Abstract
Introduction
Olfactory loss is a debilitating symptom of chronic rhinosinusitis (CRS). The pathophysiology of inflammatory olfactory dysfunction likely involves both conductive and sensorineural components. To study the interaction of CRS-associated inflammatory cytokines with the olfactory epithelium, a transgenic mouse model was developed that allows temporally-controlled local gene expression. Interferon-gamma (IFN-γ) is a prototypical Th1 cytokine linked to non-polypoid CRS, as well as sinonasal viral and bacterial infections. In this study, the effects of chronic IFN-γ expression on olfactory histology and function were investigated.
Methods
IFN-γ secretion by olfactory sustentacular cells was induced in the transgenic mouse. Viability and gross behavior were unaffected. Mice were sacrificed after 6 weeks of IFN-γ expression, and olfactory tissue was studied by histology, immunohistochemistry, and electro-olfactogram (EOG). Findings were compared with uninduced littermates.
Results
IFN-γ expression did not result in alteration of the normal histologic architecture of the neuroepithelium or lamina propria. However, EOG recordings demonstrated a significant decrease in odorant responses after IFN-γ expression. In addition, a marked increase in submucosal CD45-positive cells was observed, the majority of which were CD3 and CD4 positive lymphocytes.
Conclusion
Chronic IFN-γ expression in the mouse olfactory epithelium results in diminished odorant responsiveness, despite the absence of inflammatory tissue damage. This suggests a direct effect of IFN-γ on olfactory neuron function that may underlie olfactory loss in CRSsNP or viral infections. The infiltration of submucosal lymphocytes raises the possibility that other downstream cytokines also contribute to olfactory dysfunction.
Keywords: olfactory loss, rhinosinusitis, interferon gamma, transgenic model
Introduction/Background
Olfactory dysfunction is an often underappreciated symptom of chronic rhinosinusitis (CRS) that has a particularly negative impact on quality of life 1-3. The mechanisms underlying the loss of the sense of smell in CRS are incompletely understood, but appear to be more complex than simple airflow obstruction to the olfactory cleft. Most likely, pathologic alterations in peripheral olfactory structure and physiology due to chronic inflammation play a significant role 4. The olfactory neuroepithelium resides at an important immunological barrier within the nasal cavity in contact with the external environment, and thus it is vulnerable to both exogenous injury and endogenous host defensive processes 5. Fortunately, olfactory neurons and their progenitors are resilient and have a remarkable capacity for regeneration that is unique within the nervous system. This capacity for regeneration may not be unlimited, however, and it is believed that longstanding tissue damage ultimately results in replacement of the specialized olfactory neuroepithelium with respiratory mucosa, leading to permanent dysfunction 4. That being said, even when olfactory epithelium integrity is maintained, patients with CRS may experience waxing and waning olfactory dysfunction that is relatively rapidly reversed with systemic corticosteroids. These properties suggest that mechanisms other than tissue damage, perhaps related to local cytokine-mediated inflammation, contribute to olfactory dysfunction.
In order to study the role of cytokines in human CRS-associated olfactory loss, a transgenic mouse model was developed in which olfactory sustentacular cells can be induced to express inflammatory cytokines 6-9. Initial studies with the inducible olfactory inflammation (IOI) model demonstrated that expression of the CRS-associated cytokine, tumor necrosis factor alpha (TNF-α), resulted in a progressive inflammatory infiltrate that mimicked aspects of mucosal inflammation in CRS, including an inflammatory cell infiltrate and a downstream cascade of other cytokine mediators described in CRS. TNF-α-induced chronic olfactory inflammation is characterized by progressive neuronal apoptosis, suppression of normal olfactory regeneration, and decreased electrophysiologic response to odorants 6, 8. The decrease in electrical olfactory responses occurs weeks prior to the neuronal loss, suggesting a functional effect of inflammation on intact neurons. Moreover, while systemic corticosteroids improve the degree of olfactory inflammation in the IOI(TNF-α) mouse, odorant responses remain diminished9. This finding supports previous in vitro evidence that TNF- α has direct effects on olfactory neurons and their progenitors 10, 11
In recent years, an ever-expanding number of cytokines have been identified in the sinonasal mucosa, both in health and disease. There is no universal mediator profile associated with CRS, but the clinical categorization of CRS based on the presence or absence of nasal polyps (CRSwNP and CRSsNP, respectively) allows differentiation on the basis of Th2 cytokines. Specifically, while both forms of CRS are associated with Th1-type inflammatory mediators, CRSwNP is distinguished by a mixed profile that prominently includes Th2 cytokines. Interferon-γ (IFN- γ) is the prototypical Th1 cytokine, and its expression in the sinonasal tract is well established 12-14. The sole type II interferon, IFN- γ has diverse roles in orchestrating immune system function during infection. It modulates the inflammatory state by synergizing or antagonizing the effects of other cytokines, growth factors, and innate immune pattern-recognition receptors. In addition to key roles driving sinonasal mucosal immune responses to rhinoviruses15, IFN- γ expression has been linked to the immune response to sinonasal biofilms 16.
The loss of the sense of smell in CRS is extremely prevalent in CRSwNP, but also occurs with significant frequency in CRSsNP17, 18. Given that Th1 inflammation is common to both forms of CRS, it is reasonable to postulate that IFN- γ-mediated cytokine pathways may play a role in the pathogenesis of CRS-associated olfactory loss. In this study, we utilize the IOI mouse model to investigate the impact of chronic IFN- γ expression on the structure and function of the olfactory epithelium.
Materials & Methods
Inducible Olfactory Inflammation (IOI) INF-γ Mouse
The creation of the IOI mouse line has been described previously 6. Briefly, the reverse tetracycline transactivator gene was knocked into the olfactory-specific cyp2g1 coding region generating a cyp2g1-rtTA strain. This line was crossed with a line containing the INF-γ gene under the control of a tetracycline-responsive element (TRE- INF-γ) (The Jackson Lab, Bar Harbor ME) to generate the inducible inflammation model IOI(INF-γ). Doxycycline (DOX) was used to induce INFγ expression in adult mice between the ages of 6 and 8 weeks old.
Histologic Analysis
After sacrifice by CO2 inhalation, the mice were decapitated and the heads were fixed and decalcified by immersion in TBD2 solution (Shandon, Pittsburgh, PA) for 24 hours. The heads were embedded in paraffin, and 6-μm sections were obtained and collected on glass slides for hemotoxylin and eosin staining. For frozen section analysis, the mice were anesthetized by an intraperitoneal injection of 100 mg/kg of ketamine hydrocholoride/xylazine hydrochloride solution (Sigma, St. Louis, MO), before intracardiac perfusion with 4% paraformaldehyde. The olfactory tissue was then dissected, postfixed in 4% paraformaldehyde overnight, and transferred to a solution of 30% sucrose and 250 mM of EDTA for 48 hours. The decalcified heads were then infiltrated with TFM tissue freezing medium (Triangle Biomedical Sciences, Inc., Durham, NC) and frozen on dry ice into a plastic mold. Sections of mouse olfactory tissue in OCT were cut on a cryostat (12 μm), placed on Super-frost plus slides (Fisher Scientific, Pittsburgh, PA), and dried 4° C overnight. Slides were stored -80°C for future staining.
Immunohistochemistry
Cryostat sections were washed in phosphate-buffered saline (PBS) and were blocked for 1 hour in PBS containing 10% normal goat serum. Slides were incubated overnight at 4°C in 5% normal serum containing primary antibody to CD45 (1:100, eBiosciences, San Diego CA, #14-0451), anti-mouse CD3(1:100, eBiosciences, San Diego CA, #14-0032), and anti-mouse CD4(1:100, eBiosciences, San Diego CA, #14-0041), NCAM (1:1000, Millipore, Billerica, MA). For anti-keratin 5 staining (1:500, Covance, Princeton, NJ), an antigen retrieval step was performed prior to washing: slides were microwaved at 60% power (1000 MW) for 10 minutes in 0.01M Citrate Buffer, pH 6.0. Primary antibodies were detected using 1:200 dilution of fluorescent tagged secondary antibodies (Alexa-fluor, Invitrogen, Carlsbad, CA; Dylight, Jackson ImmunoResearch, West Grove, PA). Each sample was counterstained by the nuclear stain, DAPI (Vector Labs, Burlingame, CA). Z-stack images were obtained using a LSM510 confocal microscope (Carl Zeiss Micro-imaging). Measurements of relative area coverage and cell counts were performed using ImageJ 1.46 software (public domain, NIH, Bethesda MD).
Bromodeoxyuridine Labeling
Mice were injected intraperitoneally with bromodeoxyuridine (BrdU) (Sigma, St. Louis, MO), 50 μg/g of body weight, 2 hours before sacrifice. Cryostat sections were then incubated with 3 N HCl for 30 minutes and treated with proteinase K in Tris-Edta buffer for 10 minutes before immunostaining with rat anti-BrdU antibody (1:100, Abcam, Cambridge, MA). Primary antibodies were detected using a fluorescent tagged secondary antibody (Alexa-fluor, Invitrogen, Carlsbad, CA). Each sample was counterstained by the nuclear stain, DAPI (Vector Labs, Burlingame, CA). Images were collected using a LSM510 confocal microscope.
Electro-olfactogram (EOG)
EOGs were performed according to previously published methods. 6,7,9 The medial surface of the olfactory turbinates was prepared for recording after the mouse was sacrificed using CO2. Odorant solutions (Aldrich, St. Louis, MO) were prepared in DMSO and diluted with water to the working concentration just before EOG recording. Test odorants for air delivery were prepared at liquid concentrations of 10−3 [final DMSO concentration of 0.2% (v/v)], and diluted to 10−4 and 10−5 M concentrations. Responses to DMSO diluent alone were measured. Odorant stimulation was delivered in the vapor phase as a 100-millisecond pulse by injection into the continuous stream of humidified air. The odorant stimulus pathway was cleaned by air between each stimulus presentation with a minimum interval of 1 minute between two adjacent stimuli. Field potentials were measured with two electrodes, placed on either turbinate IIb or turbinate III, to acquire simultaneous recordings. Data was analyzed with Clampfit (Axon Instruments, Union City, CA). Peak amplitudes were determined from pre-pulse baseline, but no other data normalizations were performed.
Quantification of interferon-γ expression
Nasal lavage fluid was collected by perfusing 250 μl of PBS over the bisected nasal cavity. The fluid was stored at 80°C for subsequent analysis. IFN-γ was quantified using an ELISA kit according to the manufacturer's instructions (R&D Systems).
Statistical Analysis
Raw data was entered into spreadsheet software for statistical analysis (Excel; Microsoft Corp, Redmond, Wash). Data are expressed as mean ± SEM. Statistical comparisons between wild type and IOI(IFN-γ) mice were performed using two-tailed t-tests. Differences were considered statistically significant at P<0.05.
Results
In the absence of inflammation, the olfactory epithelium (OE) demonstrates an apical single layer of sustentacular cells overlying a densely packed layer of olfactory sensory neurons and their progenitors (figure 1, top left panel). Multipotent stem cells (horizontal basal cells and globose cells) reside in the most basal aspect of the neuronal layer. Below the basement membrane in the subepithelium are well-demarcated axon bundles. In the absence of doxycycline administration, the olfactory histologic phenotypes of IOI(IFN- γ) mice are identically normal. After 6 weeks of doxycycline induction, IFN- γ is highly expressed and detectable in nasal lavage fluid (Figure 1C). TNF-α was undetectable in the lavage fluid by ELISA (data not shown). At this time point in the IOI(IFN-γ) mice, the OE remains intact and grossly normal in appearance, with intact sustentacular cells and neuron layer. (Figure 1A). In the subepithelium, there is disruption in the architecture with inflammatory cell infiltration and compression of the axon bundles, more evident in the higher magnification images. To better visualize the changes suggested by the histologic sections, immunohistochemical staining was performed to visualize olfactory neurons and their axon bundles with an antibody to neural cell adhesion molecules (NCAM). (Figure 1B) In the absence of inflammation, NCAM is highly expressed in the cytoplasm of the olfactory neurons within the OE and in the axon bundles within the subepithelium. NCAM staining remains normal after 6 weeks of IFN- γ expression, although the axons bundles appear distorted by the subepithelial inflammatory infiltrate.
Figure 1.
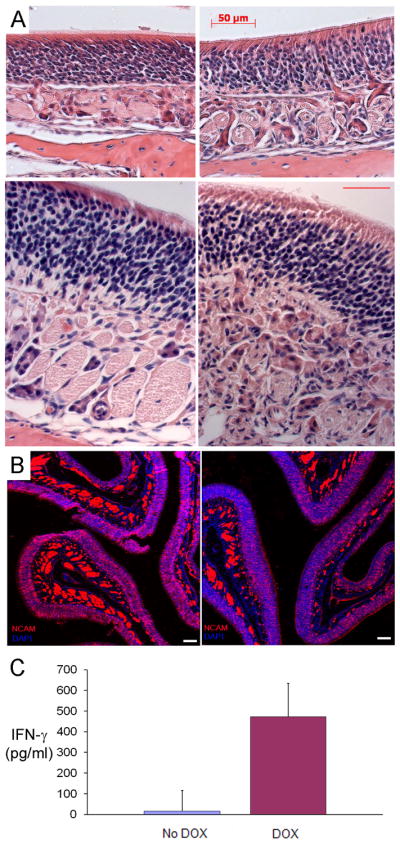
A) Hematoxylin and eosin staining of the olfactory epithelium in control (left panels) and IFN-γ mice (right panels), after 6 weeks DOX induction. Upper panels 20×, lower panels 40 × (scale bar=25μ); B) NCAM immunostaining in control (left) and IFN-γ mice (right) after 6 weeks DOX induction. Blue staining is the nuclear marker DAPI. Scale bar = 50μ; C) IFN-γ levels in the nasal lavage fluid after DOX-induction as determined by ELISA.
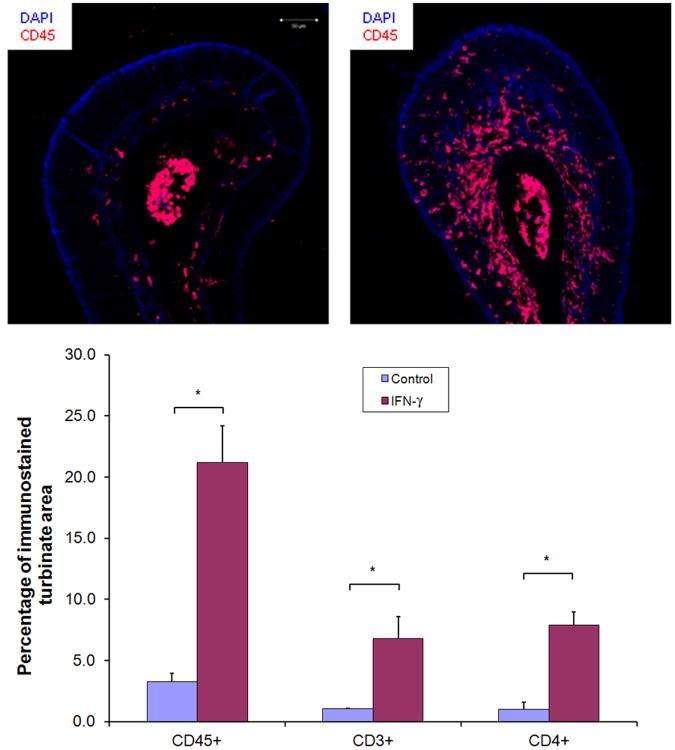
An antibody to the CD45 common leukocyte antigen19 was used to visualize nucleated cells of hematopoietic origin within the olfactory turbinate, to better demarcate the inflammatory cell infiltrate (figure 2). The pattern of antibody staining for K5, a marker of progenitor horizontal basal cells, and for BrdU, a marker used for demonstrating cell proliferation, were both unchanged between control and IOI(IFN- γ) mice. (data not shown)
Figure 2.
Top panels: Immunostaining of control (left) and IFN-γ (right) for the common leukocyte antigen CD45, which labels nucleated hematopoietic cells. Lower panel: Percentage of turbinate area immunolabeled for CD45, CD3, and CD4 in control vs IFN-γ mice. N = 3 mice per group. * p<0.05.
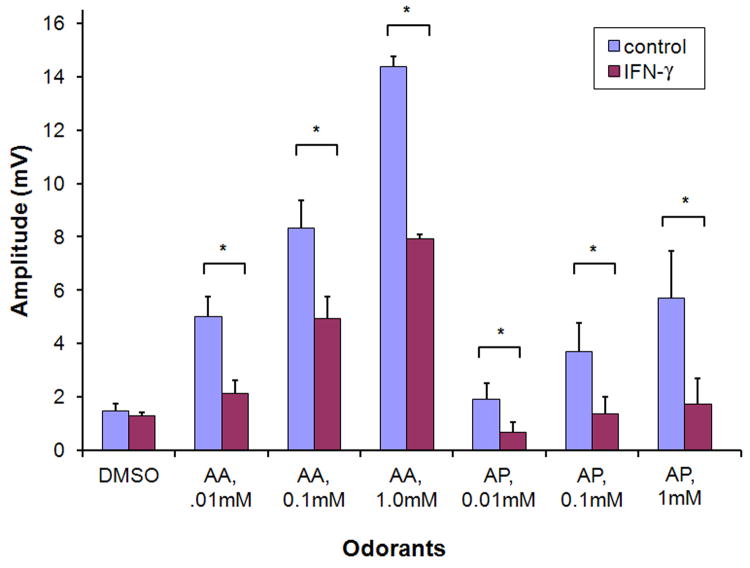
The effect of IFN- γ on sensory function was assessed by electro-olfactogram (EOG) recordings (figure 3). After 6 weeks DOX administration, odorant responses in the IOI(IFN- γ) mouse were significantly reduced, to an average amplitude 40-60% that of control.
Figure 3.
Electro-olfactogram quantification of odorant responses in control and IOI(IFN-γ) mice after 6 weeks DOX induction. AA= amyl acetate; AP=acetophenone. Results expressed as the mean of 4 separate EOG recordings from each group. * p < 0.05.
Discussion
In the present study, we show that chronic expression of IFN- γ in the mouse olfactory epithelium results in prominent lymphocytic inflammation, without the progressive loss of olfactory neurons that is seen when TNF-α is similarly expressed. Despite the preservation of a histologically intact olfactory neuron layer, a significant decrease in electrophysiologic responses to odorants is observed in the IOI(IFN-γ) mouse. This finding provides additional support to the concept that the steroid-sensitive hyposmia in CRS patients may relate to direct effects of CRS-associated inflammatory cytokines on olfactory neuron function.
The observation that significant phenotypic differences exist between chronic olfactory inflammation induced by IFN- γ and by TNF-α is potentially informative. Further characterization and comparison of inflammatory infiltrate compositions and resulting cytokine profiles may help explain the differential effects on neurons and progenitor populations. Future studies will make use of systemic corticosteroids to inhibit cytokine gene expression downstream of IFN-γ may help differentiate indirect effects of IFN-γ on olfactory neuron function. Similarly, conditional knockout mice in which the IFN-γ receptor is absent on olfactory neurons can be generated and used to provide additional evidence for a direct effect of IFN-γ on this cell population. By contrasting the consequences of different initiating cytokines in the IOI model, common pathways may emerge that provide a mechanism of interference with odorant signal transduction in neurons.
The role of IFN- γ in the olfactory system has not previously been explored in any in vitro or in vivo model system. Given the clinical entity of post-viral anosmia and the central function of IFN- γ in anti-viral immunity, a relationship between interferon signaling and olfactory dysfunction is intriguing. Such an interaction may be suggested by reports of anosmia induced by interferon therapy for hepatitis C20-22. While the presence of IFN- γ in the human olfactory epithelium has not been investigated, it is likely that local production of interferons is critical to host defense against entry of neurotropic viruses, such as influenza A and herpes simplex virus. For this reason, any olfactory loss treatment strategy that might involve antagonism of interferons in this tissue must be considered carefully.
At present, much remains unknown about the cause of human inflammation-associated olfactory dysfunction. In part, this stems from the relative inaccessibility of the olfactory cleft and the difficulty of repeatedly obtaining sufficient quantities of biopsy samples from individual subjects. The IOI transgenic mouse model greatly facilitates investigation of cellular and molecular pathways that would be nearly impossible to explore in human subjects. With this genetic approach, individual cytokines or combination of cytokines can be expressed in a temporally-controlled fashion, in order to dissect the web of overlapping signaling pathways that interact between co-existing populations of immune cells and sensory neurons within the nasal cavity. As CRS is a complex and multifactorial disease that is already in progress at the time of presentation, models like the IOI mouse are necessary to study specific mediators in isolation and to view events that occur at the initial stages of olfactory inflammation or during its resolution. Moreover, powerful genetic tools available in mice, such as knock-out strains and gene reporter constructs, can be leveraged to further elucidate mechanisms and refine pathophysiologic hypotheses.
Olfactory loss in the setting of CRS is a frustrating problem that patients often find particularly disabling. Systemic steroids reduce inflammation and may improve olfaction transiently 23, but the benefit of the long-term use of these agents is outweighed by the risks of significant health complications. While endoscopic sinus surgery is highly successful in improving the symptoms of CRS and allows for more effective delivery of topical anti-inflammatory agents, there is limited demonstrated benefit of surgery in improving olfaction 24. Given time, it is hoped that insights derived from basic research in model systems may inform development of novel therapies targeting key cytokines and their inflammatory signaling pathways, which perhaps cross-talk with sensory neurons to cause olfactory dysfunction.
Conclusion
The IOI transgenic mouse provides a unique model to study mechanisms underlying human olfactory dysfunction. IFN- γ is the prototypical Th1 cytokine, associated with sinonasal inflammation in CRS, particularly in the setting of infection. Chronic expression of IFN- γ in the olfactory epithelium initiates a local inflammatory state and diminished olfactory function. Unlike the inflammation induced by TNF-α, the expression of IFN- γ does not result in the death of olfactory neurons or the inhibition neurogenesis. This provides further evidence that inflammatory cytokines may interact directly with olfactory neurons to decrease their odorant responsiveness. Further studies are needed to fully elucidate the complex interplay between the immune system and the olfactory epithelium in animal models and ultimately in CRS patients.
Acknowledgments
Research supported by NIH DC009026 (A.P.L.).
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Presented at the Annual Meeting of the American Rhinologic Society, September 8, 2012, Washington, DC.
References
- 1.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005 Feb;125(2):116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 2.Seo HS, Jeon KJ, Hummel T, Min BC. Influences of olfactory impairment on depression, cognitive performance, and quality of life in Korean elderly. Eur Arch Otorhinolaryngol. 2009 Nov;266(11):1739–1745. doi: 10.1007/s00405-009-1001-0. [DOI] [PubMed] [Google Scholar]
- 3.Wrobel BB, Leopold DA. Olfactory and sensory attributes of the nose. Otolaryngol Clin North Am. 2005 Dec;38(6):1163–1170. doi: 10.1016/j.otc.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Doty RL, Mishra A. Olfaction and its alteration by nasal obstruction, rhinitis, and rhinosinusitis. Laryngoscope. 2001 Mar;111(3):409–423. doi: 10.1097/00005537-200103000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doty RL. The olfactory system and its disorders. Semin Neurol. 2009 Feb;29(1):74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- 6.Lane AP, Turner J, May L, Reed R. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010 Feb 10;30(6):2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner JH, Liang KL, May L, Lane AP. Tumor necrosis factor alpha inhibits olfactory regeneration in a transgenic model of chronic rhinosinusitis-associated olfactory loss. Am J Rhinol Allergy. 2010 Sep-Oct;24(5):336–340. doi: 10.2500/ajra.2010.24.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JH, May L, Reed RR, Lane AP. Reversible loss of neuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy. 2010 May-Jun;24(3):192–196. doi: 10.2500/ajra.2010.24.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan B, May LA, Lane AP. The role of TNF-alpha in inflammatory olfactory loss. Laryngoscope. 2011 Nov;121(11):2481–2486. doi: 10.1002/lary.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farbman AI, Buchholz JA, Suzuki Y, Coines A, Speert D. A molecular basis of cell death in olfactory epithelium. J Comp Neurol. 1999 Nov 22;414(3):306–314. doi: 10.1002/(sici)1096-9861(19991122)414:3<306::aid-cne2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki Y, Farbman AI. Tumor necrosis factor-alpha-induced apoptosis in olfactory epithelium in vitro: possible roles of caspase 1 (ICE), caspase 2 (ICH-1), and caspase 3 (CPP32) Exp Neurol. 2000 Sep;165(1):35–45. doi: 10.1006/exnr.2000.7465. [DOI] [PubMed] [Google Scholar]
- 12.Otto BA, Wenzel SE. The role of cytokines in chronic rhinosinusitis with nasal polyps. Curr Opin Otolaryngol Head Neck Surg. 2008 Jun;16(3):270–274. doi: 10.1097/MOO.0b013e3282fb2885. [DOI] [PubMed] [Google Scholar]
- 13.Cho KS, Kim CS, Lee HS, Seo SK, Park HY, Roh HJ. Role of interferon-gamma-producing t cells in the pathogenesis of chronic rhinosinusitis with nasal polyps associated with staphylococcal superantigen. J Otolaryngol Head Neck Surg. 2010 Oct;39(5):600–605. [PubMed] [Google Scholar]
- 14.Han JK. Subclassification of chronic rhinosinusitis. Laryngoscope. 2013 Mar;123(2):S15–27. doi: 10.1002/lary.23979. [DOI] [PubMed] [Google Scholar]
- 15.Jornot L, Cordey S, Caruso A, et al. T lymphocytes promote the antiviral and inflammatory responses of airway epithelial cells. PLoS One. 2011;6(10):e26293. doi: 10.1371/journal.pone.0026293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hekiert AM, Kofonow JM, Doghramji L, et al. Biofilms correlate with TH1 inflammation in the sinonasal tissue of patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009 Oct;141(4):448–453. doi: 10.1016/j.otohns.2009.06.090. [DOI] [PubMed] [Google Scholar]
- 17.Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2012 Jan;123(1):57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 18.Mori E, Matsuwaki Y, Mitsuyama C, Okushi T, Nakajima T, Moriyama H. Risk factors for olfactory dysfunction in chronic rhinosinusitis. Auris Nasus Larynx. 2013 Feb 16; doi: 10.1016/j.anl.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Dahlke MH, Larsen SR, Rasko JE, Schlitt HJ. The biology of CD45 and its use as a therapeutic target. Leuk Lymphoma. 2004 Feb;45(2):229–236. doi: 10.1080/1042819031000151932. [DOI] [PubMed] [Google Scholar]
- 20.Cocquyt VF, Van Belle SJ. Anosmia associated with alpha-interferon treatment. Ann Oncol. 1994 Nov;5(9):863. doi: 10.1093/oxfordjournals.annonc.a059023. [DOI] [PubMed] [Google Scholar]
- 21.Kraus I, Vitezic D. Anosmia induced with alpha interferon in a patient with chronic hepatitis C. Int J Clin Pharmacol Ther. 2000 Jul;38(7):360–361. doi: 10.5414/cpp38360. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama S, Hirayama C, Kadowaki Y, Sagayama A, Omura H, Nakamoto M. Interferon-induced anosmia in a patient with chronic hepatitis C. Am J Gastroenterol. 1998 Jan;93(1):122–123. doi: 10.1111/j.1572-0241.1998.122_c.x. [DOI] [PubMed] [Google Scholar]
- 23.Stevens MH. Steroid-dependent anosmia. Laryngoscope. 2001 Feb;111(2):200–203. doi: 10.1097/00005537-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Jiang RS, Lu FJ, Liang KL, et al. Olfactory function in patients with chronic rhinosinusitis before and after functional endoscopic sinus surgery. Am J Rhinol. 2008 Jul-Aug;22(4):445–448. doi: 10.2500/ajr.2008.22.3195. [DOI] [PubMed] [Google Scholar]