parous at wave 2, we excluded women who reported that they had given birth or had full-term pregnancies that ended in 1996 or before.
Outcome: incidence model
The main outcome was incident obesity between wave 3 and wave 4. At both waves, obesity was defined as body mass index (BMI) ≥ 30.0 kg/m2 (15), based on measured height and weight. We restricted the analysis to women who were non-obese (BMI < 30.0 kg/m2) at wave 3.
Exclusions: incidence model
The analysis sample was restricted to women who were nulliparous in the wave 2 interview year and non-obese at wave 2 and wave 3. There were 7,294 female respondents at wave 4. Of those, 4770 were also present at wave 1, wave 2, and wave 3 (65.4%). We excluded 332 women who were pregnant at wave 4 (n=330, 7.4%) or who reported having given birth within the past 6 months of the wave 4 interview (n=5, <1%), including 3 women fitting both criteria, because temporary pregnancy-related weight gain could lead to misclassification of the obesity outcome. An additional 198 women were excluded because they were pregnant at wave 3. Of the remaining 4,253 women, 148 (3.4%) were missing obesity status at wave 2, wave 3, or wave 4: at wave 2, 33 respondents (<1%) were missing obesity status, at wave 3, 61 (1.4%) were missing obesity status, and at wave 4, 62 (1.4%) were missing obesity status. Of the remaining 4,105 women, 1199 (29.2%) were excluded because they were obese at wave 2 (n=533, 13%), parous at wave 2 (n=203, 4.9%), or became obese between wave 2 and wave 3 (n=533, 13%). The final analysis sample consisted of 2,906 women, 61% of the women present at all the waves of data collection.
Data Analysis
Propensity-score matching is a method of confounding control that attempts to balance the distribution of confounders in the “treated” (parous) and “untreated” (nulliparous) groups (16). One advantage of propensity-score techniques over traditional regression is that propensity scores reduce the dimensionality of confounders: a single propensity score summarizes information from many covariates (17). Second, propensity-score methods allow one to more easily detect “non-positivity,” when there are covariate patterns or combinations of the values of observed confounders where either none or all women are parous and in whom the effect of parity can thus not be validly estimated (18).
First, to calculate propensity scores, we fit a logistic regression model in which the main exposure, incident parity, was the outcome. The predictor variables were childhood demographic characteristics (described in next section). Next, each parous woman was matched to one or more nulliparous women who had similar propensity scores (19). We used nearest-neighbor matching to estimate the average treatment effect in the treated (ATT) respondents, parous women. Our main analysis matched nulliparous women to every parous woman within calipers of 0.1 times the logit of the propensity score. Finally, in this matched population, 7-year obesity incidence between wave 3 and wave 4 among the parous women was compared to obesity incidence among matched nulliparous women. Each estimate of incidence difference had 95% Wald confidence intervals (19).
Confounding control: Predictors in propensity score model
For predictor variables, we chose confounders that temporally are likely to precede the respondent’s development of obesity and parity and logically are unlikely to be affected by the respondent’s incident obesity (Figure 1). For instance, for most respondents, self-identified racial classification was set in childhood before childbearing or obesity onset and remained stable throughout adulthood (20). Another example is parental education: because most parents would have completed their education by age 25 years (21), parental education is unlikely to be affected by the respondent’s incident obesity or parity. In contrast, we did not include factors such as the respondent’s adult income or marital status that could be shared effects of parity and obesity. For instance, the likelihood of being married is affected by both a woman’s childbearing and her obesity status (22, 23). We assumed that adjusting for these shared effects could cause collider stratification bias, a type of induced selection bias (24, 25); however we did conduct analyses to evaluate the extent of this potential source of bias.
Figure 1.
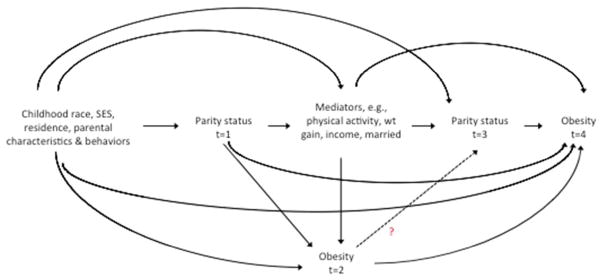
Simplified Directed Acyclic Graph of relationship between childbearing and obesity, National Longitudinal Study of Adolescent Health, 1994/5–2008
The logistic model to predict “exposure” included demographic and childhood characteristics that we hypothesized were on backdoor pathways between parity and obesity status (Figure 1). The model included the following covariates assessed at baseline (1994–1995): unique indicator of the school attended, age, race/ethnicity, immigration status, U.S. census region, parental education, urbanicity status of the school attended, and interactions and transformations of these terms, described below. Specifically, the following variables were included in the exposure model: unique ID of school attended at wave 1; age; age2; nominal 4-level census region (South, Northeast, Midwest, West); nominal school urbanicity (rural, urban, suburban); interaction of census region and urbanicity; nominal 6-level parental education; Black race; interaction of Black race and parental education; non-U.S.-born immigrant; interaction of immigrant and Black race; Mexican ethnicity; Cuban ethnicity; Puerto Rican ethnicity; Central American ethnicity; other Hispanic ethnicity; mixed Hispanic ethnicity; interaction of immigrant and Mexican ethnicity; interaction of immigrant and Cuban ethnicity; five interaction terms of immigrant and all Hispanic ethnicities except “mixed Hispanic ethnicity”; Chinese ethnicity; Filipino ethnicity; Japanese ethnicity; Asian Indian ethnicity; Korean ethnicity; Vietnamese ethnicity; “other” Asian Ethnicity; and mixed Asian ethnicities.
Supplemental analyses
To examine whether propensity score matching resulted in a different conclusion than a conventional regression model with covariate adjustment, we fit supplemental regression models to estimate the adjusted association between parity and obesity. First, we fit regression models using a “standard” set of covariates used in previous regression modeling of this topic (7). These covariates were age, age2, rural/urban/suburban residence at baseline, 4-level race/ethnicity (Black, White, Hispanic, other), and a 3-level variable for mother’s education.
Finally, we conducted a prevalence analysis, estimating the association between parity at wave 4 and obesity at wave 4 without exclusions for earlier parity or obesity status. Generally, incidence analyses are preferred over prevalence analyses because prevalence analyses may be subject to bias from causation and differential duration of prevalence of the outcome (26). However, the exclusions required for incidence analysis may limit generalizability and cause selection bias (24). By estimating the association using analyses subject to different sources of bias, we explored the robustness of our findings. Moreover, as described in the Discussion, we believe that reverse causation and differential outcome duration are unlikely to have biased the results of this prevalence analysis.
RESULTS
Table 1 shows characteristics of the analysis sample for incident obesity before and after matching. About one-fifth of the respondents (n=569, 21%) became parous between wave 2 and wave 3. Before matching, the parous and nulliparous women differed on childhood demographic and community factors. Women who became parous were disproportionately more likely to grow up in the South or a rural area and have parents who did not complete high school. In general, Black and Hispanic women were more likely to become parous than were White or Asian women. The distributions of propensity scores for women who experienced incident parity between wave 2 and wave 3 and those who remained nulliparous at wave 3 showed overlap and little evidence of non-positivity (not shown). After 1:1 matching with replacement, differences between parous and matched nulliparous women were reduced across most variables (Table 1). Because we did not believe that the distributions of these variables would be identical in the parous and nulliparous groups, we do not present any p-values testing that null hypothesis (27).
Table 1.
Descriptive characteristics of analysis sample by parity status, before and after matching by propensity scores, National Longitudinal Study of Adolescent Health, 1994–2008
| Unmatched | Matched 1:1 with replacement | |||
|---|---|---|---|---|
| Parous | Nulliparous | Parous | Nulliparous | |
| N | 569 | 2162 | 568 | 568 |
| Age, wave 4 (mean, years) | 28.4 | 27.9 | 28.4 | 28.4 |
| US-born | 94.6% | 93.6% | 94.5% | 95.8% |
| Foreign-born (ref) | 5.4% | 6.4% | 5.5% | 4.2% |
| REGION (wave I) | ||||
| West | 22.5% | 25.2% | 22.5% | 21.0% |
| Midwest | 27.8% | 29.7% | 27.8% | 29.8% |
| Northeast | 7.9% | 11.1% | 7.9% | 8.8% |
| South (ref) | 41.8% | 34.0% | 41.7% | 40.5% |
| Urbanicity (wave 1) | ||||
| Rural | 24.8% | 16.6% | 24.6% | 26.2% |
| Suburban | 49.9% | 53.3% | 50.0% | 47.7% |
| Urban (ref) | 25.3% | 30.1% | 25.4% | 26.1% |
| Highest parental education, wave 1 & wave 2 | ||||
| No high school degree | 17.6% | 7.6% | 17.4% | 19.0% |
| High school graduate/GED | 37.1% | 25.7% | 37.1% | 37.1% |
| Some college | 14.1% | 13.0% | 14.1% | 12.1% |
| Vocational schooling | 8.6% | 8.4% | 8.6% | 8.1% |
| College graduate (ref) | 17.7% | 27.2% | 17.8% | 19.0% |
| Graduate/Prof school | 4.9% | 18.1% | 4.9% | 4.6% |
| Race (Black/White) | ||||
| White (ref) | 63.8% | 70.3% | 63.9% | 62.7% |
| Black | 26.5% | 19.7% | 26.4% | 28.5% |
| Other | 9.7% | 10.0% | 9.7% | 8.8% |
| Hispanic ethnicities | ||||
| Mexican | 11.8% | 6.3% | 11.8% | 13.2% |
| Cuban | 1.1% | 2.4% | 1.1% | 0.9% |
| Puerto-Rican | 2.1% | 1.5% | 2.1% | 1.4% |
| Central/South American | 1.4% | 1.6% | 1.4% | 1.1% |
| Other Hispanic | 1.9% | 0.9% | 1.9% | 0.7% |
| Mixed Hispanic ethnicities | 0.7% | 0.6% | 0.7% | 0.4% |
| Not Hispanic (ref) | 81.0% | 86.8% | 81.0% | 82.4% |
| Asian ethnicities | ||||
| Chinese | 0.4% | 2.3% | 0.4% | 0.4% |
| Filipino | 2.3% | 3.3% | 2.3% | 1.6% |
| Japanese | 0.2% | 0.3% | 0.2% | 0.2% |
| Asian-Indian | 0.2% | 0.2% | 0.2% | 0.2% |
| Korean | 0.0% | 0.0% | 0.0% | 0.0% |
| Vietnamese | 0.2% | 0.2% | 0.2% | 0.2% |
| Other | 1.2% | 0.8% | 1.2% | 1.8% |
| Mixed Asian ethnicities | 0.2% | 0.7% | 0.2% | 0.2% |
| Not Asian (ref) | 95.4% | 92.2% | 95.4% | 95.6% |
Differences in obesity incidence in matched samples
Table 2 shows differences in 7-year obesity incidence between parous and nulliparous women before and after propensity-score matching. Before matching, obesity incidence was 3.2 percentage points greater in parous versus nulliparous women: 19.3% of parous women became obese between wave 3 and wave 4 while only 16.1% of nulliparous women became obese. Nearly all parous women could be matched to a nulliparous woman, suggesting that the positivity assumption was not violated in this analysis (18). In 1:1 matching with no replacement, the estimated difference in obesity incidence was reduced to 0.0 percentage points. In 1:1 matching with replacement, the estimated incidence difference was 0.9 percentage points.
Table 2.
Differences in 7-year obesity incidence between incident parous and nulliparous women, before and after propensity-score matching, National Longitudinal Study of Adolescent Health, 1996–2008
| Matching scheme, caliper | Total N | Parous N | Obesity incidence | Incidence difference (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Parous | Nulliparous | ||||
| Unmatched | 2731 | 569 | 19.3% | 16.1% | 3.2 (−0.3, 6.6) |
| 1:1 matching, no replacement | 2715 | 553 | 19.7% | 19.7% | 0.0 (−4.7, 4.7) |
| 1:1 matching, with replacement | 2730 | 568 | 19.4% | 18.5% | 0.9 (−4.9, 6.7) |
Supplemental regression analyses
We conducted regression analyses analogous to the matching analyses. Generalized linear models with a binomial family distribution and log and identity links that included the same covariates included in the propensity-score model would not converge after 100 iterations of log-likelihood maximization. However, the unadjusted and adjusted estimates that could be fit were similar to the results from matching with replacement. The main difference was that the confidence intervals were more conservative in the propensity-score matching analysis.
Analyses of prevalent parity and prevalent obesity
We also performed a supplemental propensity-score analysis of the relationship between prevalent parity and prevalent obesity. As expected, about half the women (53%) were parous at wave 4. As shown in Table 3, the unadjusted prevalence difference in obesity was 7.3 percentage points (95% CI: 4.9, 9.6). The difference was reduced to 0.6 percentage points (95% CI: −2.2, 3.3) and −0.9 percentage points (95% CI: −4.9, 3.2), respectively, for 1:1 matching without replacement and 1:1 matching with replacement. Regression results also suggested null associations after adjustment.
Table 3.
Differences in obesity prevalence between parous and nulliparous women, before and after propensity-score matching, National Longitudinal Study of Adolescent Health, wave 4 (2008)
| Matching scheme, caliper | Total N | Parous N | Obesity prevalence | Prevalence difference (95% CI) | |
|---|---|---|---|---|---|
|
| |||||
| Parous | Nulliparous | ||||
| Unmatched | 6601 | 3501 | 40.9% | 33.6% | 7.3 (4.9, 9.6) |
| 1:1 matching, no replacement | 5438 | 2338 | 38.9% | 38.3% | 0.6 (−2.2, 3.3) |
| 1:1 matching, with replacement | 6592 | 3492 | 40.8% | 41.7% | −0.9 (−4.9, 3.2) |
DISCUSSION
We hypothesized that there would be a positive association between childbearing and obesity prevalence in a contemporary population of U.S. women. However, in both incidence and prevalence analyses, we estimated only a small, inconsequential association between parity and obesity. In other words, if these associations are similar in magnitude to the true causal effect, it appears that there would have been little difference in obesity incidence in parous women in their late 20s and early 30s if they had remained nulliparous.
Most of the literature on the relationship between parity and weight status concerns post-partum weight retention in the first 12 months after birth (28). These studies of short-term weight retention may not reflect longer-term effects of childbearing on weight gain (29). For example, while a woman’s weight may be greater a year after childbirth than before childbearing, the difference may not reflect a causal effect of childbearing on weight gain. Instead, the weight gain may be attributable to secular trends or aging: had the woman remained nulliparous, she may have still gained weight over time.
Ecological evidence supports the findings of the present study. Over the past 35 years, age at first birth has increased, rates of adolescent pregnancy have decreased, and lifetime parity has decreased even as obesity prevalence among adolescent girls and young women has increased dramatically (2, 30). Further, in countries with low rates of adult weight gain, there is little post-partum weight retention (31). However, some observational studies of the association between childbearing and excess weight gain have found positive associations (6–9). The studies finding associations may have had limited ability to control confounding (6–9) and measurement bias from self-reported height and weight (7, 8). In particular, due to the interrelated timing of incident obesity and incident parity in a woman’s life, many studies exclude large proportions of their samples, i.e., 50%, to conduct incidence analyses. The danger of this strategy is that it could induce selection bias that produces confounding of unpredictable magnitude and direction (25).
We hypothesized that greater confounding control would significantly affect results, reducing the magnitude of a positive association between childbearing and obesity. However, in the incidence analysis, unadjusted associations were only weakly positive. Therefore, our results may not differ from previous findings of positive relationships because of confounding control; instead the association between childbearing and obesity may be weaker in contemporary cohorts.
It is notable that the unadjusted difference in incident obesity in parous versus unmatched nulliparous women was not statistically significant. By itself, restriction to a select population of non-obese, nulliparous young women yielded reduced differences in confounding factors between the parous and nulliparous women remaining in the sample. Despite the lack of statistical difference in the unmatched sample, it was still important to perform the propensity-score analysis. First, the analysis controlled for residual confounding, generating more valid estimates of the causal relationship between childbearing and obesity. Compared to the estimate in the unadjusted sample, the adjusted estimates were attenuated to the null, strengthening our main conclusion. A second rationale for conducting the propensity-score analysis is that propensity-score matching allows one to more easily detect “non-positivity,” when there are covariate patterns or combinations of the values of observed confounders in whom the effect of parity cannot be validly estimated. Our analysis indicated that positivity was not a major problem here, further strengthening our confidence in the results.
The lack of associations in the present study could be interpreted in three ways. One explanation is that there is no effect of childbearing on obesity incidence. Under this interpretation, many new mothers who experience post-partum weight retention were already on a trajectory of weight gain and would have become obese over the long term, even if they had not had a child. Contemporary U.S. women experience great weight gain in their teens, 20s, and 30s regardless of pregnancy status (32, 33).
A second interpretation of the results is that there is heterogeneity of the effect of childbearing on excess weight gain: while childbearing may increase obesity risk in some women, it would decrease risk in another group of women. Under this interpretation, the marginal, or overall, effect estimated by this study masks elevated risks and protective effects in population subgroups. For instance, we plan to conduct additional analyses stratified by demographic characteristics, such as race/ethnicity. If there is an identifiable group of women for whom childbearing increases obesity risk, then it is important to identify factors that can be modified during pregnancy or post-partum. Additionally, women who have lower post-partum weight versus pre-partum weight may be a target for interventions to maintain weight loss in new mothers (3). Finally, a third interpretation of the null results is that the observed associations are biased estimates of the true effect of childbearing on obesity risk.
This analysis focuses more on the biological rather than social effects of pregnancy and childbearing. For example, we restrict to biological mothers and include full-term pregnancies that end in stillbirth. Nevertheless, we recognize that the social aspects of childbearing are inseparable from the biological aspects. In fact, the lifestyle changes associated with childrearing may be more important than the physiological processes (34).
Our estimate could include multiple indirect effects, or pathways of action, including effects of childbearing and childrearing on eating behaviors, disposable income, and other risk factors for obesity.
This study has four major strengths. First, we analyzed data from a large, diverse sample of contemporary women. Second, to decrease bias from confounding and evaluate non-positivity, we performed propensity-score matching on a rich set of pre-pregnancy maternal characteristics. Additionally, the ATT propensity-score matching estimated translatable effect estimates, incidence and prevalence differences relevant to target populations of contemporary young parous women. Third, we had objectively measured data on height and weight. Finally, we explored the possible influence of selection bias and inadequate confounding control in our incidence analysis by conducting supplemental analyses of prevalent parity and obesity.
In the analysis of incident obesity, generalizability could be limited because exclusions narrow the target population to women who experienced relatively early childbearing and are also somewhat resistant to obesity. Additionally, the selection bias could affect validity (24, 35). For example, if parity causes obesity, then in the incidence analysis’s restricted sample of women who were non-obese at wave 3, parous women are those who managed to prevent obesity despite childbearing; therefore they are uniquely resistant to weight gain. As a result, a positive effect of parity on weight gain could be underestimated unless one were able to control for all the factors that predispose non-obese, parous women to be resistant to parity-associated weight gain.
The analysis of prevalent obesity was not subject to these biases and produced similar results as the analysis of incident obesity. Additionally, parous and nulliparous women in the prevalence analysis had more similar distributions of propensity scores (supplemental Figures 1 & 2). They were likely more exchangeable than in the incidence analysis due to being near the U.S. median age of first birth (36, 37) at wave 4 (age range: 24 to 32 years). Near the median age for first birth, there are many newly parous women who are similar to nulliparous women who will become parous in the near future and vice versa. However, prevalence difference is a valid estimate of the effect of childbearing (versus delaying childbearing) on obesity prevalence only if there is no differential outcome duration nor reverse causation. We believe differential outcome duration is likely to be small because obesity is generally a persistent state in U.S. adolescents and adults (33). Moreover, those at highest risk of obesity tend to have longest duration, which would bias results upward. In contrast, reverse causation could bias the estimate downward if obesity decreases parity risk. However, while obesity is associated with lower fecundity in clinical settings (38), protective effects of obesity on parity may be negligible at the population level in contemporary women (39).
There are limitations to our analysis. First, our matching analysis did not account for the complex survey sampling of the study. However, we attempted to account for the probability of sampling and retention corrected by the survey weights by calculating propensity scores using the variables that comprised the sample weights, i.e., race/ethnicity, parental education, the interaction of race/ethnicity and parental education, and age. Further, controlling for clustering would probably have increased the magnitude of the standard errors, not affecting our interpretation of results. Second, to construct non-pregnant, non-obese samples for the incidence analysis required excluding a large proportion of eligible women because of their obesity or parity status at waves 2 and 3. Third, we only accounted for the relative timing of incident parity and incident obesity in crude time intervals, defined by the three time points at which data were collected on obesity status by the parent study. In particular, in the incidence analysis, we did not take into account the incident parity between wave 3 and wave 4 of women who were nulliparous at wave 3. Additional restriction for births occurring between w3 and w4 could introduce selection bias and, moreover, make it impossible to ensure that parity occurred before obesity onset. However, failing to consider the wave 3-wave 4 incident parity could have biased our results towards the null. Finally, this study does not disentangle the physiological, social, and psychological mechanisms through which parity could affect obesity risk.
Future research may investigate whether childbearing has differential effects on women, increasing prevalence for some women while decreasing it for others. If heterogeneity exists, it is important to investigate mechanisms by which childbearing is protective in some women while putting others at risk. Potential targets for investigation include risk factors for gestational weight gain like pre-pregnancy BMI, race and ethnicity, single versus partnered status, socioeconomic status, sleep patterns, mental health, eating habits, physical activity, breast-feeding behavior, and smoking resumption or continued cessation. Future research should also examine more specific measures of adiposity or body composition and more long-term post-partum weight retention. Finally, in most longitudinal studies, the timing of incident obesity is unobserved, making it difficult to control for reverse causation without potentially inducing selection bias. To more precisely control for the complex timing of parity and obesity will require time-to-event analyses with many measures of weight to get at more precise timing of obesity. In summary, this analysis of the relationship between parity and obesity provided little evidence that childbearing contributes to obesity prevalence in young U.S. women. Childbearing may not affect population-level obesity incidence in a diverse, contemporary population of young U.S. women.
Supplementary Material
What is already known about this subject
Previous research on childbearing’s association with women’s obesity risk examined births to women in the 1970s and 1980s and produced mixed results.
As rates of obesity among women of childbearing age have increased since the 1980s, the effect of childbearing on women’s obesity risk may have changed.
What this study adds
This is the first study to attempt to estimate the effect of childbearing on women’s obesity risk in an ethnically diverse population of women giving birth in the late 1990s and early 2000s.
This study found little evidence that childbearing increased obesity risk in parous women in their late 20s and early 30s.
The association between childbearing and women’s obesity risk may have diminished as obesity rates have increased among young women.
Acknowledgments
The authors thank Kevin Bradway, Tomek Koszylko and Bo Rin Kim for programming and administrative assistance.
WRR conceived the research question, performed data analysis, interpreted results, and drafted the manuscript. MCC also performed data analysis and contributed to interpretation of results. KJH, TS, and AMSR contributed to interpretation of the findings and manuscript preparation.
WRR was supported by the University of Michigan Robert Wood Johnson Foundation Health and Society Scholar’s small grant program, the National Cancer Institute (1K01CA172717-01), and University Cancer Research Funds at the Lineberger Cancer Center at UNC-Chapel Hill. KJH was funded through a VA HSR&D QUERI Career Development Award (CDA 11-261) at the VA Greater Los Angeles and received additional support from the VA Office of Academic Affiliations. TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) from the National Institute on Aging at the National Institutes of Health. He also receives research funding as Principal Investigator of the UNC-DEcIDE center from the Agency for Healthcare Research and Quality. TS does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology and from unrestricted research grants from pharmaceutical companies (GlaxoSmithKline, Merck, Sanofi) to the Department of Epidemiology, University of North Carolina at Chapel Hill.
This research uses data from Add Health, a program project directed by Kathleen Mullan Harris and designed by J. Richard Udry, Peter S. Bearman, and Kathleen Mullan Harris at the University of North Carolina at Chapel Hill, and funded by grant P01-HD31921 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, with cooperative funding from 23 other federal agencies and foundations. Special acknowledgment is due Ronald R. Rindfuss and Barbara Entwisle for assistance in the original design. Information on how to obtain the Add Health data files is available on the Add Health website (http://www.cpc.unc.edu/addhealth). No direct support was received from grant P01-HD31921 for this analysis.
Footnotes
CONFLICTS OF INTEREST
None declared.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int J Obes Relat Metab Disord. 1998 Jan;22(1):39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):491–7. doi: 10.1001/jama.2012.39. Epub 2012/01/19. eng. [DOI] [PubMed] [Google Scholar]
- 3.Gunderson EP. Childbearing and obesity in women: weight before, during, and after pregnancy. Obstet Gynecol Clin North Am. 2009 Jun;36(2):317–32. ix. doi: 10.1016/j.ogc.2009.04.001. Epub 2009/06/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. The American Journal of Clinical Nutrition. 2011 Nov 1;94(5):1225–31. doi: 10.3945/ajcn.111.015289. [DOI] [PubMed] [Google Scholar]
- 5.Siega-Riz AM, Viswanathan M, Moos MK, Deierlein A, Mumford S, Knaack J, et al. A systematic review of outcomes of maternal weight gain according to the Institute of Medicine recommendations: birthweight, fetal growth, and postpartum weight retention. Am J Obstet Gynecol. 2009 Oct;201(4):339, e1–14. doi: 10.1016/j.ajog.2009.07.002. Epub 2009/10/01. eng. [DOI] [PubMed] [Google Scholar]
- 6.Gunderson EP, Murtaugh MA, Lewis CE, Quesenberry CP, West DS, Sidney S. Excess gains in weight and waist circumference associated with childbearing: The Coronary Artery Risk Development in Young Adults Study (CARDIA) Int J Obes Relat Metab Disord. 2004 Apr;28(4):525–35. doi: 10.1038/sj.ijo.0802551. Epub 2004/02/11. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis EM, Zyzanski SJ, Olson CM, Stange KC, Horwitz RI. Racial, ethnic, and socioeconomic differences in the incidence of obesity related to childbirth. Am J Public Health. 2009 Feb;99(2):294–9. doi: 10.2105/AJPH.2007.132373. Epub 2008/12/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg L, Palmer JR, Wise LA, Horton NJ, Kumanyika SK, Adams-Campbell LL. A prospective study of the effect of childbearing on weight gain in African-American women. Obes Res. 2003 Dec;11(12):1526–35. doi: 10.1038/oby.2003.204. Epub 2003/12/25. eng. [DOI] [PubMed] [Google Scholar]
- 9.Brown WJ, Hockey R, Dobson AJ. Effects of Having a Baby on Weight Gain. American Journal of Preventive Medicine. 2010;38(2):163–70. doi: 10.1016/j.amepre.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DF, Madans J, Pamuk E, Flegal KM, Kendrick JS, Serdula MK. A prospective study of childbearing and 10-year weight gain in US white women 25 to 45 years of age. Int J Obes Relat Metab Disord. 1994 Aug;18(8):561–9. Epub 1994/08/01. eng. [PubMed] [Google Scholar]
- 11.Wolfe WS, Sobal J, Olson CM, Frongillo EA., Jr Parity-associated body weight: modification by sociodemographic and behavioral factors. Obes Res. 1997 Mar;5(2):131–41. doi: 10.1002/j.1550-8528.1997.tb00653.x. Epub 1997/03/01. eng. [DOI] [PubMed] [Google Scholar]
- 12.Brown JE, Kaye SA, Folsom AR. Parity-related weight change in women. Int J Obes Relat Metab Disord. 1992 Sep;16(9):627–31. Epub 1992/09/01. eng. [PubMed] [Google Scholar]
- 13.Siega-Riz AM. Prepregnancy obesity: determinants, consequences, and solutions. Adv Nutr. 2012 Jan;3(1):105–7. doi: 10.3945/an.111.001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris KM. Design Features of Add Health. Chapel Hill, NC: Carolna Population Center, University of North Carolina; 2011. p. 18. Available from: https://http://www.cpc.unc.edu/projects/addhealth/data/guides/designpaperWI-IV.pdf. [Google Scholar]
- 15.World Health Organization. Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organization technical report series. 1995;854:1–452. Epub 1995/01/01. eng. [PubMed] [Google Scholar]
- 16.Rubin DB. For Objective Causal Inference, Design Trumps Analysis. Annals of Applied Statistics. 2008 Sep;2(3):808–40. [Google Scholar]
- 17.Sturmer T, Schneeweiss S, Brookhart MA, Rothman KJ, Avorn J, Glynn RJ. Analytic strategies to adjust confounding using exposure propensity scores and disease risk scores: nonsteroidal antiinflammatory drugs and short-term mortality in the elderly. Am J Epidemiol. 2005 May 1;161(9):891–8. doi: 10.1093/aje/kwi106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messer LC, Oakes JM, Mason S. Effects of socioeconomic and racial residential segregation on preterm birth: a cautionary tale of structural confounding. American journal of epidemiology. 2010 Mar 15;171(6):664–73. doi: 10.1093/aje/kwp435. Epub 2010/02/09. eng. [DOI] [PubMed] [Google Scholar]
- 19.Leuven E, Sianesi B. PSMATCH2: State module to perform full Mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing 2003. 2012 May 17; Available from: http://ideas.repec.org/c/boc/bocode/s432001.html.
- 20.Harris DR, Sim JS. Who is Multiracial? Assessing the Complexity of Lived Race. American Sociological Review. 2002;67(4):614–27. [Google Scholar]
- 21.Crissey SR Bureau USC, editor. Current Population Reports. 2009. Educational attainment in the United States: 2007. [Google Scholar]
- 22.Oreffice S, Quintana-Domeque C. Anthropometry and socioeconomics among couples: Evidence in the United States. Economics & Human Biology. 2010;8(3):373–84. doi: 10.1016/j.ehb.2010.05.001. Epub 2010 Jun 2. [DOI] [PubMed] [Google Scholar]
- 23.Manning WD, Smock PJ. Why marry? Race and the transition to marriage among cohabitors. Demography. 1995 Nov;32(4):509–20. [PubMed] [Google Scholar]
- 24.Hernan MA, Hernandez-Diaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004 Sep;15(5):615–25. doi: 10.1097/01.ede.0000135174.63482.43. Epub 2004/08/17. eng. [DOI] [PubMed] [Google Scholar]
- 25.Whitcomb BW, Schisterman EF, Perkins NJ, Platt RW. Quantification of collider-stratification bias and the birthweight paradox. Paediatr Perinat Epidemiol. 2009 Sep;23(5):394–402. doi: 10.1111/j.1365-3016.2009.01053.x. Epub 2009/08/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 46–8. [Google Scholar]
- 27.Poole C. Low P-values or narrow confidence intervals: which are more durable? Epidemiology. 2001 May;12(3):291–4. doi: 10.1097/00001648-200105000-00005. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt NM, Nicholson WK, Schmitt J. The association of pregnancy and the development of obesity - results of a systematic review and meta-analysis on the natural history of postpartum weight retention. Int J Obes (Lond) 2007 Nov;31(11):1642–51. doi: 10.1038/sj.ijo.0803655. Epub 2007/07/04. eng. [DOI] [PubMed] [Google Scholar]
- 29.Nehring I, Schmoll S, Beyerlein A, Hauner H, von Kries R. Gestational weight gain and long-term postpartum weight retention: a meta-analysis. Am J Clin Nutr. 2011 Nov;94(5):1225–31. doi: 10.3945/ajcn.111.015289. Epub 2011/09/16. eng. [DOI] [PubMed] [Google Scholar]
- 30.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA : the journal of the American Medical Association. 2012 Feb 1;307(5):483–90. doi: 10.1001/jama.2012.40. Epub 2012/01/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Onyango AW, Nommsen-Rivers L, Siyam A, Borghi E, de Onis M, Garza C, et al. Post-partum weight change patterns in the WHO Multicentre Growth Reference Study. Matern Child Nutr. 2011 Jul;7(3):228–40. doi: 10.1111/j.1740-8709.2010.00295.x. Epub 2011/02/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis CE, Jacobs DR, Jr, McCreath H, Kiefe CI, Schreiner PJ, Smith DE, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000 Jun 15;151(12):1172–81. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 33.Gordon-Larsen P, The NS, Adair LS. Longitudinal trends in obesity in the United States from adolescence to the third decade of life. Obesity (Silver Spring) 2010 Sep;18(9):1801–4. doi: 10.1038/oby.2009.451. Epub 2009/12/26. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossner S, Ohlin A. Pregnancy as a risk factor for obesity: lessons from the Stockholm Pregnancy and Weight Development Study. Obes Res. 1995 Sep;3(Suppl 2):267s–75s. doi: 10.1002/j.1550-8528.1995.tb00473.x. Epub 1995/09/01. eng. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Diaz S, Schisterman EF, Hernan MA. The birth weight “paradox” uncovered? Am J Epidemiol. 2006 Dec 1;164(11):1115–20. doi: 10.1093/aje/kwj275. Epub 2006/08/26. eng. [DOI] [PubMed] [Google Scholar]
- 36.Martinez G, Daniels K, Chandra A. Fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. National health statistics reports. 2012 Apr;12(51):1–28. [PubMed] [Google Scholar]
- 37.Hamilton BE, Hoyert DL, Martin JA, Strobino DM, Guyer B. Annual summary of vital statistics: 2010–2011. Pediatrics. 2013 Mar;131(3):548–58. doi: 10.1542/peds.2012-3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Metwally M, Li TC, Ledger WL. The impact of obesity on female reproductive function. Obes Rev. 2007 Nov;8(6):515–23. doi: 10.1111/j.1467-789X.2007.00406.x. [DOI] [PubMed] [Google Scholar]
- 39.Frisco ML, Weden MM, Lippert AM, Burnett KD. The multidimensional relationship between early adult body weight and women’s childbearing experiences. Soc Sci Med. 2012 Jun;74(11):1703–11. doi: 10.1016/j.socscimed.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


