Abstract
Alongside the well-known chemical modes of cell-cell communication, we find an important and powerful system of bioelectrical signaling: changes in the resting voltage potential (Vmem) of the plasma membrane driven by ion channels, pumps and gap junctions. Slow Vmem changes in all cells serve as a highly conserved, information-bearing pathway that regulates cell proliferation, migration and differentiation. In embryonic and regenerative pattern formation and in the disorganization of neoplasia, bioelectrical cues serve as mediators of large-scale anatomical polarity, organ identity and positional information. Recent developments have resulted in tools that enable a high-resolution analysis of these biophysical signals and their linkage with upstream and downstream canonical genetic pathways. Here, we provide an overview for the study of bioelectric signaling, focusing on state-of-the-art approaches that use molecular physiology and developmental genetics to probe the roles of bioelectric events functionally. We highlight the logic, strategies and well-developed technologies that any group of researchers can employ to identify and dissect ionic signaling components in their own work and thus to help crack the bioelectric code. The dissection of bioelectric events as instructive signals enabling the orchestration of cell behaviors into large-scale coherent patterning programs will enrich on-going work in diverse areas of biology, as biophysical factors become incorporated into our systems-level understanding of cell interactions.
Keywords: Ion, Voltage gradient, Gap junction, Morphogenesis
Introduction to endogenous bioelectricity
Embryonic patterning, regenerative repair and suppression of cancerous disorganization all require continuous signal exchange among cells, tissues and organ systems within the body. Alongside well-known biochemical cues, there exists an important and fascinating system of bioelectrical communication. The segregation and flow of charges achieved by ion fluxes through ion channel and pump proteins give rise to a transmembrane voltage potential (usually on the order of −50 mV, inside negative) and the parallel arrangement of cells with transporters localized to specific domains results in epithelial batteries driving trans-epithelial potentials (McCaig et al. 2005; Robinson 1989). All cells, not just excitable neurons and muscle, generate and receive bioelectrical signals encoded in changes in transmembrane potential and ion fluxes that change on a time-scale of seconds to days. These biophysical events are a crucial component of the cellular conversations that enable large-scale pattern formation and morphostasis. Tools have been developed over the last 10 years that enable an unprecedented degree of molecular-level insight into the functioning of these signals and their linkage to canonical genetic pathways. However, the properties of bioelectrical cues are different from the workings of biochemical signaling modalities familiar to most workers in molecular and cell biology today. Here, we discuss the logic, strategies and recent technologies that provide a roadmap for any group of researchers to investigate bioelectrical signals in their favorite system.
The strategies and concepts that we map out are applicable to a wide array of work in developmental, regenerative and cancer biology (plus toxicology, evolutionary biology and neuroscience). However, the aspects specifically excluded from consideration in this chapter should be noted.
First, we focus on multicellular contexts. Voltage gradients regulate important events at the level of individual cell behavior (Table 1), controlling cell number (proliferation and apoptosis), cell shape (orientation and outgrowth), cell position (migration and galvanotaxis) and cell differentiation (or de-differentiation), often over-riding competing chemical cues (Rajnicek et al. 2007; Zhao 2009). A number of recent reviews (Blackiston et al. 2010; McCaig et al. 2005; McCaig et al. 2009; Reid et al. 2005; Sundelacruz et al. 2009; Yao et al. 2011) and protocols (Song et al. 2007) have covered these data; they are mentioned only to illustrate the full complement of responses that one may observe (and study) in response to specific instances of bioelectric cell-cell communication. Significant progress has been made in dissecting the molecular mechanisms of electric-field-based guidance of cell motility and orientation in the context of wound healing (McCaig et al. 2009; Nuccitelli 2003; Ozkucur et al. 2011; Pan and Borgens 2010; Robinson and Messerli 1996; Zhao 2009). Here, we focus on strategies applicable to dissecting roles of bioelectrical signals as one component of the morphogenetic field of instructive information (an important part of communication between any cell and the host organism).
Table 1.
Cell-level properties/behaviors controlled by bioelectric events
| Cell Behavior | References |
|---|---|
| Proliferation and cell cycle progression |
Arcangeli et al. 1993; Binggeli and Weinstein 1986; Cone 1970; Cone 1971; Cone 1974; Cone and Cone 1976; Cone and Tongier 1971; Cone and Tongier 1973; Liebau et al. 2006; MacFarlane and Sontheimer 2000; Morokuma et al. 2008a; Rouzaire-Dubois et al. 1993; Stillwell et al. 1973; Wonderlin and Strobl 1996 |
| Apoptosis | Lang et al. 2005; Lauritzen et al. 2003; Miki et al. 2001; Wang et al. 1999 |
| Migration and orientation |
Anderson 1951; Fraser et al. 2005; Hyman and Bellamy 1922; McCaig et al. 2005; Pullar and Isseroff 2005; Schwab 2001; Schwab et al. 1995; Stump and Robinson 1983; Yan et al. 2009; Zhao et al. 1997 |
| Differentiation |
Barth and Barth 1974a; Barth and Barth 1974b; Hinard et al. 2008; Konig et al. 2006; Lange et al. 2011; Sundelacruz et al. 2008 |
| De-differentiation | Cone and Cone 1976; Cone and Tongier 1971; Harrington and Becker 1973; Stillwell et al. 1973 |
Second, bioelectric cues as defined here specifically do not include: action potentials in excitable cells (millisecond-scale spiking in nerve and muscle), the effects of exogenous electromagnetic field exposure and the fascinating literature on developmental roles of the geomagnetic field, charge transfer in DNA, static charges and electromagnetic communication among cells via ultraweak photon emission (see Table 2). Instead, we focus on slow changes of transmembrane potential (Vmem) in all cells. The sources of these bioelectrical gradients are ion channels and pumps that underlie the movement of chloride, sodium, potassium and hydrogen ions. The large literature on calcium signaling (Jaffe 1999; Slusarski and Pelegri 2007) is not discussed here, because it signals at extremely low concentrations; its effects are mediated by its chemical nature, not its contribution to transmembrane potential.
Table 2.
Biophysical modes of cell-cell communication not included in this chapter. Whereas this review focuses on voltage potential across the cell membrane, numerous other types of bioelectrical signals exist (Cifra et al. 2011; Levin 2003; Rossi et al. 2011) and may underlie poorly understood channels for cell-cell communication in vivo
The voltage gradients set up by cells result in endogenous electric fields that can mediate long-range communication (Borgens et al. 1989; Jaffe and Nuccitelli 1977; Nuccitelli 1995; Nuccitelli et al. 1986; Robinson 1989; Robinson and Messerli 1996, 2003). In addition to the channels and pumps, gap junction (GJ) proteins allow the direct exchange of small molecules (including ions) between the cytoplasm of adjacent cells, thus establishing local domains of iso-potential cell groups (Fitzharris and Baltz 2006) that might encode cell identity (Guthrie et al. 1994). GJs (Levin 2007a; Nicholson 2003) not only augment the ability of cells to sense extracellular electric signals from their neighbors (Cooper 1984) but also partition cell fields into functional domains, for example, when delimiting regions of neurogenic precursors in the spinal cord (Russo et al. 2008).
The large body of data demonstrating the importance of bioelectrical signals in patterning is unfamiliar to several generations of modern cell and developmental biologists. This is largely because the rise of molecular biology has focused attention on the development of tools, protocols and concepts most suited to the study of biochemical signals, making it extremely difficult to dissect the genetic basis and downstream targets of discrete bioelectric events. The last decade has seen a resurgence of the field of bio-electricity as new techniques in molecular physiology have enabled high-resolution functional approaches. This work has implicated voltage gradients as instructive signals in novel aspects of patterning, has allowed the mechanistic connection of biophysical events with canonical upstream and downstream genetic pathways and has revealed bio-electric parameters as convenient “control knobs” on cells and tissues that can be exploited for important advances in biomedical intervention.
Preparing to investigate a bioelectrical pathway: thinking differently
Transmembrane voltage potential is an instructive parameter for patterning and not simply a housekeeping property. One of the remarkable findings in this field is that bioelectrical cues are distinct from the metabolic gradients proposed by Child (1941): it is usually possible experimentally to dissociate the housekeeping functions of bioelectric properties from their more subtle patterning roles. Much as modulation can transmit information on top of a strong carrier wave, targeted artificial perturbation of Vmem usually results not in death or un-interpretable dysmorphias but in specific coherent changes of large-scale patterning (Adams et al. 2007; Beane et al. 2011; Blackiston et al. 2011; Pai et al. 2012; Vandenberg et al. 2011). Thus, it is possible to functionally perturb Vmem and to characterize phenotypes that are not unduly confounded by generalized toxicity or pleiotropic effects.
It will be helpful for those entering this field to familiarize themselves with the “classics” (Altizer et al. 2001; Borgens et al. 1989; Borgens 1982; Borgens and Shi 1995; Burr 1944; Burr and Northrop 1935; Hotary and Robinson 1992, 1994; Jaffe 1981, 1982; Jenkins et al. 1996; Marsh and Beams 1947, 1949, 1952; Nuccitelli 1980, 1992; Robinson and Messerli 1996; Woodruff and Telfer 1980). Work over the last 50 years has provided strong functional data illustrating the way in which multicellular systems utilize bioelectric signals for a wide range of morphogenetic processes. While pre-dating molecular-genetic tools, this body of data serves to illustrate general principles and wide conservation and applicability of bioelectric signals and to provide an important complement to today’s focus on genetic networks and chemical gradients.
These data have been recently reviewed (Adams 2008; Levin 2009a, 2011a; McCaig et al. 2009) and a small sample is shown in Table 3, illustrating the known roles of bioelectric signals in multicellular structures. Although many modern workers do not think about bioelectric signaling as a cohesive field, connections between molecular genetic pathways and ion flows are unavoidably being forged by new data, such as ion channel genes coming up as hits in microarray and network analyses and drugs identified in small molecule screens that turn out to be ion channel modulators, such as salinomycin (Gupta et al. 2009). Several channelopathies with morphogenetic or neoplastic phenotypes have now been discovered by unbiased approaches (Tables 4, 5), although ion transporters are usually deprioritized in comparative microarray experiments, because cell and molecular biologists are not yet accustomed to thinking in terms of voltage itself as an instructive signal. By highlighting the techniques and tools now available and by illustrating strategies for integrating bioelectrical signals with mainstream pathways, we hope that workers in multiple sub-fields will find it easier to design and implement investigations of the regulatory roles of ion currents and voltage gradients in their favorite patterning or mis-patterning problem.
Table 3.
Data on endogenous bioelectric signal roles in morphogenesis. Studies in which bioelectric parameters have been functionally shown to have an instructive patterning role
| Role | Species/system | References |
|---|---|---|
| Cellular polarization (anatomical asymmetry of cell or epithelium) |
Alga Fucus, yeast | Jaffe 1982; Minc and Chang 2010 |
| Migration of neurons and positional information | Chick, Amphibia | Pan and Borgens 2010; Shi and Borgens 1995 |
| Patterning in gastrulation, neurulation and organogenesis |
Chick, axolotl, frog |
Adams et al. 2006; Borgens and Shi 1995; Hotary and Robinson 1992; Levin et al. 2002; Shi and Borgens 1995; Stern 1982 |
| Directional transport of maternal components into the oocyte |
Moth, Drosophila | Woodruff 2005 |
| Growth control and size determination | Segmented worms | Kurtz and Schrank 1955 |
| Neural differentiation | Xenopus embryo | Lange et al. 2011; Uzman et al. 1998 |
| Polarity during regeneration | Planaria, plants, and annelids | Beane et al. 2011; Bentrup et al. 1967; Marsh and Beams 1947, 1949, 1950, 1952; Novak and Bentrup 1972; Novak and Sirnoval 1975) |
| Induction of limb and spinal cord regeneration | Amphibia | Borgens 1986; Borgens et al. 1990, 1986 |
| Control of gene expression and anatomy in craniofacial patterning |
Xenopus embryo | Vandenberg et al. 2011 |
| Induction of eye development | Xenopus embryo | Pai et al. 2012 |
Table 4.
Genetic data identifying patterning roles for ion channels or gap junctions. Although single gene mutation approaches are not well-suited to uncovering roles for resting voltage potential (Vmem) because of the high degree of compensation and redundancy among ion channel family members, a number of ion-transport regulators have been identified in unbiased screens for morphogenetic mutants. Comprehensive screens for bioelectric signaling in development will require methods in which Vmem is systematically altered in distinct cell types, among discrete ranges of voltage, for example, by using tight physiological or optical control of genetically misexpressed transporters
| Protein | Morphogenetic role | Species | Reference |
|---|---|---|---|
| TMEM16A chloride channel | Tracheal morphogenesis | Mouse | Rock et al. 2008 |
| Kir7.1 potassium channel | Melanosome development | Zebrafish | Iwashita et al. 2006 |
| KCNH2 potassium channel | Cardiac morphology | Mouse | Teng et al. 2008 |
| Cx41.8 gap junction | Pigmentation pattern | Zebrafish | Watanabe et al. 2006 |
| Cx43 gap junction | Fin regeneration | Zebrafish | Hoptak-Solga et al. 2008 |
| Cx43 gap junction | Fin-size regulation | Zebrafish | Iovine et al. 2005 |
| Kir2.1 potassium channel | Craniofacial morphogenesis (Andersen-Tawil syndrome) | Mouse | Bendahhou et al. 2003 |
Table 5.
Genetic data identifying roles for ion channels or gap junctions in cancer. Several ion transporters are now recognized as causal agents in carcinogenesis, consistent with the role of Vmem in regulating cell proliferation, migration and differentiation
| Protein | Species | Reference |
|---|---|---|
| NaV1.5 sodium channel | Human | House et al. 2010; Onkal and Djamgoz 2009 |
| EAG-1 potassium channel | Human | Pardo et al. 1999 |
| KCNK9 potassium channel | Mouse | Pei et al. 2003 |
| Ductin (proton V-ATPase component) |
Mouse | Saito et al. 1998 |
| SLC5A8 sodium/butyrate transporter |
Human | Gupta et al. 2006 |
| KCNE2 potassium channel | Mouse | Roepke et al. 2010 |
In formulating testable models of voltage-based control of cell behavior, several unique features of this modality should be kept in mind. Bioelectric signals are fundamentally epigenetic, in the sense of Waddington’s Epigenetic Landscape (not in the more modern and restrictive sense of chromatin modification); they underlie physiological heterogeneity (Brock et al. 2009) and are a component of the system-level stable state that directly reflects Waddington’s original concept of the relationship between genotype and anatomical phenotype (Huang et al. 2009). Only recently have the tools appropriate to the rather unusual properties of bioelectrical signals become available; their use requires a somewhat different mindset, integrating physiological and biophysical concepts that are distinct from those familiar to molecular developmental biologists.
For example, a traditional genetic screen or a regulatory network/microarray analysis might implicate a specific ion channel gene in some process of interest. It is then natural to think of this gene as being intrinsically tied to a given phenotype. However, this kind of mapping is often illusory as it is the physiological effect of that protein, not its genetic identity, that is important for the phenotype. Indeed, one of the main strategies illustrated below is the use of deletion and rescue experiments (swapping one channel for a completely different one) to identify Vmem roles that are functionally independent of the protein machinery that created the endogenous potential gradient.
What might bioelectric signals be doing? Vmem changes and ion flows are components of long-range signaling pathways among cells that occur during embryogenesis and regeneration. They can regulate the transport of small signaling molecules in and out of cells (as occurs for the electrophoretic transport of maternal serotonin among early embryonic blastomeres during left-right patterning (Fukumoto et al. 2005b) or cause the release of diffusible secondary messenger molecules from specific cell populations, as occurs during the voltage-mediated neoplastic-like transformation of pigment cells (Blackiston et al. 2011). Vmem changes in adjacent cells can propagate over long distances via conventional gap-junctional paths (Esser et al. 2006) or the more exotic nanotubes, namely, narrow cytoplasmic structures that have a GJ at their base and that can conduct electrical signals between cells as a kind of nanowire (Wang et al. 2010) but which remain to be investigated in complex tissues. The Vmem levels of key cell groups mediate large-scale polarity along major body axes, including head-tail (planarian regeneration; Beane et al. 2011), left-right (embryogenesis; Aw and Levin 2009) and base-tip (pollen-tube outgrowth; Certal et al. 2008; Michard et al. 2009) and carry positional information for the guidance of migratory cell types (Shi and Borgens 1995).
Interestingly, voltage changes also encode master-regulator-like signals that initiate complete, highly orchestrated, self-limiting downstream patterning cascades such as tail regeneration (Adams et al. 2007; Tseng et al. 2010). For example, the activation of Xenopus tail regeneration by a yeast proton transporter illustrates that signals of extremely low information content can induce responses in the host that are far too complex to bioengineer directly, the micromanagement of the construction of a limb or an eye from individual cell types being currently beyond reach. This is an extremely attractive property for biomedicine, because it suggests that desirable tissue outcomes can be induced long before we know everything needed to directly micromanage the assembly of a complex organ or appendage (Levin 2009b, 2011b). Although a large body of literature exists showing the promise of applied electric fields in biomedicine (Albert and Wong 1991; Shapiro et al. 2005; Walters 2010; Yasuda 1974, 1977), the recently developed molecular-genetic techniques for bioelectric modulation have not yet been applied in human patients. They are currently being used to dissect these pathways in animal model systems and may offer unprecedented levels of spatio-temporal control of physiological signals that regulate cell growth and organ anatomy.
To understand the manner in which bioelectrical signals participate in the specification of anatomy, we need to keep in mind that bioelectric networks, like gene networks, can exhibit non-linear behavior with surprising dynamics. For example, changes in membrane voltage gradients affect the function of voltage-sensitive ion channels, which in turn alters membrane potentials further, implementing a dynamical system with multiple attractor states (Gallaher et al. 2010; Sachdeva et al. 2010). Likewise, GJs shape electrical properties of cell groups and are themselves sensitive to changes in transmembrane potential and pH. This offers rich opportunities for cell groups to use ion flows to implement both positive- and negative-feedback mechanisms. The former, such as that created by the hydrogen/potassium exchanger regulation via potassium-sensitive nuclear factor kappa B (Zhang and Kone 2002), can be used to amplify small physiological signals, whereas the latter, such as that created by the depolarization-induced activation of the hyperpolarizing V-ATPase pump (Jouhou et al. 2007), can be employed to ensure the robustness of patterning against perturbations. This can often be ignored for simplicity and Vmem regulation can be treated as a self-contained module (see Fig. 1 for a representation of the relationship between transcriptional and biophysical controls). However, a great deal of interesting biology is open territory for those interested in understanding the “order for free” (Kauffman 1993) that arises from the movement of ions.
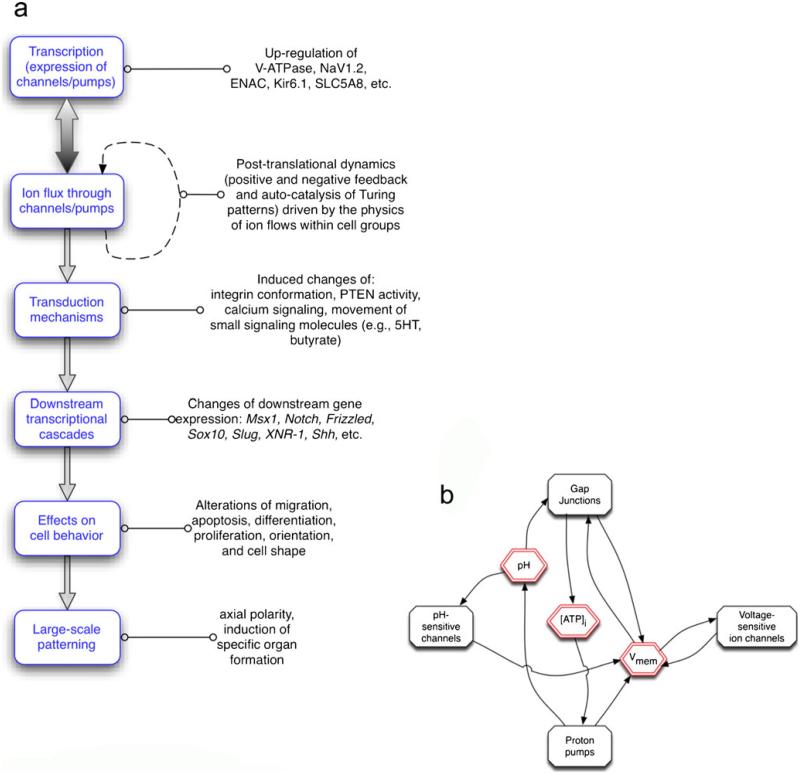
Fig. 1.
Overview of bioelectric signaling in cell regulation. a Transcriptional events (up- or down-regulation of specific ion channel/pump genes) establish the complement of ion translocators expressed in any cell. Their activity results in changes of ion content and transmembrane potential, which is transduced by a number of mechanisms that couple biophysical events to changes of the expression of target genes. These in turn also control key cell behaviors and large-scale anatomical properties such as axial patterning and organ specification/morphogenesis. b Interplay between physiological properties (red) and specific proteins (black). An important aspect of physiological signaling is the multiple feedback loops that occur because of the physiological (post-translational) gating of channels and pumps. For example, the level of gap-junctional connectivity determines the way that voltage spreads between adjacent cells but gap junctions are themselves regulated by voltage gradient. Thus, cells and cell groups can implement self-organizing spatially distributed stable patterns (e.g., auto-catalytic Turing-Child dynamics) even in the absence of differential transcription or genetic prepattern. Cell fields can thus be described as complex dynamical systems with multiple stable attractors, supporting symmetry breaking, hysteresis (memory) and the amplification of stochastic heterogeneity (Vmem resting voltage potential)
Ion transporters are gated post-translationally; thus, bioelectrical signals derive some of their behavior from the intrinsic physics governing the movement of charged molecules in electric fields. Whereas such physiological dynamics ultimately do indeed feed into transcriptional programs, we should note that order and heterogeneity can be generated among cells with identical levels of protein expression in the absence of changes in mRNA and protein levels. Much as action potentials and calcium fertilization waves in eggs drive fronts of moving signals across the cell surface without needing changes of transcription or translation, multicellular networks implementing voltage-sensitive GJs and ion channels can establish complex regionalizations of Vmem and GJ coupling as a kind of autocatalytic process.
As has long been known, some chemical systems can support self-organizing waves of activity; for example, the Belousov-Zhabotinski (Bansagi et al. 2011; Yoshida 2010) reaction exhibits stunning patterns of dynamically moving colors that reveal changes of concentration of various reaction products across a well-mixed volume of medium. A classic paper by Alan Turing (Turing 1952) proposed that spatially periodic, temporally stable patterns emerging from the instability of a homogeneous steady state represent a mechanism for morphogenesis in living systems. Recent work (Agladze et al. 1993; Sawai et al. 2000; Schiffmann 2011) has analyzed the means by which the chemical gradients formed by a system of diffusing compounds (a long-range inhibitor plus a short-range activator) can also self-catalyze the creation of a pattern within a homogeneous cell field (Schiffmann 1991). Such symmetry-breaking and spatial self-organization might underlie anterior-posterior pattern formation during Drosophila embryogenesis, Hydra regeneration, amphibian organizer signaling and pigment patterns in the epidermis of numerous animals (Kondo 2002; Kondo et al. 2009; Meinhardt 2000, 2001; Nakamasu et al. 2009). Importantly, these Turing fields are not exclusively implemented by chemical diffusion; the fundamental properties required for the self-generation of patterns can also be implemented by bioelectric signaling mechanisms (Boettiger et al. 2009; Boettiger and Oster 2009; Oster 1988).
Fields of homogeneous cells that are able to generate and respond to ionic signals could thus support Turing-Child dynamics; this implies that many different cell types can form an “excitable medium” within which waves, stable compartments and other patterns can be set up purely by dynamics of physiology and do not require a pre-existing transcriptional prepattern (but themselves set up down-stream differential gene expression domains by inducing or repressing specific transcriptional readouts). Moreover, systems of ion channels can implement hysteresis and occupy one of two discrete Vmem states by physiological signals (Gallaher et al. 2010); any such bi-stable system is potentially a memory element (storing 1 bit of information) revealing that the bioelectric properties of all cells could potentially store information in the real-time dynamics of current flows, as occurs in computer memory. These unusual aspects of bioelectrical pathways are important not only because they occur in vivo and must ultimately be incorporated into our understanding of endogenous pattern formation mechanisms but also because they can be exploited in bioengineering, artificial life and synthetic biology approaches that seek to exploit computational abilities of tissues to extend beyond that found in nature in order to build robust cybernetic devices.
One difficulty of current approaches for understanding and modulating bioelectrical signal pathways is that physiologically derived patterning is largely invisible to widespread protein or mRNA profiling techniques. Cells with the exact same complement of channel proteins can be in different physiological states, because channels can be gated by other physiological signals and cells with very different channel gene profiles can be in the same physiological state, because the same Vmem and pH range can be achieved by many different ion fluxes. For example, screens based on knockouts, morpholinos, RNA interference and protein or mRNA profiling would have entirely missed all of the early events of the left-right patterning in the frog, because this is a system that uses a voltage gradient to distribute small molecule morphogens and is entirely driven by maternal proteins and the physics of electrophoresis for many hours before zygotic transcription even begins (Levin 2006; Levin and Palmer 2007). Likewise, isolated or sorted cell populations that appear pure by molecular markers turn out to include several discrete groups with distinct physiological properties (Yu et al. 2002).
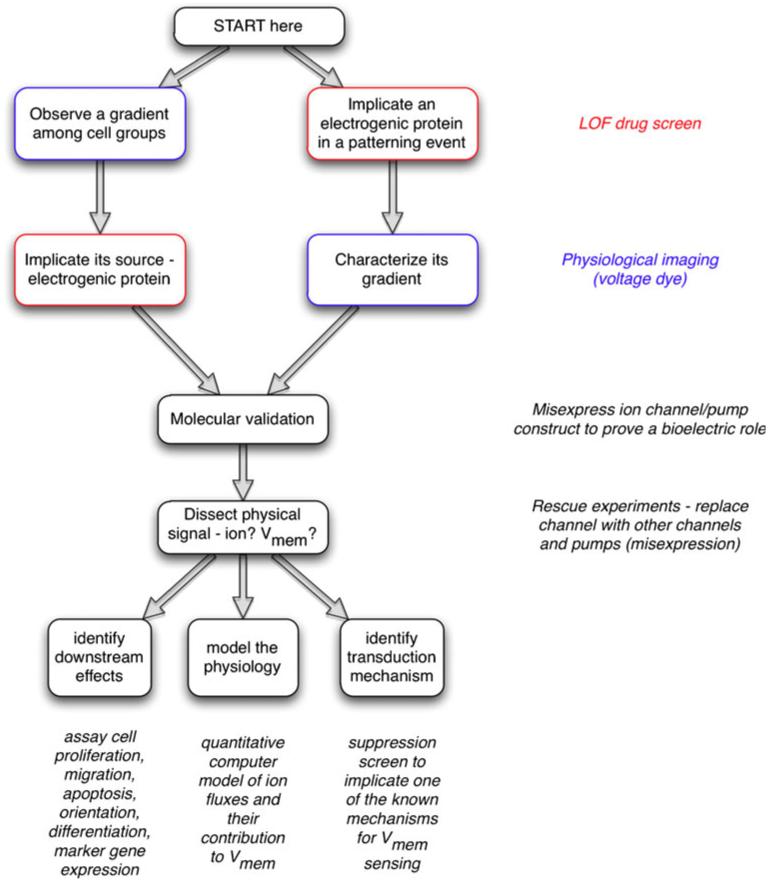
How does one begin to investigate a bioelectrical signal in a system? An overall strategic map is shown in Fig. 2 (and details for each step are discussed below). One usually starts either by (1) detecting an interesting bioelectrical gradient and then implicating a specific ion channel/pump in its production (by using a loss-of-function drug screen), or (2) implicating a specific channel/pump protein in some patterning event of interest and, then, motivated by the functional data that suggest this ion flux is actually important, characterizing the physiological gradients produced by it. This chemical-genetics work is followed by molecular validation of the pharmacological results: misexpression of channel and pumps (or their mutants) by using gene-specific molecular genetic reagents to confirm that bioelectric signals do indeed matter in the phenomenon under study and that at least one ion-translocator protein can be used as an entry-point into its molecular investigation.
Fig. 2.
Strategy for investigating bioelectric signaling. One often begins by detecting an interesting bioelectrical gradient during some process and hypothesizing that this voltage or ion flow pattern is functionally important. In this case, the next step is to develop a robust assay recapitulating this process and to conduct a loss-of-function hierarchical drug screen to narrow down a list of candidate transporters that might underlie the observed gradient. In contrast, a specific transporter candidate might be suggested by the results of a genetic screen, network analysis, or microarray comparison. Because Vmem is determined by ion flux, part of characterizing bioelectric signals is determining the endogenous translocators involved. Thus, the next step would involve the use of fluorescent voltage- or ion-reporting dyes (with and without a blocker of the channel/pump) to characterize the spatiotemporal properties of whatever gradient pattern might be produced by the transporter in question (Adams and Levin 2012a, 2012b; Oviedo et al. 2008). Molecular-genetic validation of the pharmacological data follows; a loss-of-function experiment with the knockout or misexpression of a dominant negative mutant should be used to establish that this transporter is indeed important for the phenotype under study. Next, to determine the physical process carrying instructive information, the transporter is blocked and a rescue of the phenotype is attempted with other transporters that distinguish among non-ionic, ion-specific and voltage-specific functions. Subsequently, this biophysical signal has to be connected to canonical pathways by (1) identifying the second-messenger mechanism(s) by which a biophysical signal is transduced into changes in cell behaviors, (2) identifying its downstream effects (which genes does it turn on/off, how does it affect cell behavior) and (3) modeling the physiology (quantitative spatial simulation of the ion flows that result in the relevant voltage gradient)
The next step is an extension of the molecular validation process: identification of the physical signal that really matters. For example, if a morphogenetic phenotype is induced by the function of a particular proton pump, is this because of the resulting hyperpolarization (exit of positive charges) or of the pH gradient? Once the physical event that carries the cell-cell communication signal is identified, three lines of investigation can be conducted, depending on where one’s interests lie. To understand how this bioelectrical signal regulates patterning outcomes, we need to (1) identify downstream effects (changes in cell behavior and gene expression that implement the morphogenetic outcome), (2) model the physiology to discover which ion flows give rise to what voltage gradients and (3) identify the transduction mechanism(s) that link biophysical events to second-messenger and transcriptional cascades (this is usually carried out with a suppression drug screen; Fig. 3).
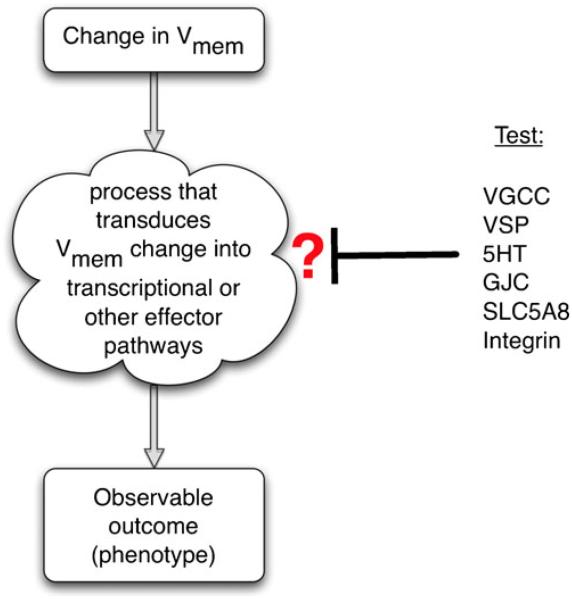
Fig. 3.
Identification of a transduction mechanism. To identify which of several possible mechanisms is involved in transducing an epigenetic (biophysical) event into a biochemical signaling pathway, a suppression screen should be performed (Blackiston et al. 2011). Each candidate transduction mechanism can be probed in turn by inhibiting it in an assay in which an induced change in transmembrane potential leads to an observable phenotype (increase in proliferation, up-regulation of a target gene, cell migration, etc.). For example, if gene X is up-regulated upon depolarization but this up-regulation does not occur in the presence of a GdCl, then voltage-gated calcium channel (VGCC) opening is implicated. If on the other hand a blocker of gap-junctional communication (GJC) abolishes the effects of depolarization, this suggests that the voltage-gated control of GJC and small molecule movement mediate the effects. Similar experiments with inhibitory drugs, dominant negative constructs and genetic deletion can be used to implicate serotonergic (5HT) signaling, butyrate-driven chromatin modifications (SLC5A8), integrin signaling, voltage-sensitive phosphatases (VSP), or other transduction mechanisms
Recent studies of the role of bioelectricity in the left-right patterning of the early frog embryo provide a simple illustration (Levin 2006; Levin et al. 2006). A pharmacological screen implicated several transporters as being required for normal asymmetry (Levin et al. 2002); fluorescent voltage dye imaging than revealed that these transporters produced a L→R gradient in transmembrane potential during early cleavages (Adams et al. 2006; Levin et al. 2002). The misexpression of various proton and potassium channels showed that these channels and pumps were indeed used endogenously as an obligate component of left-right patterning but that voltage was the crucial parameter and worked upstream of changes in the transcription of genes that controlled morphogenesis of the heart and asymmetric viscera (Adams et al. 2006; Aw et al. 2008, 2010; Morokuma et al. 2008b). A suppression screen identified serotonergic mechanisms as mediating the effect (Fukumoto et al. 2005a, 2005b) and quantitative modeling (Esser et al. 2006; Zhang and Levin 2009) revealed the way that this bioelectric parameter works to set up an embryonic gradient of serotonin. The loop was then closed by showing how the serotonin gradient regulates transcription, i.e., by binding to chromatin-modifying machinery, which results in unilateral gene expression and asymmetric morphogenesis at later stages (Carneiro et al. 2011). Below, we give specific guidance on each of these steps.
Implication of bioelectrical signals
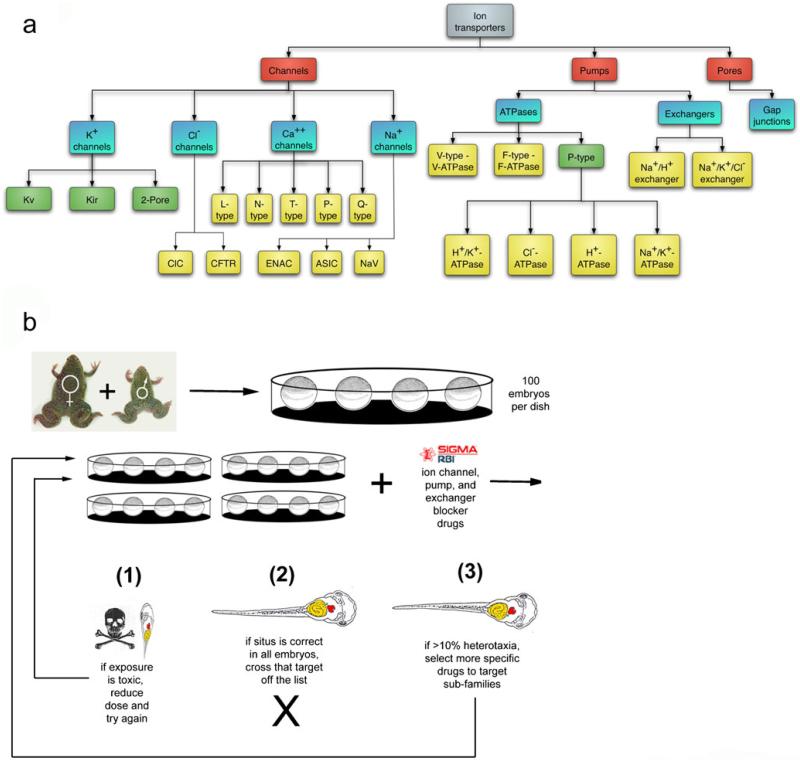
To test whether bioelectric signaling plays a role in a given patterning event (e.g., eye induction, initiation of cell movements), it is best to conduct a pharmacological screen (Fig. 4). Such screens (detailed information on reagents is given in Adams and Levin [2006a, 2006b]) inexpensively and rapidly implicate specific endogenous ion-transporter proteins in any assay. This is a chemical genetics approach that capitalizes on a tiered (least specific → more specific) tree-based distribution of channel and pump blocker compounds that enables an efficient binary search for likely candidates. The use of highly non-specific reagents at the top tier is a major advantage: for example, if barium chloride (a non-selective potassium channel blocker) does not impact the phenotypic endpoint, the whole enormous set of potassium channels can be crossed off the list and does not need to be tested. If a positive hit is obtained, then one must proceed to test the candidates by using drugs in the more-specific nodes below the one that positively impacted the assay.
Fig. 4.
Loss-of-function hierarchical drug screens. To determine whether a bioelectric process is likely to be involved in some process of interest and simultaneously to implicate specific channel/pump family(ies) as endogenous mediators of the biophysical signals, a loss-of-function pharmacological screen is performed. Known blockers of different types of channels/pumps are arranged (Adams and Levin 2006a, 2006b) in a hierarchical tree, from least specific to most specific (a). This chemical genetics approach is applied in an assay (b), such as the scoring of asymmetric positioning of internal organs following drug exposure at early embryonic stages (a screen to identify roles for ion flow in a left-right patterning during development; Adams et al. 2006; Levin et al. 2002). A negative result at any node in the tree means that the nodes of drugs (and their matching protein targets) below that point do not have to be pursued further, whereas a positive result (an observable change in the assay outcome) leads to the testing of the nodes below. This screen does not saturate (since not every transporter is blocked by a known drug) and in some (surprisingly rare) cases, pleiotropic functions of a given transporter lead to a toxicity phenotype that precludes a clean yes/no answer. Nevertheless, the existence (and continued development) of a plethora of pharmacological reagents and the demonstrated possibility of dissociating subtle patterning functions of ion flows from housekeeping roles combine to make this a powerful, rapid and inexpensive method to identify a manageable number of candidates for further molecular analysis and to motivate investigations of bioelectric components in a process of interest. Figure in panel a is taken from Adams and Levin 2006a, Fig. 1, with permission of John Wiley and Sons. Figure in panel b is taken from Levin et al. 2006, Fig. 3, with permission of S. Karger AG, Basel
Usually, no more than 5–10 compounds need to be tried in the chosen assay in order to home in on an ion-transporter type. Such screens probing native bioelectrical mechanisms have resulted in the identification of channels and pumps as novel components of left-right patterning (Adams et al. 2006; Levin 2006), anterior-posterior polarity determination in planarian regeneration (Beane et al. 2011; Nogi and Levin 2005), tail regeneration in Xenopus tadpoles (Adams et al. 2007; Tseng et al. 2010) and mammalian stem cell regulation (Gupta et al. 2009; Lange et al. 2011; Sundelacruz et al. 2008). The robust and continuing development of novel compounds targeting ion channels will further improve the power and efficiency of these screens over time. Although any target implicated by pharmacological approaches needs to be confirmed by using gene-specific molecular reagents (see below), this approach has the advantage that it rapidly tests whether any aspect of bioelectricity is involved and suggests a manageable number of candidate genes for molecular validation. Hierarchical pharmacological blockade also has the advantage that, unlike knockdowns of individual genes, it greatly reduces problems of compensation (masking phenotypes as other family members substitute for the one channel knocked out in a traditional screen). When performed correctly, the output of this analysis is a candidate (or set of candidates) for the endogenous protein that drives a given ion flux important for some particular assay.
Characterization of bioelectrical properties in vivo
Overview of physiological profiling
The first step to dissecting a bioelectric signal is the characterization of the spatio-temporal distributions of ionic parameters in vivo and a determination of their correlation with anatomical and genetic patterning events. This is akin to the mRNA and protein levels revealed by in situ hybridization and immunohistochemistry in conventional molecular embryology. However, unlike protein and mRNA localization, physiological properties cannot be studied in fixed samples: reporters must be used in the living state. Specifically, the imaging of voltage gradient and ion concentration patterns within tissues (or whole embryos) can be used for: (1) revealing the boundaries of iso-potential cell groups (compartments or embryonic fields), (2) identifying the distributions of Vmem gradients among cells that regionalize the tissue as a pre-pattern for gene expression, (3) revealing the existence of physiological heterogeneity among cells that might seem identical at the anatomic or even genetic levels, (4) identifying special cells such as stem cells, precursor/progenitor cells and cancer cells by their unique physiological signature and (5) identifying special developmental stages or events (e.g., initiation of a regenerative program) by alterations in steady-state ionic properties.
Tools for the characterization of bioelectrical events now include highly sensitive ion-selective extracellular electrode probes (Reid et al. 2007; Smith et al. 2007) that reveal ion flux at the cell membrane, microelectrode arrays (Aryasomayajula et al. 2010) and techniques that report the content of individual ion species such as protons (Tantama et al. 2011) and sodium (Tseng et al. 2010). Voltage gradients can now be visualized in three-dimensional time-lapse recordings by using fluorescent reporters of transmembrane potential such as the cell-permeable dyes DiSBAC2(3) and CC2-DMPE (Adams et al. 2006; Oviedo et al. 2008; Ozkucur et al. 2010), reporter proteins (Baker et al. 2008; Mutoh et al. 2011; Shen et al. 2011; Tsutsui et al. 2008a) and more exotic nano-scale materials (Tyner et al. 2007), suitable for use in any optically accessible tissue (Steinberg et al. 2007; Yun et al. 2007). These are a significant addition to traditional electrophysiology, because whole organs can be imaged at once and the data can be collected over long time periods in moving samples without needing to perforate each individual cell membrane. GJ paths can be revealed by the introduction of fluorescent small molecules followed by imaging (Bittman et al. 2004; Coelho and Kosher 1991a, 1991b; Levin and Mercola 1998; Nagajski et al. 1989) and by more elegant techniques such as photo-uncaging of GJ-permeable fluorescent molecules (Bedner et al. 2003; Braet et al. 2003; Guo et al. 2008; Yang and Li 2009) to map out GJ-coupled cell groups and junctional isolation boundaries.
Although many of these reagents have been optimized for rapid (neuronal) electrical signals (Akemann et al. 2010; Perron et al. 2009a), they are adaptable to the slower dynamics of patterning events because of their modular nature. Versatile genetically encoded voltage sensors are now available (Akemann et al. 2009, 2010; Baker et al. 2008; Gautam et al. 2009; Mutoh et al. 2011; Perron et al. 2009a, 2009b): fluorescence resonance energy transfer (FRET)-based proteins with plasma-membrane targeting and red-shifted fluorescence (for better penetration of longer-wavelength light), e.g., VSFP2.3 (Lundby et al. 2008; Murata et al. 2005; Mutoh et al. 2009). Several studies have now explored the endogenous patterns of ion flows during morphogenetic events (Ozkucur et al. 2010; Vandenberg et al. 2011) or the behavior of stem cells (Stroh et al. 2010) and this technique is finding its way into medicine for minimally invasive diagnosis by identifying cells with abnormal growth potential revealed by their specific physiological signature (Ishiguro et al. 2010).
The sample under study needs to be either transfected with DNA expressing a genetically encoded reporter protein or soaked in a medium containing fluorescent reporter dyes. When performed after a transporter has been implicated, these techniques are used to test a specific hypothesis: that this transporter sets up a physiological gradient that carries instructive information in some assay. For example, voltage and sodium reporter dyes have been used to characterize the bioelectrical states of regeneration blastemas during amphibian tail regeneration following a drug screen (Adams et al. 2007; Ozkucur et al. 2010; Tseng et al. 2010). On the other hand, any interesting event (e.g., organ induction, gastrulation, neurulation) can be imaged in an unbiased physiological “screen” to look for gradients and novel patterns that might be suggestive of patterning information otherwise invisible in the tissue.
Over 50 years ago, spatial patterns of bioelectric parameters (e.g., voltage difference between specific locations) were observed quantitatively to predict anatomical features developing at much later timepoints and possibly thus to control morphogenesis as a kind of subtle scaffold (Burr and Hovland 1937; Burr and Sinnott 1944; Lund 1947). However, we can now probe the instructive nature of such physiological gradients at molecular resolution, one example involving craniofacial patterning in Xenopus embryos. By using voltage-reporter dyes and timelapse microscopy, a map was made of the bioelectric gradients during the formation of the vertebrate face (Vandenberg et al. 2011). A complex regionalization of voltage gradient (natively driven by differences in the activity of the V-ATPase proton pump) demarcated the interior of the neural tube and the future mouth, while thin bilateral crescents on the edge of the face marked the position of the first pharyngeal pouch. These bioelectrically unique regions match the expression patterns of key genes that regulate the differentiation and migration of tissues in the face and the position of invaginating epithelium. Artificially perturbing the pattern of the voltage domains results in changes in the expression of important patterning genes, such as Slug, Mitf and Frizzled3 and in the subsequent characteristic defects in the morphology of craniofacial structures. Such spatio-temporal profiling of native physiology, combined with the detailed characterization of anatomical and molecular-genetic perturbation of the boundaries of the hyperpolarization domains, has revealed a superb example of a physiological state serving as a prepattern for regions of gene expression, much as transcriptional domains can act as prepatterns for subsequent anatomy (e.g., the Hox code). Similar findings have been reported for the formation of the vertebrate eye during which regions of endogenous hyperpolarization demarcate a small subset of Pax6-positive cells alone fated to become eyes; the signal has been shown to be instructive, as experimental manipulations designed to put other cells into the same Vmem range have been able to induce eye formation anywhere in the body (Pai et al. 2012). A convergence of modeling and molecular physiology data will be able to define intervention protocols to alter such patterns at will and thus potentially to provide the tools to repair a variety of craniofacial birth defects.
Specific principles of imaging transmembrane potential
The ability to measure Vmem has improved significantly with the invention of fluorescent Vmem reporting dyes (Adams and Levin 2012a). Classical electrode-based electrophysiology techniques are still the standard for absolute measurements of average plasma-membrane potential; however, dyes allow researchers to observe bioelectrical phenomena at both spatial and temporal scales that are impossible when using electrodes: (1) entire fields and volumes of cells can be measured simultaneously, so that cell-cell interactions and population dynamics can be imaged; (2) subcellular resolution can be achieved and thus the bioelectrical activities of various membranes and of various membrane regions can be parsed and their distinct contributions to cell physiology and behavior can be investigated; (3) long-duration observations are relatively simple, even for samples that move, making it possible to perform longitudinal studies of Vmem changes in response to external stimuli or natural changes, such as growth and/or development. The techniques are relatively simple and can be performed without specialized equipment, aspects that add even more to the power of these dyes to yield important data for our understanding of bioelectric signals and signatures.
The dyes work as reporters because the intensity of their fluorescence varies with membrane potential. Although the mechanisms are not completely understood, there are currently two ways that this is believed to occur: (1) a charged dye molecule accumulates or disperses, or moves within the membrane, in proportion to the charge on the membrane (Wolff et al. 2003), or (2) the color or intensity of a fluorophore varies as a protein undergoes conformational changes in response to membrane potential (Lundby et al. 2010). The oxonol dyes, such as DiBAC and DiSBAC are examples of the former. These negatively charged molecules enter the cell and associate with the positively charged membrane of de-polarized cells. Conversely, when the membrane is negative, these molecules leave the cell and cease to be fluorescent. The number of molecules fluorescing determines the brightness of the signal and thus signal intensity (usually measured on a scale of 0–255 or 0–4095) is a readout of the charge on the membrane. Mermaid is an example of a protein-based voltage reporter (Tsutsui et al. 2008b). With this reporter, a change in Vmem is “felt” by a voltage-sensitive domain that changes its conformation thus separating two fluorescent proteins or bringing them closer together, which results in FRET. Thus, the intensities of donor and acceptor fluorescence are a measure of Vmem. Other FRET-based techniques employ a donor, often the coumarin derivative CC2-DMPE, which is stationary in the outer leaflet of the membrane and a mobile acceptor, often an oxonol (Gonzalez and Tsien 1997). This coupling of dyes takes advantage of two different ways to measure Vmem: the fluorescence of the acceptor dye, which increases with de-polarization and the FRET between the two dyes, which increases with hyperpolarization. The two sets of signals can then be combined by normalizing one to the other (taking the ratio), thus filtering out some of the inevitable systematic error.
Choice of the dye to use involves knowledge of as much as possible about the dyes and the imaging equipment available. The first choice will often be a dye for which the appropriate filter cubes are already in the microscope. There are two general categories of dye; the fast- and the slow-response dyes. Fast dyes, such as Di-ANEPPS, can be used to image action potentials and other millisecond-range phenomena. The slow dyes have been used for long-running and/or time-lapse studies of Vmem. Dyes are also available as both impermeant and permeant forms, so their means of administration should be considered. Dyes also bleach at different rates and some even self-quench if used at too high a concentration. Whatever dye or set of dyes is chosen, the primary literature should be searched for any information available about the dye. Some commonly used dyes have been found to cause artifacts (Mennerick et al. 2010; Morimoto et al. 2007) and new information is becoming available rapidly and so a literature search is important to avoid having to rely entirely on the manufacturer’s data.
Crucially, all of the dyes must be tested and titered for each new cell type and as with any technique, appropriate controls must be run. The most important control is to confirm, by rational manipulation of Vmem with ionophores and by variation of external ion concentrations, that the change in dye intensity is in the right direction. The ideal extension of this control is an actual calibration of the dye. This can be a complicated task, as the best way to achieve it is to monitor dye intensity while simultaneously measuring Vmem by using an electrode. Another approach is to control Vmem by using the Goldman-Katz-Hodgkin equation to guide changes in external ion concentrations. In many situations, a report of the relative Vmem is sufficient to address fundamental and fascinating questions about cells and cell populations.
Imaging samples correctly is a critical aspect of using these dyes, as they are by nature quantitative. This means that many of the default capabilities of image analysis and manipulation programs must be ignored. In other words, some automatic functions, especially the ones that “improve” the images by changing pixel values, must be turned off. Two other critical aspects should be taken into account with imaging fluorescent dyes. The first is to optimize exposure by being sure to take advantage of the full range of intensities that can be resolved by your camera. The second is that the images must be corrected in two ways and only those two ways. The first is darkfield correction, which corrects for electronic noise in the system. The second is flatfield, which corrects for uneven illumination and other systematic distortions in the imaging machinery. Again, these two corrections are necessary and sufficient. No other manipulations that affect pixel intensity should be performed.
If the imaging has been correctly performed, analysis is relatively straightforward. The intensities in the various regions of an image can be measured by the software and reported. The difficult part here is defining a region of interest (ROI), which must be carried out without bias; the chosen method should be reported together with the results.
Functional experiments to test instructive roles
Identification of instructive roles for bioelectric events requires that changes in Vmem are inducible on demand in vivo, in a spatio-temporally controlled manner, to link these to cell- and tissue-level outcomes, as is routinely performed in knockdown and overexpression experiments for biochemical signals. Loss-of-function experiments performed by inhibiting or knocking down a specific channel or pump that underlies a given ion flow has allowed the identification of novel roles for Vmem in embryonic left-right asymmetry (Adams et al. 2006; Levin et al. 2002), tadpole tail regeneration (Adams et al. 2007; Tseng et al. 2010) and muscle cell differentiation (Hinard et al. 2008; Konig et al. 2004). The data obtained shed light on endogenous roles of ion-transport events within any given developmental or regenerative context.
Following the characterization of the Vmem, the next step is functional perturbation to test specific hypotheses. For example, if the imaging data suggest that a Vmem depolarization is responsible for a particular event (e.g., induction of organ primordium expansion), then two types of experiments need to be carried out. A loss-of-function test will reveal whether the depolarization is indeed required; for this, a hyperpolarizing channel (e.g., Kir2.1, Kv1.5) or pump (PMA-1.2) should be introduced shortly before the initiation of the event and if the hypothesis is correct, will block it. A gain-of-function test will reveal whether the depolarization is sufficient for the event; prior to the event’s occurrence (or in a state in which the event does not normally occur, such as a non-regenerative developmental stage in the case of voltage-directed regeneration), the tissue should be transfected with a depolarizing construct such as EXP-1 (a cation channel) or NaV1.2. These molecular reagents are part of a large toolkit (some of which are available from our laboratory upon request). They can be introduced, for example, by lipofection, electroporation, direct mRNA or DNA injection, or viral infection and should carry a fluorescent fusion protein to mark the cells in which the required ion flow change is expected to occur (especially important for detecting non-cell-autonomous effects). In all cases, the intended voltage change should be directly confirmed by using the measuring techniques described above, as some cells will use native transporters to attempt to counteract the voltage change being experimentally induced. When choosing the marker fluorophore, one should remember to consider the wavelengths that will be used to measure voltage, as overlaps will confound the data.
Direct application of electric fields is another technique that is well-suited to the study of cell responses to physiological-strength electric signals in vitro (Wang and Zhao 2010; Zhao 2009); however, the complex impedance of living tissues makes this difficult to control when applied in vivo. Highly targeted gain-of-function experiments should be performed by using well-characterized ion-transporter proteins to induce known changes in ion content and transmembrane potential in specific cells. For example, misexpression of the P-type (single protein) proton pump has recently been shown to be sufficient to induce regeneration of the tadpole tail when the native V-ATPase (a 13-subunit V-type H+ pump complex, normally required for regenerative response) is inhibited (Adams et al. 2007). Likewise, the manipulation of a regulated chloride channel and rescue experiments with potassium channels have allowed tight experimental control of Vmem in frog embryo cells, revealing a role for transmembrane potential in triggering a neoplastic-like transition in neural crest (stem cell) derivatives (Blackiston et al. 2011; Morokuma et al. 2008a).
Whereas genetic perturbation has the highest potential to yield high-resolution information about bioelectric patterning control, the uncertainties of gene therapy (and the use in model systems in which transgenesis is difficult, such as planaria or non-genetic model systems) and the difficulty of controlling the timing of activity require that pharmacological techniques also be developed for use in biomedical strategies; these can take advantage of extant drugs that target natively expressed channels and pumps to effect the necessary changes in transmembrane potential (Reid et al. 2011). One recent example is the design of a sodium ionophore cocktail that induced full regeneration of the tadpole tail (a complex neuromuscular appendage including spinal cord) in a range of non-regenerative conditions after just 1 h of exposure (Tseng et al. 2010); another is the use of proton/potassium exchanger and chloride channel modulators to control the regenerative polarity of planaria: fragments that normally develop a head and tail at their appropriate locations can be induced at will to reprogram blastema growth thereby resulting in double-head or no-head regenerates (Beane et al. 2011).
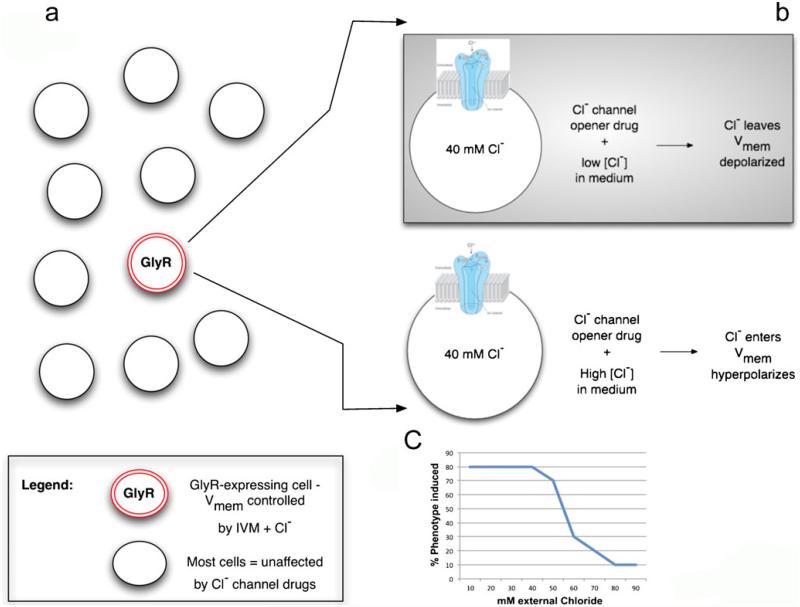
Misexpression of constitutive depolarizers and hyperpolarizers can be a powerful technique but often spatio-temporal regulation is desired. Moreover, it is advantageous to be able to control the degree of Vmem change, which is especially important in cases where a specific Vmem level might be crucial for determining tissue outcome. Spatial regulation can be achieved by the control of the number/location of cells transfected with the perturbing channel or by using light activation in the technique of optogenetics with light-gated depolarizing channelrhodopsins and hyperpolarizing halorhodopsins (Gradinaru et al. 2010; Knopfel et al. 2010; Liu and Tonegawa 2010). Temporal control, together with a significant degree of voltage regulation, can be achieved by taking advantage of drug-gated chloride channels (Fig. 5). Cells expressing the glycine-gated chloride channel can be exposed to a specific GlyCl channel opener (Ivermectin) and their voltage controlled simply by varying the concentration of chloride in the medium (since the open channel will allow experimental control of the amount of negative Cl− ions inside the cell).
Fig. 5.
Pharmacological strategy for specific control of Vmem. To control Vmem experimentally, the best strategy is first to express a convenient ion channel in cell(s) of interest (or make sure it is expressed there natively); alternatively, an ionophore can be used if the targeting of specific cell subpopulations is not needed. Then, the channel is opened with a specific pharmacological activator and the extracellular concentration of that ion is varied in the medium. For example, in a field of embryonic cells, some express the glycine-gated chloride channel GlyR (a). When targeted by Ivermectin (IVM), a specific GlyR opener (b), these cells can be depolarized in medium of low chloride content (because negative chloride ions will tend to leave the cells via the open channel down their concentration gradient) and hyperpolarized in a medium of high chloride content (since the negative Cl− ions will enter the cells). By varying the chloride level, one can observe, for example, the disappearance of a depolarization-induced phenotype, as the Vmem is driven toward hyperpolarization by the high chloride content (c typical result of a hypothetical experiment). To use this method quantitatively to measure or create absolute values of Vmem, it helps to know the internal and external concentrations of all the ions and their permeability coefficients. Then, the Goldman-Katz-Hodgkin equation can be used to calculate Vmem
Similar strategies can be implemented with any channel for which a specific opener/activator exists, such as sodium channels, by simply manipulating the relevant extracellular ion concentration. The trick also requires knowledge of the approximate intracellular concentration of ions in the tissue of interest and the use of salts that do not contain a counter ion: for example, when manipulating potassium, one should use K+-gluconate, not KCl, to avoid the confounding effects of chloride transport.
Isolation of the information-bearing component of bioelectric signaling
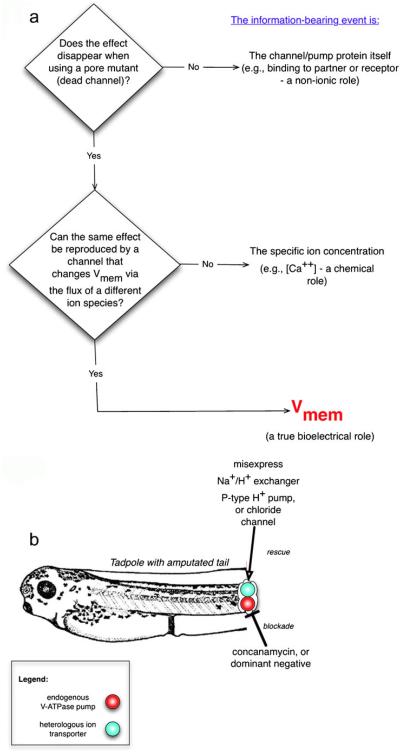
Having used the misexpression of well-characterized constructs to confirm a role for ion transporters, one can then dissect their mechanism of action. The first step is to determine their physical mode of action, which usually falls into three possible categories: an ion-independent role (binding partner for other proteins, for example), an ion-specific role (e.g., pH), or a true voltage role (Fig. 6). These can be distinguished experimentally with a set of rescue experiments.
Fig. 6.
Isolation of the information-bearing aspect of an ion-translocator function. In any given assay (process involving cell signaling), the function of a given ion transporter can be dissected by inhibiting the transporter and attempting to rescue the process with several distinct constructs that distinguish among ion-independent roles, ion-specific roles, or voltage-dependent roles (a). If a rescue can be made with an inactive (e.g., pore-mutant) form of the channel, then a non-ion-passing function (e.g., binding partner for some other protein) is implicated. If a rescue can be made with only the same channel family and no others, an ion-specific role is implicated. If any transporter of a different ion but with similar effects on transmembrane potential is sufficient to reproduce the same phenotype, then voltage alone is implicated. An example is shown in b: in the amputated tadpole tail, the native V-ATPase proton pump that is required for regeneration was blocked. Misexpression of a heterologous (yeast) P-type proton pump protein (with no sequence or structure homology to the V-ATPase) rescued regeneration, indicating that the proton pumping, not some ion-independent function, carried the signal necessary to initiate regeneration. In contrast, regeneration was not rescued by the electroneutral sodium/proton exchanger, ruling out the pH gradient as the causal factor in initiating regeneration (instead implicating Vmem regulation as the information-bearing aspect of this process). A Vmem role would be directly demonstrated by misxpressing a chloride channel and controlling intracellular chloride concentration in accordance with the Goldman equation, to reveal the voltage at which regeneration is initiated
The first important requirement is to know whether a specific channel or pump protein exerts its control upon cell behavior solely by virtue of its ion-translocating function, or whether its signaling occurs by binding or titrating other proteins. A number of ion channels and pumps have now been shown to have such ion-independent roles (Paul et al. 2007; Walters et al. 2009) and so one cannot assume bioelectricity is involved until scaffolding and other such functions are ruled out. The best way to test this is to knock out the endogenous channel and attempt to rescue the phenotype with an inactive mutant, which is ideally a pore mutant that is structurally identical to the native form but does not pass ions. These are available for many types of channels (Lalli et al. 1998; van Bever et al. 2004) and “dead” forms of transporters also exist. If the phenotype is not rescued, then the information-bearing signal probably does indeed involve ion flux.
The next step is to distinguish between a “chemical” role for a given ion species (dependence on the concentration of that specific ion) and a true bioelectrical role that depends on Vmem alone. The best-known ion-specific signal is a change in pH but the concentrations of other ions can also trigger specific events; for example, DNA-structure quadruplex formation and transcription are determined by K+ levels in the cytoplasm (Beaudoin and Perreault 2008; Chen et al. 2008; Kumar et al. 2008) and nucleus (Paine et al. 1981). Likewise, the latter phase of ionic control over tail regeneration in tadpoles is dependent on sodium, which is transduced by a Na+-specific salt-inducible kinase (Tseng et al. 2010). Most transporters affect both the level of specific ions and the voltage and so rescue experiments with constructs that change one of these at a time are necessary to deduce which aspect actually matters in a given situation. For example, a plasma-membrane V-ATPase proton pump simultaneously hyperpolarizes the cell (a change of Vmem), acidifies the extracellular milieu and alkalinizes the cortical cytoplasm. Being able to distinguish which of these carries instructive information for downstream effects on cell behavior is crucial. In the case of the induction of tadpole tail regeneration by a V-ATPase (Adams et al. 2007), a single-subunit P-type ATPase was first shown to be able to substitute for the multi-unit V-ATPase complex. Since the rescue was accomplished with a pump that had no sequence or structure similarity to the native pump complex, this ruled out scaffolding or binding roles dependent on protein structure and implicated proton pumping itself as being required. Then, electroneutral transporters (such as NHE, the sodium-hydrogen exchanger) were used in the rescue assay to distinguish between pH and voltage roles. The overall “rescue” strategy thus involves the inhibition or genetic deletion of the native protein responsible for the ion flow and then an attempt to recapitulate the phenotype in the assay by using DNA encoding a transporter that changes the relevant ion concentration but not the voltage (an electroneutral one), followed by the use of a transporter that changes the voltage via a completely different ion species.
Indeed, the best way to implicate voltage itself as the regulatory factor is to attempt to replicate the phenotype by using as many different transporters as possible (ideally, passing different ion species) that have the same effect on Vmem. For example, in a recent study on the neoplastic conversion of melanocytes in frog embryos, the same depolarization-induced phenotype was shown to result when a voltage gradient was reduced by chloride, proton, or potassium flux (Blackiston et al. 2011). Moreover, chloride-driven depolarization could be rescued by the co-expression of a potassium channel, indicating that the transformation of the melanocytes was truly sensitive to Vmem changes, irrespective of which ion flows induced those changes. Notably, in such cases, a physical factor (akin to cell geometry or substrate stiffness) encodes the relevant regulatory signal and not any one specific gene or protein. This is different from traditional biochemical signaling in which a cellular outcome can be tied to one particular gene whose protein is detected by a specific receptor; in this case, a physiological parameter, not a gene, codes for a phenotype.
Rescue experiments can be conducted with the many available mutants to probe additional aspects. For example, mutants defective in the voltage- or pH-gating of channels or GJs can be used in assays to reveal whether these modes of channel regulation are involved in the process of interest. Likewise, channels with altered kinetics or ion specificity can be used to probe additional mechanistic details of the bioelectric signal. Literature searches will often turn up a plethora of genetic tools (developed by physiologists working in the kidney, nervous system, inner ear, etc. who have previously undertaken the hard work of characterizing each mutant); these can be used in assays to reveal many details of the use of ion flow in any given patterning context. The identification of the specific biophysical event that is necessary and sufficient for a given response is a key component of mechanistically fleshing out the bioelectric component of any cell-cell interaction and of linking bioelectric signals to down-stream mechanisms.
Connecting to canonical signaling pathways
An understanding of the role of bioelectricity in pattern regulation requires the identification of both the molecular source of an ion flow (expression and function of a given transporter) and its consequences (amplification by second-messenger systems into biochemical and genetic cascades). The dissection of these upstream and downstream processes for any bioelectric signal allows a thorough integration of genetic and physiological processes, thereby describing cells as dynamical systems cycling between biophysical and biochemical regulatory events. How are changes in membrane potential coupled to transcriptional responses and alter cell behavior?
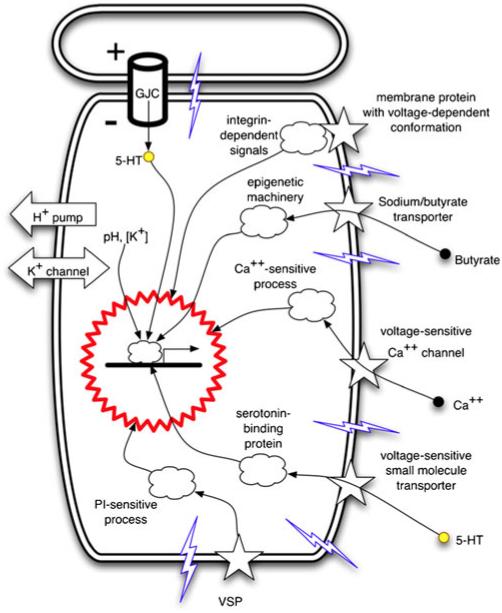
Transduction mechanisms (reviewed in detail in Levin 2007b, 2009a) that convert Vmem levels into changes of gene expression (Fig. 7) include voltage regulation of: (1) the movement of small signaling molecules across GJs (Adams et al. 2006; Esser et al. 2006; Fukumoto et al. 2005b) and through voltage-powered transporters such as the serotonin transporter, SERT (Fukumoto et al. 2005a) or the sodium/butyrate transporter (Ganapathy et al. 2008; Gupta et al. 2006; Li et al. 2003); (2) conformational changes in integrins (Arcangeli 2005; Hart 2008; Olivotto et al. 1996); (3) tyrosine phosphorylation (Holmes et al. 1997); (4) the activity of voltage-gated calcium channels that trigger calcium-dependent responses (Nakanishi and Okazawa 2006); (5) electrophoretic separation/clustering of protein complex subunits in the plane of the membrane (Cooper et al. 1989; Poo and Robinson 1977; Poo et al. 1978); (6) activity of voltage-sensitive enzymes that hydrolyze phosphoinositides upon depolarization of membrane potential (Lacroix et al. 2011; Okamura and Dixon 2011). Specific genes downstream of bioelectric events include BMP4, Notch and MSX1 during tail regeneration in Xenopus (Adams et al. 2007; Tseng et al. 2010), Rx1 and Pax6 during eye induction in Xenopus (Pai et al. 2012), Inx7 and Hox9 during the control of anterior-posterior polarity in planarian regeneration, Sox10, Slug and Nav1.7 (Blackiston et al. 2011; Brackenbury and Djamgoz 2006; Morokuma et al. 2008a) during the induction of a neoplastic phenotype, Frizzled, Mitf, Otx2 and Sox9 during craniofacial patterning (Vandenberg et al. 2011) and Sonic hedgehog and Nodal during left-right patterning in chick and frog embryos (Adams et al. 2006; Levin et al. 2002),
Fig. 7.
Known mechanisms by which transmembrane potential changes are transduced to downstream effector pathways. Changes of Vmem are physical processes that need to be converted into second messenger pathways and ultimately into transcriptional responses. Cell-autonomous mechanisms by which cells sense their depolarization or hyperpolarization include: electrophoretic transfer of small signaling molecules (Ca++, 5-HT) through gap-junctional connections (GJC); voltage potential-mediated changes of integrin conformation (leading to activation of integrin pathways); regulation of transport of small molecules (e.g., the sodium/butyrate transporter) that impact chromatin modification; activation of voltage-gated calcium channels (thus impinging on the many pathways controlled by calcium); voltage-dependent activity of small signaling molecule transporters (such as SERT, the serotonin transporter) or of phosphatases (such as the phosphatase and tensin homolog; VSP voltage-sensitive phosphatases). Illustration is taken from Levin 2007b, Fig. 1a, with permission of Elsevier
Thus, bioelectric signaling can interface with canonical pathways via calcium, phosphatases, serotonergic signaling, or chromatin modification machinery (Carneiro et al. 2011; He et al. 2011). The known set of transduction modes should be tested for any bioelectrically guided process by a suppression screen that seeks to rescue the effect of the Vmem change by the inhibition of each of these mechanisms in turn (Fig. 3). However, additional ways of coupling ionic signaling to nuclear effectors probably remain to be discovered.
Cutting edge developments
The above strategies have been used successfully and represent a relatively mature set of approaches that can be applied widely. However, the field has just begun to scratch the surface of bioelectric signaling. A number of interesting aspects have been observed but not yet investigated in detail. The hope is that the increased integration of bioelectrical components into the work of biologists will result in the development of new tools and functional data for some of these novel aspects.
Not just one Vmem: microdomains of voltage within single cells
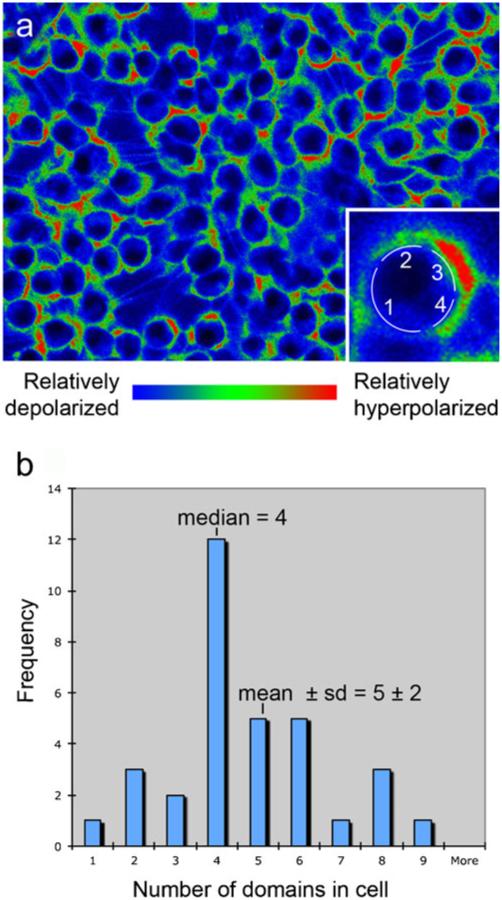
Most physiological literature reports one Vmem measurement for any given cell. However, this is simply an artifact of probing with one electrode, which necessarily reports only a single number for any cell. Imaging with membrane-voltage-reporting dyes (Adams and Levin 2012b; Oviedo et al. 2008; Vandenberg et al. 2011) reveals that, both in culture and in vivo, individual cell membranes have multiple domains with different resting membrane potentials. Unlike action potentials that last milliseconds (and that are now being imaged by using so-called “fast response” dyes), the potentials imaged with slow-response dyes are resting potentials that can last minutes to hours. Thus, whereas these domains are analogous to the regional areas of depolarization that travel down an axon, these domains are, comparatively, extremely stable. For example, COS M6 cells, cultured according to standard protocols and then stained by using a combination of CC2-DMPE and DiBAC3(4) have an average of at least four to five domains (counted conservatively around the circumference on images such as that presented in Fig. 8).
Fig. 8.
Multiple domains of membrane voltage within single cells in culture. a Monolayer of cultured COS M6 cells were imaged by using the membrane potential reporters DiBAC4(3) and CC2-DMPE (red the most negatively charged membrane, blue the least negatively charged membrane). A domain was defined by coloring the original black and white image by using a red/green/blue lookup table in IPLabs, the software used to collect the images. The image was then opened in Photoshop and any stretch of color in which the pixels were pure red, pure green, or pure blue were counted as a region. Thus, this count reveals three possible values of membrane voltage. Inset Higher magnification of a single cell showing that this cell was determined to have four domains. b Domains were counted on 33 randomly chosen cells. These cells had 5±2 (mean±SD) domains per circumference; the median number of domains was four. Thus, even on using a conservative counting protocol, these cells were found to maintain four to five voltage domains around the circumference of the cell within the plane of focus
We have also documented membrane voltage domains in the blastomeres of cleaving frog embryos (Fig. 9). Time-lapse videos of these large cells undergoing mitosis reveal a pattern to the formation and dissipation of various regions. In general, regions of hyperpolarization form almost synchronously but slightly ahead of the first appearance of a cleavage furrow. These regions then grow as cleavage proceeds. Once cleavage ends, these regions become relatively depolarized until the next cleavage furrow forms. These data are consistent with evidence for a high concentration of K+ channels in the new plasma membrane that is inserted during the cleavage process (Kline et al. 1983).
Fig. 9.
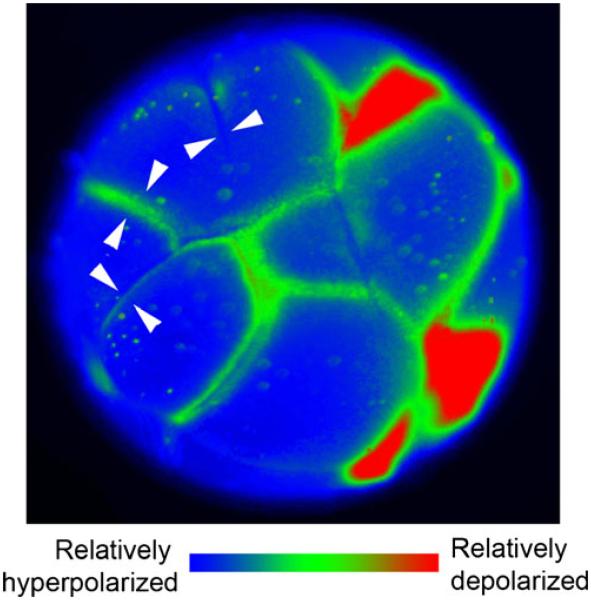
Multiple domains of membrane voltage within frog embryonic cells in vivo. This 16- to 32-cell frog embryo has been stained with the voltage-sensitive dye DiSBAC2(3). In this image, red areas are depolarized and blue are hyperpolarized. Regions near the cleavage furrows are depolarized relative to the rest of the cell. Time-lapse videos of cleavage reveal that areas of depolarization appear immediately before visible signs of the cleavage furrow, expand as the furrow moves and then become relatively hyperpolarized once cleavage is complete (white arrowhead pairs regions in which the Vmem of adjacent cells match, even though each individual cell has multiple domains)
Protists, which are nucleated single-celled organisms, also show this compartmentalization of membrane voltage (Fig. 10). By staining and time-lapse recording, we have captured not only domains of voltage but extremely fine scale domains that are maintained even as the protist changes shape and phagocytoses food particles.
Fig. 10.
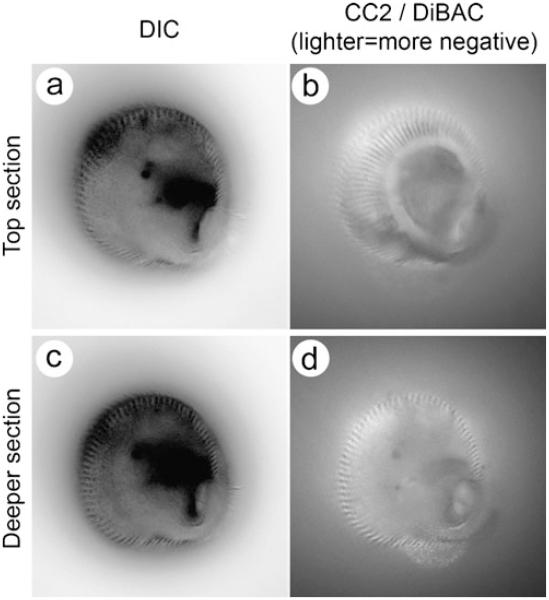
Multiple domains of membrane voltage within the single-celled ciliated protist Stentor coeruleus. An actively feeding Stentor was photographed at two different focal planes (a, b and c, d). In the differential interference contrast images left, the dark lines visible at the edges correspond to stripes of cilia that run along the cell from the oral to the aboral end. Right Images of the intricate and stable stripes of relatively hyperpolarized (lighter) and depolarized (darker) regions of the plasma membrane. The hyperpolarized stripes co-localize with the stripes of cilia. In d, the interior of the spiral feeding apparatus is visible as a spot of light surrounded by dark membrane. Despite the active phagocytosis of particles at this position on the membrane, this pattern is maintained. These fine scale differences in voltage are highly stable
The method by which adjacent domains maintain different voltage values is still largely unclear. Lipid rafts might bring different types of ion channels (O’Connell and Tamkun 2005) to different components of the cell membrane (as occurs during the polarization of epithelial cells) but we do not know how the cell avoids short-circuits across the underlying cytoplasm that would equalize the Vmem levels among neighboring domains.
Although individual cells were considered to have more than a single transmembrane potential value as early as 1983 (Kline et al. 1983), these domains can only now be readily detected and characterized over time in vivo in patterning systems by using fluorescent reporter dyes. A cell surface is a manifold that can encode enormous amounts of data for itself, such as a combinatorial code for cell identity (Silver 1996), or for its neighbors. The regulated changing of those patterns, such as in dividing blastomeres, adds an order of magnitude more potential for creating signals and reagents need to be developed to allow the functional modification of microdomains of voltage within cells. The importance of this discovery lies in the implication that the membrane voltage of a non-neural cell can be stably patterned and thus can carry significantly more developmental information than was previously thought (Davies et al. 2006; Martens et al. 2004; Wallace 2007).
Even greater potential: time varying membrane voltage
Several other directions probably offer the opportunity for fundamental advances. First, although the Vmem signals change slowly, they do change as a function of time (Pai et al. 2012; Vandenberg et al. 2011). Thus, we need to ask whether any aspect of cell communication is actually encoded in variations of Vmem around its resting value (frequency or amplitude modulation, perhaps). Such datasets can now be obtained and a wide variety of systems should be analyzed to mine the physiological data for patterns that might indicate novel control mechanisms and aspects of non-genetic (physiological) heterogeneity. Likewise, the use of multiple physiological dyes in experiments involving fluorescence-activated cell sorting should identify subpopulations within “pure” stem and other cell types that differ in key bioelectric properties, as has been observed for human umbilical vein endothelial cells (Yu et al. 2002). Such experiments on dissociated cells will clearly highlight properties that are cell-autonomous versus those physiological conditions that can only be maintained as a group phenomenon of cell-cell communication.
More broadly, large datasets of voltage and ion-content measurements need to be gathered in a wide range of cells and model species, so that the relationship between these bioelectric parameters and tissue outcomes can be fleshed out in quantitative models. The data need to encompass not only the plasma membrane but also intracellular membranes such as the poorly understood nuclear envelope potential (Bustamante 1994; Bustamante et al. 1994; Mazzanti et al. 2001; Yamashita 2011), which might have important implications for transcriptional control. Perhaps voltage reporter dyes can be developed with targeting domains for nuclear membranes and conversely, depolarizing and hyperpolarizing ion channels with nuclear targeting signals should be developed to probe functionally the roles of such physiological parameters.
An important area for future advances involves the harnessing of the techniques of optogenetics (Arrenberg et al. 2010; Schultheis et al. 2011; Wyart et al. 2009), in which ion flow through light-sensitive channels can be controlled with extremely tight spatio-temporal specificity in order to control non-excitable cells. Whereas the reagents are currently optimized for the control of rapid spiking in nerve and muscle (Lin 2011), constructs such as the step-function opsins can probably be made to regulate Vmem in any cell of interest. Indeed, optogenetic lines in tractable organisms such as zebrafish (Arrenberg et al. 2009) and mouse (Tomita et al. 2009) already exist, although expression is limited to the nervous system and therefore more broadly relevant lines need to be created. Several research groups are working to create transgenic Xenopus laevis lines in which any cell or tissue of interest will express light-sensitive hyperpolarizing/depolarizing channels, allowing ready access to bioelectrical experiments in cell, developmental, or regenerative biology by using LED (light-emitting diode) arrays (Grossman et al. 2010) or other patterned light sources. A promising new chemical approach confers photoregulation upon existing potassium channels (Fortin et al. 2008; Fortin et al. 2011) and is thus ideal both for probing endogenous channel roles and for biomedical applications to control growth bioelectrically without the need for transgenesis.
Future directions and opportunities: translating the bioelectric language of cell conversations
The understanding of biophysical controls will have many implications for basic and applied biology. Such regulation is an essential part of endogenous control systems enabling self-assembly during embryogenesis and represent an important target for evolutionary change (enabling robustness and modularity). Learning to control biophysical parameters that guide cell-cell interactions will greatly improve interventions in regenerative medicine (especially given the master-regulator properties of ionic signals for complex organ formation). The same Vmem change can be induced by any number of channels; for example, in the tadpole, a yeast P-type H+ pump (which is not present in vertebrates) can induce regeneration during non-regenerative conditions (Adams et al. 2007). Thus, biomedical applications can potentially use the most convenient of many channels or pumps to achieve a desired change in cell physiology and tissue outcome.
The most transformative applications of this field will require a mechanistic understanding of the derivation of patterning information from the real-time dynamics of physiological networks. Much as early computer memories stored bits as the directions of current flow within individual coils of wire, morphogenetic information can readily be encoded in the paths of ion flow within and among cells. Ion-channel circuits with hysteresis (memory) properties (Bennekou et al. 2004; Gallaher et al. 2010; Sachdeva et al. 2010; Spray et al. 1981) and the use of GJs and ion channels by non-neuronal cells to form nerve-like computational networks (Krishnan et al. 2008; Turner et al. 2002) have previously been described. Nevertheless, how would transient physiological events result in stable changes in anatomy?
Brief changes of physiological polarity can cause permanent reversal of anatomical polarity, for example, as can occur during the dorso-ventral patterning of embryonic chick blastoderm (Stern and MacKenzie 1983; Stern 1987, 1991). In addition to embryonic morphogenesis, regenerative pattern formation in adult animals is now known to depend on information stored physiologically. Recent work has shown that transient perturbation of the physiological state in a flatworm (brief interruption in cell-cell communication via GJs) permanently and radically alters its large-scale anatomy, despite its DNA sequence remaining unchanged (Oviedo et al. 2010): after subsequent injury, such as amputation of the head but without further physiological manipulation, such worms regenerate with the induced pattern! These results illustrate several interesting points. First, manipulation of the real-time physiological state can over-ride the genetically encoded pattern: the animals acquire a perpetually two-headed bodyplan, despite the genome of the planarian still containing instructions for building a normal one-headed worm. The ability of bioelectric signals to over-ride more canonical cues has also been shown during mesenchymal stem cell differentiation (Sundelacruz et al. 2008) and in corneal wound healing assays (Zhao 2009): in cases in which competing chemical and bioelectrical signals for differentiation or cell motility are provided together, the resulting cell behavior indicates that, at least in these cases, ionic controls trump oppositely directed chemical cues. Second, the identification of a specific physiological perturbation that permanently changes the shape to which injured animals regenerate might enable molecular dissection of the mechanisms by which “target morphology” can be stored during patterning.
Importantly, bioelectrical mechanisms offer exciting new building blocks for synthetic biology. Alongside the chemical and transcriptional modules in the current toolkit of workers in this field (Bashor et al. 2010; Purnick and Weiss 2009), the addition of bioelectric signaling modules, i.e., DNA cassettes encoding ion channels/pumps and transducers of bioelectrical signals to and from genetic endpoints, will allow the exploitation of transmembrane potential, ion flows and self-generated electric fields radically to expand the capabilities of hybrid biological-engineered constructs. Taking advantage of the bioelectric regulation of proliferation, orientation and migration will allow synthetic biology to move beyond the substrates of bacterial/yeast soups and toward the programming of the control of the self-assembly of three-dimensional patterns (Davies 2008): synthetic morphology approaches for achieving the desired level of control over growth and form in complex multi-cellular tissues. Moreover, the bioelectrical implementation of information-processing, memory and signaling in non-neural tissues promises the ability to create highly parallelized, robust, computational tissues, such as suggested by the studies of electrically mediated memory in plants and bone (Davies 2004; Szechynska-Hebda et al. 2010). These “biocomputers” could support algorithms with abilities far different from those possible with the linear Von-Neumann architecture upon which current computers are based, in which one instruction at a time is executed. The unique properties of three-dimensional electrical networks with complex feedback and analog signaling modes (as contrasted with the digital spiking of neurons) offer interesting tools for the bioengineer; the use of bioelectric components in ways that go beyond developmental programs that presently exist in the biological world will be an essential part of the field of synthetic morphology (Davies 2008), a field with capabilities that we have barely begun to explore.
Concluding remarks
The widely conserved, multi-scale, instructive capacity of bioelectric events makes ion flows and voltage gradients powerful modalities for the control of growth and form. Recent discoveries have begun to shed light on the interplay between genetic and biophysical signals and numerous tools are now available that lower the barriers for any research group to probe the interconnections between biophysical and genetic factors at the molecular level. Many opportunities exist for discovering bioelectrical controls in developmental, regenerative and cancer biology and for the production of new tools and methodologies that will uncover entirely new aspects of this field. Moreover, the development of specific strategies for the analysis and control of physiological information storage is sure to open exciting new vistas in regenerative medicine, bioengineering and synthetic biology.
Acknowledgments
We thank the members of the Levin laboratory and numerous members of the bioelectricity, physiology and neurobiology communities for many useful discussions.
M.L. is grateful for support from the NIH (EY018168, AR061988, GM078484, AR055993), the G. Harold and Leila Y. Mathers Charitable Foundation and the Telemedicine and Advanced Technology Research Center (TATRC) at the U.S. Army Medical Research and Materiel Command (USAMRMC) through award W81XWH-10-2-0058. D.S.A. gratefully acknowledges NIH K22-DE016633.
References
- Adams DS. A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng. 2008;14:1461–1468. doi: 10.1089/ten.tea.2008.0080. [DOI] [PubMed] [Google Scholar]
- Adams DS, Levin M. Inverse drug screens: a rapid and inexpensive method for implicating molecular targets. Genesis. 2006a;44:530–540. doi: 10.1002/dvg.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Strategies and techniques for investigation of biophysical signals in patterning. In: Whitman M, Sater AK, editors. Analysis of growth factor signaling in embryos. CRC Press; Boca Raton: 2006b. pp. 177–262. [Google Scholar]
- Adams DS, Levin M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harbor Protoc. 2012a doi: 10.1101/pdb.top067710. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harbor Protoc. 2012b doi: 10.1101/pdb.prot067702. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+−V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Agladze K, Budriene L, Ivanitsky G, Krinsky V, Shakhbazyan V, Tsyganov M. Wave mechanisms of pattern formation in microbial populations. Proc Biol Sci. 1993;253:131–135. doi: 10.1098/rspb.1993.0092. [DOI] [PubMed] [Google Scholar]
- Akemann W, Lundby A, Mutoh H, Knopfel T. Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophys J. 2009;96:3959–3976. doi: 10.1016/j.bpj.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akemann W, Mutoh H, Perron A, Rossier J, Knopfel T. Imaging brain electric signals with genetically targeted voltage-sensitive fluorescent proteins. Nat Methods. 2010;7:643–649. doi: 10.1038/nmeth.1479. [DOI] [PubMed] [Google Scholar]
- Albert SF, Wong E. Electrical stimulation of bone repair. Clin Podiatr Med Surg. 1991;8:923–935. [PubMed] [Google Scholar]
- Altizer A, Moriarty L, Bell S, Schreiner C, Scott W, Borgens R. Endogenous electric current is associated with normal development of the vertebrate limb. Dev Dyn. 2001;221:391–401. doi: 10.1002/dvdy.1158. [DOI] [PubMed] [Google Scholar]
- Anderson JD. Galvanotaxis of slime mold. J Gen Physiol. 1951;35:1–16. doi: 10.1085/jgp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–232. [PubMed] [Google Scholar]
- Arcangeli A, Carla M, Bene M, Becchetti A, Wanke E, Olivotto M. Polar/apolar compounds induce leukemia cell differentiation by modulating cell-surface potential. Proc Natl Acad Sci USA. 1993;90:5858–5862. doi: 10.1073/pnas.90.12.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Del Bene F, Baier H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci USA. 2009;106:17968–17973. doi: 10.1073/pnas.0906252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrenberg AB, Stainier DY, Baier H, Huisken J. Optogenetic control of cardiac function. Science. 2010;330:971–974. doi: 10.1126/science.1195929. [DOI] [PubMed] [Google Scholar]
- Aryasomayajula A, Derix J, Perike S, Gerlach G, Funk RH. DC microelectrode array for investigating the intracellular ion changes. Biosens Bioelectron. 2010;26:1268–1272. doi: 10.1016/j.bios.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Asashima M, Shimada K, Pfeiffer CJ. Magnetic shielding induces early developmental abnormalities in the newt, Cynops pyrrhogaster. Bioelectromagnetics. 1991;12:215–224. doi: 10.1002/bem.2250120403. [DOI] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H, K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol. 2010;346:39–53. doi: 10.1016/j.ydbio.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Mutoh H, Dimitrov D, Akemann W, Perron A, Iwamoto Y, Jin L, Cohen LB, Isacoff EY, Pieribone VA, Hughes T, Knopfel T. Genetically encoded fluorescent sensors of membrane potential. Brain Cell Biol. 2008;36:53–67. doi: 10.1007/s11068-008-9026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansagi T, Jr, Vanag VK, Epstein IR. Tomography of reaction–diffusion microemulsions reveals three-dimensional Turing patterns. Science. 2011;331:1309–1312. doi: 10.1126/science.1200815. [DOI] [PubMed] [Google Scholar]
- Barth LG, Barth LJ. Ionic regulation of embryonic induction and cell differentiation in Rana pipiens. Dev Biol. 1974a;39:1–22. doi: 10.1016/s0012-1606(74)80004-7. [DOI] [PubMed] [Google Scholar]
- Barth LJ, Barth LG. Effect of the potassium ion on induction of notochord from gastrula ectoderm of Rana pipiens. Biol Bull. 1974b;146:313–325. doi: 10.2307/1540407. [DOI] [PubMed] [Google Scholar]
- Bashor CJ, Horwitz AA, Peisajovich SG, Lim WA. Rewiring cells: synthetic biology as a tool to interrogate the organizational principles of living systems. Annu Rev Biophys. 2010;39:515–537. doi: 10.1146/annurev.biophys.050708.133652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Adams DS, Levin M. A chemical genetics approach reveals H, K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudoin JD, Perreault JP. Potassium ions modulate a G-quadruplex-ribozyme's activity. RNA. 2008;14:1018–1025. doi: 10.1261/rna.963908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedner P, Niessen H, Odermatt B, Willecke K, Harz H. A method to determine the relative cAMP permeability of connexin channels. Exp Cell Res. 2003;291:25–35. doi: 10.1016/s0014-4827(03)00323-9. [DOI] [PubMed] [Google Scholar]
- Beech JA. Bioelectric potential gradients may initiate cell cycling: ELF and zeta potential gradients may mimic this effect. Bioelectromagnetics. 1997;18:341–348. doi: 10.1002/(sici)1521-186x(1997)18:5<341::aid-bem1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, Ptacek LJ. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J Biol Chem. 2003;278:51779–51785. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- Bennekou P, Barksmann TL, Jensen LR, Kristensen BI, Christophersen P. Voltage activation and hysteresis of the non-selective voltage-dependent channel in the intact human red cell. Bioelectro-chemistry. 2004;62:181–185. doi: 10.1016/j.bioelechem.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Bentrup F, Sandan T, Jaffe L. Induction of polarity in Fucus eggs by potassium ion gradients. Protoplasma. 1967;64:254–266. [Google Scholar]
- van Bever L, Poitry S, Faure C, Norman RI, Roatti A, Baertschi AJ. Pore loop-mutated rat KIR6.1 and KIR6.2 suppress KATP current in rat cardiomyocytes. Am J Physiol Heart Circ Physiol. 2004;287:H850–859. doi: 10.1152/ajpheart.00054.2004. [DOI] [PubMed] [Google Scholar]
- Binggeli R, Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Bittman KS, Panzer JA, Balice-Gordon RJ. Patterns of cell-cell coupling in embryonic spinal cord studied via ballistic delivery of gap-junction-permeable dyes. J Comp Neurol. 2004;477:273–285. doi: 10.1002/cne.20253. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Shomrat T, Nicolas CL, Granata C, Levin M. A second-generation device for automated training and quantitative behavior analyses of molecularly-tractable model organisms. PLoS One. 2010;5:e14370. doi: 10.1371/journal.pone.0014370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger AN, Oster G. Emergent complexity in simple neural systems. Commun Integr Biol. 2009;2:467–470. doi: 10.4161/cib.2.6.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger A, Ermentrout B, Oster G. The neural origins of shell structure and pattern in aquatic mollusks. Proc National Acad Sci USA. 2009;106:6837–6842. doi: 10.1073/pnas.0810311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB. What is the role of naturally produced electric current in vertebrate regeneration and healing. Int Rev Cytol. 1982;76:245–298. doi: 10.1016/s0074-7696(08)61793-3. [DOI] [PubMed] [Google Scholar]
- Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Prog Clin Biol Res. 1986;210:239–250. [PubMed] [Google Scholar]
- Borgens RB, Shi R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn. 1995;203:456–467. doi: 10.1002/aja.1002030408. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol. 1990;296:634–653. doi: 10.1002/cne.902960409. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, Murphy DJ. Axonal regeneration in spinal cord injury: a perspective and new technique. J Comp Neurol. 1986;250:157–167. doi: 10.1002/cne.902500203. [DOI] [PubMed] [Google Scholar]
- Borgens R, Robinson K, Vanable J, McGinnis M. Electric fields in vertebrate repair. Liss; New York: 1989. [Google Scholar]
- Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol (Lond) 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium. 2003;33:37–48. doi: 10.1016/s0143-4160(02)00180-x. [DOI] [PubMed] [Google Scholar]
- Brock A, Chang H, Huang S. Non-genetic heterogeneity−a mutation-independent driving force for the somatic evolution of tumours. Nat Rev Genet. 2009;10:336–342. doi: 10.1038/nrg2556. [DOI] [PubMed] [Google Scholar]
- Burr HS. The meaning of bioelectric potentials. Yale J Biol Med. 1944;16:353. [PMC free article] [PubMed] [Google Scholar]
- Burr HS, Hovland CI. Bio-electric correlates of development in Amblystoma. Yale J Biol Med. 1937;9:540–549. [PMC free article] [PubMed] [Google Scholar]
- Burr HS, Northrop F. The electrodynamic theory of life. Q Rev Biol. 1935;10:322–333. [Google Scholar]
- Burr HS, Sinnott EW. Electrical correlates of form in cucurbit fruits. Am J Botany. 1944;31:249–253. [Google Scholar]
- Bustamante JO. Nuclear electrophysiology. J Membr Biol. 1994;138:105–112. doi: 10.1007/BF00232638. [DOI] [PubMed] [Google Scholar]
- Bustamante JO, Liepins A, Hanover JA. Nuclear pore complex ion channels (review) Mol Membr Biol. 1994;11:141–150. doi: 10.3109/09687689409162232. [DOI] [PubMed] [Google Scholar]
- Bustamante JO, Hanover JA, Liepins A. The ion channel behavior of the nuclear pore complex. J Membr Biol. 1995;146:239–251. doi: 10.1007/BF00233944. [DOI] [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguez-Leon J, Wu HM, Cheung AY, Feijo JA. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell. 2008;20:614–634. doi: 10.1105/tpc.106.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Liang J, Tian X, Liu X. G-quadruplex structure: a target for anticancer therapy and a probe for detection of potassium. Biochemistry (Mosc) 2008;73:853–861. doi: 10.1134/s0006297908080026. [DOI] [PubMed] [Google Scholar]
- Child CM. Patterns and problems of development. University of Chicago Press; Chicago: 1941. [Google Scholar]
- Cifra M, Fields JZ, Farhadi A. Electromagnetic cellular interactions. Prog Biophys Mol Biol. 2011;105:223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Coelho CN, Kosher RA. Gap junctional communication during limb cartilage differentiation. Dev Biol. 1991a;144:47–53. doi: 10.1016/0012-1606(91)90477-k. [DOI] [PubMed] [Google Scholar]
- Coelho CN, Kosher RA. A gradient of gap junctional communication along the anterior-posterior axis of the developing chick limb bud. Dev Biol. 1991b;148:529–535. doi: 10.1016/0012-1606(91)90271-4. [DOI] [PubMed] [Google Scholar]
- Cone CD. Variation of the transmembrane potential level as a basic mechanism of mitosis control. Oncology. 1970;24:438–470. doi: 10.1159/000224545. [DOI] [PubMed] [Google Scholar]
- Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- Cone CD. The role of the surface electrical transmembrane potential in normal and malignant mitogenesis. Ann NY Acad Sci. 1974;238:420–435. doi: 10.1111/j.1749-6632.1974.tb26808.x. [DOI] [PubMed] [Google Scholar]
- Cone CD, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192:155–158. doi: 10.1126/science.56781. [DOI] [PubMed] [Google Scholar]
- Cone CD, Tongier M. Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971;25:168–182. doi: 10.1159/000224567. [DOI] [PubMed] [Google Scholar]
- Cone CD, Tongier M. Contact inhibition of division: involvement of the electrical transmembrane potential. J Cell Physiol. 1973;82:373–386. doi: 10.1002/jcp.1040820307. [DOI] [PubMed] [Google Scholar]
- Cooper MS. Gap junctions increase the sensitivity of tissue cells to exogenous electric fields. J Theor Biol. 1984;111:123–130. doi: 10.1016/s0022-5193(84)80200-3. [DOI] [PubMed] [Google Scholar]
- Cooper MS, Miller JP, Fraser SE. Electrophoretic repatterning of charged cytoplasmic molecules within tissues coupled by gap junctions by externally applied electric fields. Dev Biol. 1989;132:179–188. doi: 10.1016/0012-1606(89)90216-9. [DOI] [PubMed] [Google Scholar]
- Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, Koch D, Pratt WS, Saibil HR, Dolphin AC. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E. New functions for electrical signals in plants. New Phytol. 2004;161:607–610. doi: 10.1111/j.1469-8137.2003.01018.x. [DOI] [PubMed] [Google Scholar]
- Davies JA. Synthetic morphology: prospects for engineered, self-constructing anatomies. J Anat. 2008;212:707–719. doi: 10.1111/j.1469-7580.2008.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mattei M, Gagliano N, Moscheni C, Dellavia C, Calastrini C, Pellati A, Gioia M, Caruso A, Stabellini G. Changes in polyamines, c-myc and c-fos gene expression in osteoblast-like cells exposed to pulsed electromagnetic fields. Bioelectromagnetics. 2005;26:207–214. doi: 10.1002/bem.20068. [DOI] [PubMed] [Google Scholar]
- Dubrov A. The geomagnetic field and life. Plenum; New York: 1978. [Google Scholar]
- Elson E. Developmental control in animals and a biological role for DNA charge transfer. Prog Biophys Mol Biol. 2006;95:1–15. doi: 10.1016/j.pbiomolbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Elson E. II. Model building: an electrical theory of control of growth and development in animals, prompted by studies of exogenous magnetic field effects (paper I), and evidence of DNA current conduction, in vitro. Electromagnetic Biol Med. 2009;28:283–309. doi: 10.3109/15368370903114297. [DOI] [PubMed] [Google Scholar]
- Esser AT, Smith KC, Weaver JC, Levin M. Mathematical model of morphogen electrophoresis through gap junctions. Dev Dyn. 2006;235:2144–2159. doi: 10.1002/dvdy.20870. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Forsyth C, Banan A, Shaikh M, Engen P, Fields JZ, Keshavarzian A. Evidence for non-chemical, nonelectrical intercellular signaling in intestinal epithelial cells. Bio-electrochemistry. 2007;71:142–148. doi: 10.1016/j.bioelechem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Fels D. Cellular communication through light. PLoS One. 2009;4:e5086. doi: 10.1371/journal.pone.0005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzharris G, Baltz JM. Granulosa cells regulate intracellular pH of the murine growing oocyte via gap junctions: development of independent homeostasis during oocyte growth. Development. 2006;133:591–599. doi: 10.1242/dev.02246. [DOI] [PubMed] [Google Scholar]
- Forrester JV, Lois N, Zhao M, McCaig C. The spark of life: the role of electric fields in regulating cell behaviour using the eye as a model system. Ophthalmic Res. 2007;39:4–16. doi: 10.1159/000097901. [DOI] [PubMed] [Google Scholar]
- Fortin DL, Banghart MR, Dunn TW, Borges K, Wagenaar DA, Gaudry Q, Karakossian MH, Otis TS, Kristan WB, Trauner D, Kramer RH. Photochemical control of endogenous ion channels and cellular excitability. Nat Methods. 2008;5:331–338. doi: 10.1038/nmeth.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin DL, Dunn TW, Kramer RH. Engineering light-regulated ion channels. Cold Spring Harb Protoc. 2011;2011:579–585. doi: 10.1101/pdb.top112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Funk RH, Monsees T, Ozkucur N. Electromagnetic effects− from cell biology to medicine. Prog Histochem Cytochem. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Funk RH, Monsees TK. Effects of electromagnetic fields on cells: physiological and therapeutical approaches and molecular mechanisms of interaction. A review. Cells Tissues Organs. 2006;182:59–78. doi: 10.1159/000093061. [DOI] [PubMed] [Google Scholar]
- Gallaher J, Bier M, van Heukelom JS. First order phase transition and hysteresis in a cell's maintenance of the membrane potential−an essential role for the inward potassium rectifiers. Biosystems. 2010;101:149–155. doi: 10.1016/j.biosystems.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, Prasad PD. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10:193–199. doi: 10.1208/s12248-008-9022-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam SG, Perron A, Mutoh H, Knopfel T. Exploration of fluorescent protein voltage probes based on circularly permuted fluorescent proteins. Front Neuroeng. 2009;2:14. doi: 10.3389/neuro.16.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmitrov J, Gmitrova A. Geomagnetic field effect on cardiovascular regulation. Bioelectromagnetics. 2004;25:92–101. doi: 10.1002/bem.10173. [DOI] [PubMed] [Google Scholar]
- Gonzalez JE, Tsien RY. Improved indicators of cell membrane potential that use fluorescence resonance energy transfer. Chem Biol. 1997;4:269–277. doi: 10.1016/s1074-5521(97)90070-3. [DOI] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Berlinguer Palmini R, Gong Z, Drakakis EM, Neil MA, Dawson MD, Burrone J, Degenaar P. Multi-site optical excitation using ChR2 and micro-LED array. J Neural Eng. 2010;7:16004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- Guo YM, Chen S, Shetty P, Zheng G, Lin R, Li WH. Imaging dynamic cell-cell junctional coupling in vivo using Trojan-LAMP. Nat Methods. 2008;5:835–841. doi: 10.1038/nmeth.1238. [DOI] [PubMed] [Google Scholar]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwitsch AA. A historical review of the problem of mitogenetic radiation. Experientia. 1988;44:545–550. doi: 10.1007/BF01953301. [DOI] [PubMed] [Google Scholar]
- Guthrie PB, Lee RE, Rehder V, Schmidt MF, Kater SB. Self-recognition: a constraint on the formation of electrical coupling in neurons. J Neurosci. 1994;14:1477–1485. doi: 10.1523/JNEUROSCI.14-03-01477.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington DB, Becker RO. Electrical stimulation of RNA and protein synthesis in the frog erythrocyte. Exp Cell Res. 1973;76:95–98. doi: 10.1016/0014-4827(73)90423-0. [DOI] [PubMed] [Google Scholar]
- Harrison C, Funnel B. Relationship of paleomagnetic reversals and micropaleontology in two late Caenozoic cores from the Pacific Ocean. Nature. 1964;204:566. [Google Scholar]
- Hart FX. The mechanical transduction of physiological strength electric fields. Bioelectromagnetics. 2008;29:447–455. doi: 10.1002/bem.20411. [DOI] [PubMed] [Google Scholar]
- He XB, Yi SH, Rhee YH, Kim H, Han YM, Lee SH, Lee H, Park CH, Lee YS, Richardson E, Kim BW. Prolonged membrane depolarization enhances midbrain dopamine neuron differentiation via epigenetic histone modifications. Stem Cells. 2011;29:1861–1873. doi: 10.1002/stem.739. [DOI] [PubMed] [Google Scholar]
- Hinard V, Belin D, Konig S, Bader CR, Bernheim L. Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242. Development. 2008;135:859–867. doi: 10.1242/dev.011387. [DOI] [PubMed] [Google Scholar]
- Holmes TC, Berman K, Swartz JE, Dagan D, Levitan IB. Expression of voltage-gated potassium channels decreases cellular protein tyrosine phosphorylation. J Neurosci. 1997;17:8964–8974. doi: 10.1523/JNEUROSCI.17-23-08964.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptak-Solga AD, Nielsen S, Jain I, Thummel R, Hyde DR, Iovine MK. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev Biol. 2008;317:541–548. doi: 10.1016/j.ydbio.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166:789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, Stuart JM, Lee NH. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ernberg I, Kauffman S. Cancer attractors: a systems view of tumors from a gene network dynamics and developmental perspective. Semin Cell Dev Biol. 2009;20:869–876. doi: 10.1016/j.semcdb.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman L, Bellamy A. Studies on the correlation between metabolic gradients, electrical gradients, and galvanotaxis I. Biol Bull. 1922;XLIII:313–347. [Google Scholar]
- Iovine MK, Higgins EP, Hindes A, Coblitz B, Johnson SL. Mutations in connexin43 (GJA1) perturb bone growth in zebra-fish fins. Dev Biol. 2005;278:208–219. doi: 10.1016/j.ydbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Ando T, Watanabe O, Goto H. Novel application of low pH-dependent fluorescent dyes to examine colitis. BMC Gastroenterol. 2010;10:4. doi: 10.1186/1471-230X-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL, Kurachi Y, Okada N, Kondo S. Pigment pattern in jaguar/obelix zebra-fish is caused by a Kir7.1 mutation: implications for the regulation of melanosome movement. PLoS Genet. 2006;2:e197. doi: 10.1371/journal.pgen.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond Biol. 1981;295:553–566. doi: 10.1098/rstb.1981.0160. [DOI] [PubMed] [Google Scholar]
- Jaffe L. Developmental currents, voltages, and gradients. In: Subtelny S, editor. Developmental order: its origin and regulation. Liss; New York: 1982. pp. 183–215. [Google Scholar]
- Jaffe LF. Organization of early development by calcium patterns. Bioessays. 1999;21:657–667. doi: 10.1002/(SICI)1521-1878(199908)21:8<657::AID-BIES5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jenkins LS, Duerstock BS, Borgens RB. Reduction of the current of injury leaving the amputation inhibits limb regeneration in the red spotted newt. Dev Biol. 1996;178:251–262. doi: 10.1006/dbio.1996.0216. [DOI] [PubMed] [Google Scholar]
- Jenrow KA, Smith CH, Liboff AR. Weak extremely-low-frequency magnetic field-induced regeneration anomalies in the planarian Dugesia tigrina. Bioelectromagnetics. 1996;17:467–474. doi: 10.1002/(SICI)1521-186X(1996)17:6<467::AID-BEM6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Jouhou H, Yamamoto K, Homma A, Hara M, Kaneko A, Yamada M. Depolarization of isolated horizontal cells of fish acidifies their immediate surrounding by activating V-ATPase. J Physiol (Lond) 2007;585:401–412. doi: 10.1113/jphysiol.2007.142646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman SA. The origins of order: self-organization and selection in evolution. Oxford University Press; New York: 1993. [Google Scholar]
- Kline D, Robinson KR, Nuccitelli R. Ion currents and membrane domains in the cleaving Xenopus egg. J Cell Biol. 1983;97:1753–1761. doi: 10.1083/jcb.97.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S. The reaction–diffusion system: a mechanism for autonomous pattern formation in the animal skin. Genes Cells. 2002;7:535–541. doi: 10.1046/j.1365-2443.2002.00543.x. [DOI] [PubMed] [Google Scholar]
- Kondo S, Iwashita M, Yamaguchi M. How animals get their skin patterns: fish pigment pattern as a live Turing wave. Int J Dev Biol. 2009;53:851–856. doi: 10.1387/ijdb.072502sk. [DOI] [PubMed] [Google Scholar]
- Konig S, Hinard V, Arnaudeau S, Holzer N, Potter G, Bader CR, Bernheim L. Membrane hyperpolarization triggers myogenin and myocyte enhancer factor-2 expression during human myoblast differentiation. J Biol Chem. 2004;279:28187–28196. doi: 10.1074/jbc.M313932200. [DOI] [PubMed] [Google Scholar]
- Konig S, Beguet A, Bader CR, Bernheim L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006;133:3107–3114. doi: 10.1242/dev.02479. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Sachdeva G, Chakravarthy VS, Radhakrishnan S. Interpreting voltage-sensitivity of gap junctions as a mechanism of cardiac memory. Math Biosci. 2008;212:132–148. doi: 10.1016/j.mbs.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Kumar N, Patowary A, Sivasubbu S, Petersen M, Maiti S. Silencing c-MYC expression by targeting quadruplex in P1 promoter using locked nucleic acid trap. Biochemistry. 2008;47:13179–13188. doi: 10.1021/bi801064j. [DOI] [PubMed] [Google Scholar]
- Kurtz I, Schrank AR. Bioelectrical properties of intact and regenerating earthworms Eisenia foetida. Physiol Zool. 1955;28:322–330. [Google Scholar]
- Lacroix J, Halaszovich CR, Schreiber DN, Leitner MG, Bezanilla F, Oliver D, Villalba-Galea CA. Controlling the activity of a phosphatase and tensin homolog (PTEN) by membrane potential. J Biol Chem. 2011;286:17945–17953. doi: 10.1074/jbc.M110.201749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli MJ, Johns DC, Janecki M, Liu Y, O’Rourke B, Marban E. Suppression of KATP currents by gene transfer of a dominant negative Kir6.2 construct. Pflugers Archiv. 1998;436:957–961. doi: 10.1007/s004240050729. [DOI] [PubMed] [Google Scholar]
- Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- Lange C, Prenninger S, Knuckles P, Taylor V, Levin M, Calegari F. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011;20:843–850. doi: 10.1089/scd.2010.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+−dependent cerebellar granule neuron apoptosis. Role of task leak K+ channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectromagnetic patterning fields: roles in embryonic development, regeneration, and neoplasm. Bioelectromagnetics. 2003;24:295–315. [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007a;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007b;17:262–271. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol. 2009a;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Regeneration: recent advances, major puzzles, and biomedical opportunities. Semin Cell Dev Biol. 2009b;20:515–516. doi: 10.1016/j.semcdb.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries. Bioessays (in press. 2011a doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. The wisdom of the body: future techniques and approaches to morphogenetic fields in regenerative medicine, developmental biology and cancer. Regen Med. 2011b;6:667–673. doi: 10.2217/rme.11.69. [DOI] [PubMed] [Google Scholar]
- Levin M, Ernst SG. Applied AC and DC magnetic fields cause alterations in the mitotic cycle of early sea urchin embryos. Bio-electromagnetics. 1995;16:231–240. doi: 10.1002/bem.2250160405. [DOI] [PubMed] [Google Scholar]
- Levin M, Ernst SG. Applied DC magnetic fields cause alterations in the time of cell divisions and developmental abnormalities in early sea urchin embryos. Bioelectromagnetics. 1997;18:255–263. doi: 10.1002/(sici)1521-186x(1997)18:3<255::aid-bem9>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+−ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Li H, Myeroff L, Smiraglia D, Romero MF, Pretlow TP, Kasturi L, Lutterbaugh J, Rerko RM, Casey G, Issa JP, Willis J, Willson JK, Plass C, Markowitz SD. SLC5A8, a sodium transporter, is a tumor suppressor gene silenced by methylation in human colon aberrant crypt foci and cancers. Proc Natl Acad Sci USA. 2003;100:8412–8417. doi: 10.1073/pnas.1430846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebau S, Propper C, Bockers T, Lehmann-Horn F, Storch A, Grissmer S, Wittekindt OH. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J Neuro-chem. 2006;99:426–437. doi: 10.1111/j.1471-4159.2006.03967.x. [DOI] [PubMed] [Google Scholar]
- Lin JY. A user’s guide to channelrhodopsin variants: features, limitations and future developments. Exp Physiol. 2011;96:19–25. doi: 10.1113/expphysiol.2009.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovitz TA, Montrose CJ, Doinov P, Brown KM, Barber M. Superimposing spatially coherent electromagnetic noise inhibits field-induced abnormalities in developing chick embryos. Bioelectromagnetics. 1994;15:105–113. doi: 10.1002/bem.2250150203. [DOI] [PubMed] [Google Scholar]
- Liu X, Tonegawa S. Optogenetics 3.0. Cell. 2010;141:22–24. doi: 10.1016/j.cell.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Lund E. Bioelectric fields and growth. University of Texas Press; Austin: 1947. [Google Scholar]
- Lundby A, Mutoh H, Dimitrov D, Akemann W, Knopfel T. Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One. 2008;3:e2514. doi: 10.1371/journal.pone.0002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Akemann W, Knöpfel T. Biophysical characterization of the fluorescent protein voltage probe VSFP2.3 based on the voltage-sensing domain of Ci-VSP. Eur Biophys J. 2010;39:1625–1635. doi: 10.1007/s00249-010-0620-0. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of growth polarity in regenerating Dugesia tigrina. Fed Proc. 1947;6:163–164. [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of axial polarity in a regenerating annelid. Anat Rec. 1949;105:513–514. [Google Scholar]
- Marsh G, Beams HW. Electrical control of growth axis in a regenerating annelid. Anat Rec. 1950;108:512–512. [Google Scholar]
- Marsh G, Beams HW. Electrical control of morphogenesis in regenerating Dugesia tigrina. I. Relation of axial polarity to field strength. J Cell Comp Physiol. 1952;39:191–211. doi: 10.1002/jcp.1030390203. [DOI] [PubMed] [Google Scholar]
- Martens JR, O'Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Models for organizer and notochord formation. C R Acad Sci III. 2000;323:23–30. doi: 10.1016/s0764-4469(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Meinhardt H. Organizer and axes formation as a self-organizing process. Int J Dev Biol. 2001;45:177–188. [PubMed] [Google Scholar]
- Mennerick S, Chisari M, Shu H-J, Taylor A, Vasek M, Eisenman LN, Zorumski CF. Diverse voltage-sensitive dyes modulate GABAAReceptor function. J Neurosci. 2010;30:2871–2879. doi: 10.1523/JNEUROSCI.5607-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijo JA. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- Miki T, Iwanaga T, Nagashima K, Ihara Y, Seino S. Roles of ATP-sensitive K+ channels in cell survival and differentiation in the endocrine pancreas. Diabetes. 2001;50(Suppl 1):S48–S51. doi: 10.2337/diabetes.50.2007.s48. [DOI] [PubMed] [Google Scholar]
- Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20:710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misakian M, Sheppard AR, Krause D, Frazier ME, Miller DL. Biological, physical, and electrical parameters for in vitro studies with ELF magnetic and electric fields: a primer. Bioelectromagnetics Suppl. 1993;2:1–73. doi: 10.1002/bem.2250140703. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Sakamoto K, Sade H, Ohya S, Muraki K, Imaizumi Y. Voltage-sensitive oxonol dyes are novel large-conductance Ca2+−activated K+ channel activators selective for beta1 and beta4 but not for beta2 subunits. Mol Pharmacol. 2007;71:1075–1088. doi: 10.1124/mol.106.031146. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci USA. 2008a;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem. 2008b;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Mutoh H, Perron A, Dimitrov D, Iwamoto Y, Akemann W, Chudakov DM, Knopfel T. Spectrally-resolved response properties of the three most advanced FRET based fluorescent protein voltage probes. PLoS One. 2009;4:e4555. doi: 10.1371/journal.pone.0004555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh H, Perron A, Akemann W, Iwamoto Y, Knopfel T. Optogenetic monitoring of membrane potentials. Exp Physiol. 2011;96:13–18. doi: 10.1113/expphysiol.2010.053942. [DOI] [PubMed] [Google Scholar]
- Nagajski D, Guthrie S, Ford C, Warner A. The correlation between patterns of dye transfer through gap junctions and future developmental fate in Xenopus. Development. 1989;105:747–752. [Google Scholar]
- Nakamasu A, Takahashi G, Kanbe A, Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proc Natl Acad Sci USA. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J Physiol (Lond) 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson BJ. Gap junctions−from cell to molecule. J Cell Sci. 2003;116:4479–4481. doi: 10.1242/jcs.00821. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Novak B, Bentrup FW. An electrophysiological study of regeneration in Acetabularia mediterranea. Planta. 1972;108:227–244. doi: 10.1007/BF00384111. [DOI] [PubMed] [Google Scholar]
- Novak B, Sirnoval C. Inhibition of regeneration of Acetabularia mediterranea enucleated posterior stalk segments by electrical isolation. Plant Sci Lett. 1975;5:183–188. [Google Scholar]
- Novikov VV, Sheiman IM, Fesenko EE. Effect of weak static and low-frequency alternating magnetic fields on the fission and regeneration of the planarian Dugesia (Girardia) tigrina. Bioelectromagnetics. 2008;29:387–393. doi: 10.1002/bem.20407. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Vibrating probe−high spatial-resolution extracellular current measurement. Fed Proc. 1980;39:2129. [Google Scholar]
- Nuccitelli R. Endogenous ionic currents and DC electric-fields in multicellular animal-tissues. Bioelectromagnetics Suppl. 1992;1:147–157. doi: 10.1002/bem.2250130714. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R. Endogenous electric fields measured in developing embryos. In: Blank M, editor. Electromagnetic fields. Biological interactions and mechanisms. Advances in chemistry. Vol. 250. ACS Publications; Washington: 1995. pp. 109–124. [Google Scholar]
- Nuccitelli R. A role for endogenous electric fields in wound healing. Curr Top Dev Biol. 2003;58:1–26. doi: 10.1016/s0070-2153(03)58001-2. [DOI] [PubMed] [Google Scholar]
- Nuccitelli R, Robinson K, Jaffe L. On electrical currents in development. Bioessays. 1986;5:292–294. doi: 10.1002/bies.950050616. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci. 2005;118:2155–2166. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology (Bethesda) 2011;26:6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- Olivotto M, Arcangeli A, Carla M, Wanke E. Electric fields at the plasma membrane level: a neglected element in the mechanisms of cell signalling. Bioessays. 1996;18:495–504. doi: 10.1002/bies.950180612. [DOI] [PubMed] [Google Scholar]
- Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Oster GF. Lateral inhibition models of developmental processes. Math Biosci. 1988;90:265–286. [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Live imaging of planarian membrane potential using DiBAC4(3) Cold Spring Harb Protoc. 2008 doi: 10.1101/pdb.prot5055. 2008:pdb.prot5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkucur N, Epperlein HH, Funk RH. Ion imaging during axolotl tail regeneration in vivo. Dev Dyn. 2010;239:2048–2057. doi: 10.1002/dvdy.22323. [DOI] [PubMed] [Google Scholar]
- Ozkucur N, Perike S, Sharma P, Funk RH. Persistent directional cell migration requires ion transport proteins as direction sensors and membrane potential differences in order to maintain directedness. BMC Cell Biol. 2011;12:4. doi: 10.1186/1471-2121-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential conrols embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine PL, Pearson TW, Tluczek LJ, Horowitz SB. Nuclear sodium and potassium. Nature. 1981;291:258–259. doi: 10.1038/291258a0. [DOI] [PubMed] [Google Scholar]
- Pan L, Borgens RB. Perpendicular organization of sympathetic neurons within a required physiological voltage. Exp Neurol. 2010;222:161–164. doi: 10.1016/j.expneurol.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Camino D del, Sanchez A, Alves F, Bruggemann A, Beckh S, Stuhmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SM, Palladino MJ, Beitel GJ. A pump-independent function of the Na, K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007;134:147–155. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron A, Mutoh H, Akemann W, Gautam SG, Dimitrov D, Iwamoto Y, Knopfel T. Second and third generation voltage-sensitive fluorescent proteins for monitoring membrane potential. Front Mol Neurosci. 2009a;2:5. doi: 10.3389/neuro.02.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perron A, Mutoh H, Launey T, Knopfel T. Red-shifted voltage-sensitive fluorescent proteins. Chem Biol. 2009b;16:1268–1277. doi: 10.1016/j.chembiol.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo M, Robinson KR. Electrophoresis of concanavalin A receptors along embryonic muscle cell membrane. Nature. 1977;265:602–605. doi: 10.1038/265602a0. [DOI] [PubMed] [Google Scholar]
- Poo MM, Poo WJ, Lam JW. Lateral electrophoresis and diffusion of Concanavalin A receptors in the membrane of embryonic muscle cell. J Cell Biol. 1978;76:483–501. doi: 10.1083/jcb.76.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp FA, Li KH, Gu Q. Recent advances in biophoton research and its applications. World Scientific Publishing; Singapore: 1992. [Google Scholar]
- Prat AG, Cantiello HF. Nuclear ion channel activity is regulated by actin filaments. Am J Physiol. 1996;270:C1532–C1543. doi: 10.1152/ajpcell.1996.270.5.C1532. [DOI] [PubMed] [Google Scholar]
- Prato FS, Robertson JA, Desjardins D, Hensel J, Thomas AW. Daily repeated magnetic field shielding induces analgesia in CD-1 mice. Bioelectromagnetics. 2005;26:109–117. doi: 10.1002/bem.20056. [DOI] [PubMed] [Google Scholar]
- Pu J, McCaig CD, Cao L, Zhao Z, Segall JE, Zhao M. EGF receptor signalling is essential for electric-field-directed migration of breast cancer cells. J Cell Sci. 2007;120:3395–3403. doi: 10.1242/jcs.002774. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Rizzo A, Isseroff RR. Beta-adrenergic receptor antagonists accelerate skin wound healing: evidence for a cate-cholamine synthesis network in the epidermis. J Biol Chem. 2006;281:21225–21235. doi: 10.1074/jbc.M601007200. [DOI] [PubMed] [Google Scholar]
- Pullar CE, Zhao M, Song B, Pu J, Reid B, Ghoghawala S, McCaig C, Isseroff RR. Beta-adrenergic receptor agonists delay while antagonists accelerate epithelial wound healing: evidence of an endogenous adrenergic network within the corneal epithelium. J Cell Physiol. 2007;211:261–272. doi: 10.1002/jcp.20934. [DOI] [PubMed] [Google Scholar]
- Purnick PE, Weiss R. The second wave of synthetic biology: from modules to systems. Nat Rev Mol Cell Biol. 2009;10:410–422. doi: 10.1038/nrm2698. [DOI] [PubMed] [Google Scholar]
- Rajnicek AM, Foubister LE, McCaig CD. Prioritising guidance cues: directional migration induced by substratum contours and electrical gradients is controlled by a rho/cdc42 switch. Dev Biol. 2007;312:448–460. doi: 10.1016/j.ydbio.2007.09.051. [DOI] [PubMed] [Google Scholar]
- Redmann K, Jenssen HL, Kohler HJ. Experimental and functional changes in transmembrane potential and zeta potential of single cultured cells. Exp Cell Res. 1974;87:281–289. doi: 10.1016/0014-4827(74)90482-0. [DOI] [PubMed] [Google Scholar]
- Reid B, Graue-Hernandez EO, Mannis MJ, Zhao M. Modulating endogenous electric currents in human corneal wounds−a novel approach of bioelectric stimulation without electrodes. Cornea. 2011;30:338–343. doi: 10.1097/ICO.0b013e3181f7f2de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc. 2007;2:661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR. Endogenous and applied electrical currents: their measurement and application. In: Borgens R, Robinson K, Vanable J, McGinnis M, editors. Electric fields in vertebrate repair. Liss; New York: 1989. pp. 1–25. [Google Scholar]
- Robinson K, Messerli M. Electric embryos: the embryonic epithelium as a generator of developmental information. In: McCaig C, editor. Nerve growth and guidance. Portland Press; Portland: 1996. pp. 131–141. [Google Scholar]
- Robinson KR, Messerli MA. Left/right, up/down: the role of endogenous electrical fields as directional signals in development, repair and invasion. Bioessays. 2003;25:759–766. doi: 10.1002/bies.10307. [DOI] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Foletti A, Magnani A, Lamponi S. New perspectives in cell communication: bioelectromagnetic interactions. Semin Cancer Biol. 2011;21:207–214. doi: 10.1016/j.semcancer.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Gerard V, Dubois JM. Involvement of K+ channels in the quercetin-induced inhibition of neuroblastoma cell growth. Pflugers Arch. 1993;423:202–205. doi: 10.1007/BF00374395. [DOI] [PubMed] [Google Scholar]
- Russo RE, Reali C, Radmilovich M, Fernandez A, Trujillo-Cenoz O. Connexin 43 delimits functional domains of neurogenic precursors in the spinal cord. J Neurosci. 2008;28:3298–3309. doi: 10.1523/JNEUROSCI.5736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva G, Kalyanasundaram K, Krishnan J, Chakravarthy VS. Bistable dynamics of cardiac cell models coupled by dynamic gap junctions linked to cardiac memory. Biol Cybern. 2010;102:109–121. doi: 10.1007/s00422-009-0352-3. [DOI] [PubMed] [Google Scholar]
- Saito T, Schlegel R, Andresson T, Yuge L, Yamamoto M, Yamasaki H. Induction of cell transformation by mutated 16 K vacuolar H+−ATPase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–1680. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- Sawai S, Maeda Y, Sawada Y. Spontaneous symmetry breaking Turing-type pattern formation in a confined Dictyostelium cell mass. Phys Rev Lett. 2000;85:2212–2215. doi: 10.1103/PhysRevLett.85.2212. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y. An hypothesis: phosphorylation fields as the source of positional information and cell differentiation−(cAMP, ATP) as the universal morphogenetic Turing couple. Prog Bio-phys Mol Biol. 1991;56:79–105. doi: 10.1016/0079-6107(91)90015-k. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y. Turing-Child field underlies spatial periodicity in Drosophila and planarians. Prog Biophys Mol Biol. 2011;105:258–269. doi: 10.1016/j.pbiomolbio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Schultheis C, Liewald JF, Bamberg E, Nagel G, Gottschalk A. Optogenetic long-term manipulation of behavior and animal development. PLoS One. 2011;6:e18766. doi: 10.1371/journal.pone.0018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol. 2001;280:F739–F747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- Schwab A, Gabriel K, Finsterwalder F, Folprecht G, Greger R, Kramer A, Oberleithner H. Polarized ion transport during migration of transformed Madin-Darby canine kidney cells. Pflugers Arch. 1995;430:802–807. doi: 10.1007/BF00386179. [DOI] [PubMed] [Google Scholar]
- Shapiro S, Borgens R, Pascuzzi R, Roos K, Groff M, Purvines S, Rodgers RB, Hagy S, Nelson P. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- Shen B, Xiang Z, Miller B, Louie G, Wang W, Noel JP, Gage FH, Wang L. Genetically encoding unnatural amino acids in neural stem cells and optically reporting voltage-sensitive domain changes in differentiated neurons. Stem Cells. 2011;29:1231–1240. doi: 10.1002/stem.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- Silver RB. Calcium, BOBs, QEDs, microdomains and a cellular decision: control of mitotic cell division in sand dollar blastomeres. Cell Calcium. 1996;20:161–179. doi: 10.1016/s0143-4160(96)90105-0. [DOI] [PubMed] [Google Scholar]
- Slusarski DC, Pelegri F. Calcium signaling in vertebrate embryonic patterning and morphogenesis. Dev Biol. 2007;307:1–13. doi: 10.1016/j.ydbio.2007.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJS, Sanger RS, Messerli MA. Principles, development and applications of self-referencing electrochemical microelectrodes to the determination of fluxes at cell membranes. In: Michael AC, editor. Methods and new frontiers in neuroscience. CRC Press; Boca Raton: 2007. pp. 373–405. [PubMed] [Google Scholar]
- Song B, Gu Y, Pu J, Reid B, Zhao Z, Zhao M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc. 2007;2:1479–1489. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- Spray DC, Harris AL, Bennett MV. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science. 1981;211:712–715. doi: 10.1126/science.6779379. [DOI] [PubMed] [Google Scholar]
- Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. Experimental reversal of polarity in chick embryo epiblast sheets in vitro. Exp Cell Res. 1982;140:468–471. doi: 10.1016/0014-4827(82)90143-4. [DOI] [PubMed] [Google Scholar]
- Stern CD. Control of epithelial polarity and induction in the early chick embryo. In: Wolff S, Berry S, editors. Mesenchymal-epithelial interactions in neural development. Springer; Berlin: 1987. pp. 91–100. [Google Scholar]
- Stern CD. The subembryonic fluid of the egg of the domestic fowl and its relationship to the early development of the embryo. In: Tullett SG, editor. Avian incubation. Butterworths; London: 1991. pp. 81–90. [Google Scholar]
- Stern C, MacKenzie D. Sodium transport and the control of epiblast polarity in the early chick embryo. J Embryol Exp Morphol. 1983;77:73–98. [PubMed] [Google Scholar]
- Stillwell EF, Cone CM, Cone CD. Stimulation of DNA synthesis in CNS neurones by sustained depolarisation. Nat New Biol. 1973;246:110–111. doi: 10.1038/newbio246110a0. [DOI] [PubMed] [Google Scholar]
- Strilic B, Eglinger J, Krieg M, Zeeb M, Axnick J, Babal P, Muller DJ, Lammert E. Electrostatic cell-surface repulsion initiates lumen formation in developing blood vessels. Curr Biol. 2010;20:2003–2009. doi: 10.1016/j.cub.2010.09.061. [DOI] [PubMed] [Google Scholar]
- Stroh A, Tsai HC, Ping Wang L, Zhang F, Kressel J, Aravanis A, Santhanam N, Deisseroth K, Konnerth A, Schneider MB. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2010 doi: 10.1002/stem.558. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump RF, Robinson KR. Xenopus neural crest cell migration in an applied electrical field. J Cell Biol. 1983;97:1226–1233. doi: 10.1083/jcb.97.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szechynska-Hebda M, Kruk J, Gorecka M, Karpinska B, Karpinski S. Evidence for light wavelength-specific photoelectrophysiological signaling and memory of excess light episodes in Arabidopsis. Plant Cell. 2010;22:2201–2218. doi: 10.1105/tpc.109.069302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai G, Reid B, Cao L, Zhao M. Electrotaxis and wound healing: experimental methods to study electric fields as a directional signal for cell migration. Methods Mol Biol. 2009;571:77–97. doi: 10.1007/978-1-60761-198-1_5. [DOI] [PubMed] [Google Scholar]
- Tantama M, Hung YP, Yellen G. Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J Am Chem Soc. 2011;133:10034–10037. doi: 10.1021/ja202902d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng GQ, Zhao X, Lees-Miller JP, Quinn FR, Li P, Rancourt DE, London B, Cross JC, Duff HJ. Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality. Circ Res. 2008;103:1483–1491. doi: 10.1161/CIRCRESAHA.108.177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofani S, Barone D, Cintorino M, Santi MM de, Ferrara A, Orlassino R, Ossola P, Peroglio F, Rolfo K, Ronchetto F. Static and ELF magnetic fields induce tumor growth inhibition and apoptosis. Bioelectromagnetics. 2001;22:419–428. doi: 10.1002/bem.69. [DOI] [PubMed] [Google Scholar]
- Tomita H, Sugano E, Fukazawa Y, Isago H, Sugiyama Y, Hiroi T, Ishizuka T, Mushiake H, Kato M, Hirabayashi M, Shigemoto R, Yawo H, Tamai M. Visual properties of transgenic rats harboring the channelrhodopsin-2 gene regulated by the thy-1.2 promoter. PLoS One. 2009:4–e7679. doi: 10.1371/journal.pone.0007679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neuro-sci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008a;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Meth. 2008b;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- Turing AM. The chemical basis of morphogenesis. Philos Trans R Soc Lond Biol. 1952;237:37–72. [Google Scholar]
- Turner CH, Robling AG, Duncan RL, Burr DB. Do bone cells behave like a neuronal network? Calcif Tissue Int. 2002;70:435–442. doi: 10.1007/s00223-001-1024-z. [DOI] [PubMed] [Google Scholar]
- Tyner KM, Kopelman R, Philbert MA. "Nanosized voltmeter" enables cellular-wide electric field mapping. Biophys J. 2007;93:1163–1174. doi: 10.1529/biophysj.106.092452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda A, Trillo MA, Chacon L, Blanco MJ, Leal J. Chick embryo development can be irreversibly altered by early exposure to weak extremely-low-frequency magnetic fields. Bioelectromagnetics. 1994;15:385–398. doi: 10.1002/bem.2250150503. [DOI] [PubMed] [Google Scholar]
- Uzman JA, Patil S, Uzgare AR, Sater AK. The role of intracellular alkalinization in the establishment of anterior neural fate in Xenopus. Dev Biol. 1998;193:10–20. doi: 10.1006/dbio.1997.8782. [DOI] [PubMed] [Google Scholar]
- Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, Campbell TJ, Breit SN. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol (Lond) 2000;529:541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD, Adams DS. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan TE, Weaver JC. Molecular change signal-to-noise criteria for interpreting experiments involving exposure of biological systems to weakly interacting electromagnetic fields. Bioelectromagnetics. 2005;26:305–322. doi: 10.1002/bem.20094. [DOI] [PubMed] [Google Scholar]
- Wainwright M. Historical and recent evidence for the existence of mitogenetic radiation. Perspect Biol Med. 1998;41:565–571. doi: 10.1353/pbm.1998.0023. [DOI] [PubMed] [Google Scholar]
- Wallace R. Neural membrane microdomains as computational systems: toward molecular modeling in the study of neural disease. Biosystems. 2007;87:20–30. doi: 10.1016/j.biosystems.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Walters BC. Oscillating field stimulation in the treatment of spinal cord injury. PM R. 2010;2:S286–291. doi: 10.1016/j.pmrj.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Walters ZS, Haworth KE, Latinkic BV. NKCC1 (SLC12a2) induces a secondary axis in Xenopus laevis embryos independently of its co-transporter function. J Physiol (Lond) 2009;587:521–529. doi: 10.1113/jphysiol.2008.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Zhao M. Regulation of tissue repair and regeneration by electric fields. Chin J Traumatol. 2010;13:55–61. [PubMed] [Google Scholar]
- Wang L, Zhou P, Craig RW, Lu L. Protection from cell death by mcl-1 is mediated by membrane hyperpolarization induced by K (+) channel activation. J Membr Biol. 1999;172:113–120. doi: 10.1007/s002329900589. [DOI] [PubMed] [Google Scholar]
- Wang X, Veruki ML, Bukoreshtliev NV, Hartveit E, Gerdes HH. Animal cells connected by nanotubes can be electrically coupled through interposed gap-junction channels. Proc Natl Acad Sci USA. 2010;107:17194–17199. doi: 10.1073/pnas.1006785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Res. 2005;65:11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff C, Fuks B, Chatelain P. Comparative study of membrane potential-sensitive fluorescent probes and their use in ion channel screening assays. J Biomol Screening. 2003;8:533–543. doi: 10.1177/1087057103257806. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Woodruff R, Telfer W. Electrophoresis of proteins in intercellular bridges. Nature. 1980;286:84–86. doi: 10.1038/286084a0. [DOI] [PubMed] [Google Scholar]
- Woodruff RI. Calmodulin transit via gap junctions is reduced in the absence of an electric field. J Insect Physiol. 2005;51:843–852. doi: 10.1016/j.jinsphys.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Wyart C, Del Bene F, Warp E, Scott EK, Trauner D, Baier H, Isacoff EY. Optogenetic dissection of a behavioural module in the vertebrate spinal cord. Nature. 2009;461:407–410. doi: 10.1038/nature08323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. Fluctuations in nuclear envelope's potential mediate synchronization of early neural activity. Biochem Biophys Res Commun. 2011;406:107–111. doi: 10.1016/j.bbrc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Yan X, Han J, Zhang Z, Wang J, Cheng Q, Gao K, Ni Y, Wang Y. Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics. 2009;30:29–35. doi: 10.1002/bem.20436. [DOI] [PubMed] [Google Scholar]
- Yang S, Li WH. Assaying dynamic cell-cell junctional communication using noninvasive and quantitative fluorescence imaging techniques: LAMP and infrared-LAMP. Nat Protoc. 2009;4:94–101. doi: 10.1038/nprot.2008.219. [DOI] [PubMed] [Google Scholar]
- Yao L, Shanley L, McCaig C, Zhao M. Small applied electric fields guide migration of hippocampal neurons. J Cell Physiol. 2008;216:527–535. doi: 10.1002/jcp.21431. [DOI] [PubMed] [Google Scholar]
- Yao L, Pandit A, Yao S, McCaig CD. Electric field-guided neuron migration: a novel approach in neurogenesis. Tissue Eng Part B Rev. 2011;17:143–153. doi: 10.1089/ten.TEB.2010.0561. [DOI] [PubMed] [Google Scholar]
- Yasuda I. Mechanical and electrical callus. Ann N Y Acad Sci. 1974;238:457–465. doi: 10.1111/j.1749-6632.1974.tb26812.x. [DOI] [PubMed] [Google Scholar]
- Yasuda I. Electrical callus and callus formation by electret. Clin Orthop Relat Res. 1977;124:53–66. [PubMed] [Google Scholar]
- Yoshida R. Self-oscillating gels driven by the Belousov-Zhabotinsky reaction as novel smart materials. Adv Mater. 2010;22:3463–3483. doi: 10.1002/adma.200904075. [DOI] [PubMed] [Google Scholar]
- Yu K, Ruan DY, Ge SY. Three electrophysiological phenotypes of cultured human umbilical vein endothelial cells. Gen Physiol Biophys. 2002;21:315–326. [PubMed] [Google Scholar]
- Yun Z, Zhengtao D, Jiachang Y, Fangqiong T, Qun W. Using cadmium telluride quantum dots as a proton flux sensor and applying to detect H9 avian influenza virus. Anal Biochem. 2007;364:122–127. doi: 10.1016/j.ab.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Zhang W, Kone BC. NF-kappaB inhibits transcription of the H (+)-K(+)-ATPase alpha(2)-subunit gene: role of histone deacetylases. Am J Physiol Renal Physiol. 2002;283:F904–F911. doi: 10.1152/ajprenal.00156.2002. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. Particle tracking model of electrophoretic morphogen movement reveals stochastic dynamics of embryonic gradient. Dev Dyn. 2009;238:1923–1935. doi: 10.1002/dvdy.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M. Electrical fields in wound healing−an overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao M, McCaig CD, Agius-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]