Abstract
Abnormal signaling through the platelet-derived growth factor receptor (PDGFR) has been proposed as a possible mechanism of spinal cord glioma initiation and progression. However, the extent of PDGFR expression in human spinal cord gliomas remains unknown. In this study we perform immunohistochemical analysis of PDGFRα expression in a series of 33 primary intramedullary spinal cord gliomas of different types and grades. PDGFRα was seen to be expressed in a significant subset of these tumors across all major glioma types including ependymoma, oligodendroglioma, pilocytic astrocytoma, astrocytoma, and glioblastoma. These results support the hypothesis that growth factor signaling through the PDGFR may be important for the development of at least a subset of human spinal cord gliomas. Further studies investigating the prognostic significance of PDGFR expression as well as the role of PDGF signaling on the development of intramedullary spinal cord gliomas are warranted.
Keywords: Astrocytoma, Ependymoma, Glioblastoma multiforme, Glioma, Intramedullary, Oligodendroglioma, Pilocytic, Spinal cord, Tumor
Introduction
Primary intramedullary spinal cord gliomas are rare but potentially devastating lesions. The mechanisms of tumorigenesis and the molecular-genetic profile of these tumors remain poorly defined. However, platelet-derived growth factor (PDGF) signaling has been proposed to play an important role in their development. In animal models, induction of PDGF-B expression in the spinal cord has been shown to initiate the formation of intramedullary gliomas [1–3]. These experimental spinal cord gliomas also express the cognate receptor PDGFRα suggesting that signaling downstream of this receptor tyrosine kinase may drive tumor formation. Additionally, evidence of PDGF receptor expression in human spinal cord ependymoma as well as responsiveness to receptor inhibition further implicates a mechanistic role for PDGF signaling in the pathogenesis of intramedullary glioma [4, 5].
Although, a number of reports have demonstrated expression of PDGF receptors in cerebral gliomas [6–10], the extent of PDGF receptor expression in spinal cord gliomas remains unknown. Recognition that PDGF and its receptors are often expressed in cerebral gliomas has provided the framework for the initiation of several clinical trials testing inhibition of this pathway in brain tumors [11–17]. The development and implementation of therapies targeted at PDGF receptor and/or downstream target inhibition in spinal cord gliomas will depend both on the feasibility of receptor profiling and on the frequency with which positive expression is observed. In this study we assess the expression status of platelet-derived growth factor receptor alpha (PDGFRα) by immunohistochemistry in a series of 33 primary spinal cord gliomas. Using this technique we characterize expression within various spinal cord gliomas of different histological types and grades.
Methods
Case selection and pathological samples
The cases for this study were identified within the neuropathology databases maintained by the Columbia University Medical Center, Department of Pathology and within the Department of Neurological Surgery Bartoli Brain Tumor Bank. Cases of primary intramedullary spinal cord glioma with sufficient paraffinized tissue for analysis obtained between 1995 and 2009 were eligible for inclusion. Patients with a diagnosis of myxopapillary ependymoma were excluded. Original surgery and pathology reports were reviewed for each case. After diagnostic confirmation was obtained by review of the original pathological slides, formalin-fixed paraffin-embedded tumor blocks were obtained. Five micron thick sections were cut and mounted on glass slides in preparation for immunohistochemical staining. All study data were processed, reviewed, and catalogued in accordance with policies of the Institutional Review Board.
Immunohistochemistry and microscopy
Glass-mounted 5 micron thick sections were de-paraffinized in xylene and rehydrated with 100% ethanol and distilled water. Antigen retrieval was performed by boiling the samples in 10 mM citrate buffer at a pH of 6. Samples were then blocked in 0.3% peroxide, washed, and blocked in 20% normal goat serum (Vector Laboratories). After washing, the samples were incubated with rabbit anti- PDGFRα (1:500; Cell Signaling). Vectastain ABC kit (Vector laboratories) and DAB chromogen were used for visualization. Sections were then counterstained with hematoxylin, washed, and dehydrated prior to cover slip mounting. Cerebral glioblastomas with known PDGFRα expression status were immunohistochemically processed in parallel with the study samples to serve as positive and negative staining controls.
All photomicrographs were obtained using a Nikon Labphot-2 microscope. Digital images were processed using NIS-Elements imaging software (Nikon). PDGFRα expression was classified as either positive or negative based on whether immunoperoxidase reactivity (brown staining) was observed during microscopic analysis. Due to the small sample sizes and the presence of focal variations in staining intensity, qualitative rather than quantitative immunohistochemical expression profiling was deemed most appropriate.
Results
Study cohort
A total of 33 histologically confirmed cases of primary intramedullary spinal cord glioma were included in this study (Table 1). The patients include 15 women and 18 men with a median age of 40 (range 2–81) years at the time of diagnosis. The pathological diagnoses include 26 WHO grade II ependymomas, 1 WHO grade II oligodendroglioma, 1 pilocytic astrocytoma, 1 anaplastic pilocytic astrocytoma, 3 WHO grade II astrocytomas, and 1 glioblastoma multiforme. The tumors had no segmental level predilection and were seen throughout the cervical, thoracic, and lumbar spinal cord.
Table 1.
Spinal cord glioma cases
| Case | Gender | Age | Diagnosis | WHO grade | Spinal cord level | PDGFRα status |
|---|---|---|---|---|---|---|
| 1 | M | 68 | Ependymoma | II | Thoracic | Positive |
| 2 | M | 21 | Ependymoma | II | Thoracolumbar | Positive |
| 3 | F | 29 | Ependymoma | II | Cervical | Positive |
| 4 | M | 48 | Ependymoma | II | Cervical | Positive |
| 5 | M | 33 | Ependymoma | II | Thoracolumbar | Positive |
| 6 | F | 71 | Ependymoma | II | Lumbar | Negative |
| 7 | F | 25 | Ependymoma | II | Lumbar | Negative |
| 8 | F | 59 | Ependymoma | II | Thoracic | Negative |
| 9 | M | 46 | Ependymoma | II | Thoracic | Negative |
| 10 | F | 44 | Ependymoma | II | Cervicothoracic | Negative |
| 11 | F | 39 | Ependymoma | II | Cervical | Negative |
| 12 | M | 60 | Ependymoma | II | Cervical | Negative |
| 13 | M | 48 | Ependymoma | II | Thoracic | Negative |
| 14 | F | 56 | Ependymoma | II | Cervical | Negative |
| 15 | M | 44 | Ependymoma | II | Thoracic | Negative |
| 16 | M | 51 | Ependymoma | II | Thoracolumbar | Negative |
| 17 | M | 71 | Ependymoma | II | Cervical | Negative |
| 18 | M | 32 | Ependymoma | II | Cervical | Negative |
| 19 | M | 40 | Ependymoma | II | Cervicothoracic | Negative |
| 20 | F | 31 | Ependymoma | II | Lumbar | Negative |
| 21 | M | 19 | Ependymoma | II | Thoracic | Negative |
| 22 | F | 19 | Ependymoma | II | Lumbar | Negative |
| 23 | M | 47 | Ependymoma | II | Cervical | Negative |
| 24 | M | 40 | Ependymoma (tanycytic) | II | Thoracic | Negative |
| 25 | M | 39 | Ependymoma (tanycytic) | II | Lumbar | Negative |
| 26 | F | 50 | Ependymoma (tanycytic) | II | Cervicothoracic | Negative |
| 27 | M | 46 | Oligodendroglioma | II | Thoracic | Positive |
| 28 | F | 8 | Pilocytic astrocytoma | I | Cervical | Negative |
| 29 | M | 81 | Anaplastic pilocytic astrocytoma | III | Cervical | Positive |
| 30 | F | 33 | Astrocytoma | II | Thoracic | Positive |
| 31 | F | 2 | Astrocytoma | II | Thoracic | Positive |
| 32 | F | 23 | Astrocytoma | II | Thoracic | Negative |
| 33 | F | 27 | Glioblastoma multiforme | IV | Thoracolumbar | Positive |
Histological and immunohistochemical analysis
PDGFRα localization and expression
A total of 10 out of 33 (30.3%) spinal cord gliomas analyzed were seen to express PDGFRα (Table 1). PDGFRα immunoreactivity localized to both the cytoplasm and cell membranes of tumor cells within positive samples. Staining of variable intensity was often seen in a discrete subset of cells. The density and distribution of PDGFRα-expressing cells varied from tumor to tumor and from region to region within tumors.
Ependymoma
Ependymoma was the most commonly identified intra-medullary spinal cord tumor within the queried tumor banks and 26 were included in this study. On histological examination all 26 tumors were classified World Health Organization (WHO) grade II ependymoma with three being subtyped tanycytic. In addition to histological phenotyping, the diagnosis of ependymoma was supported by the presence of characteristic dot-like epithelial membrane antigen (EMA) immunoreactivity in 8 of 9 tested samples (not shown). PDGFRα immunoreactivity was detected in 19% (5/26) of the spinal cord ependymoma cases studied. Representative examples are shown in Fig. 1.
Fig. 1.
Spinal cord ependymomas. Representative H&E (a, c) and corresponding PDGFRα immunoperoxidase (b, d) stained sections from cases 1 and 16, respectively are shown. Positive (b) and negative (d) PDGFRα immunoreactivity (brown) is demonstrated. Magnification in all panels 200×
Oligodendroglioma
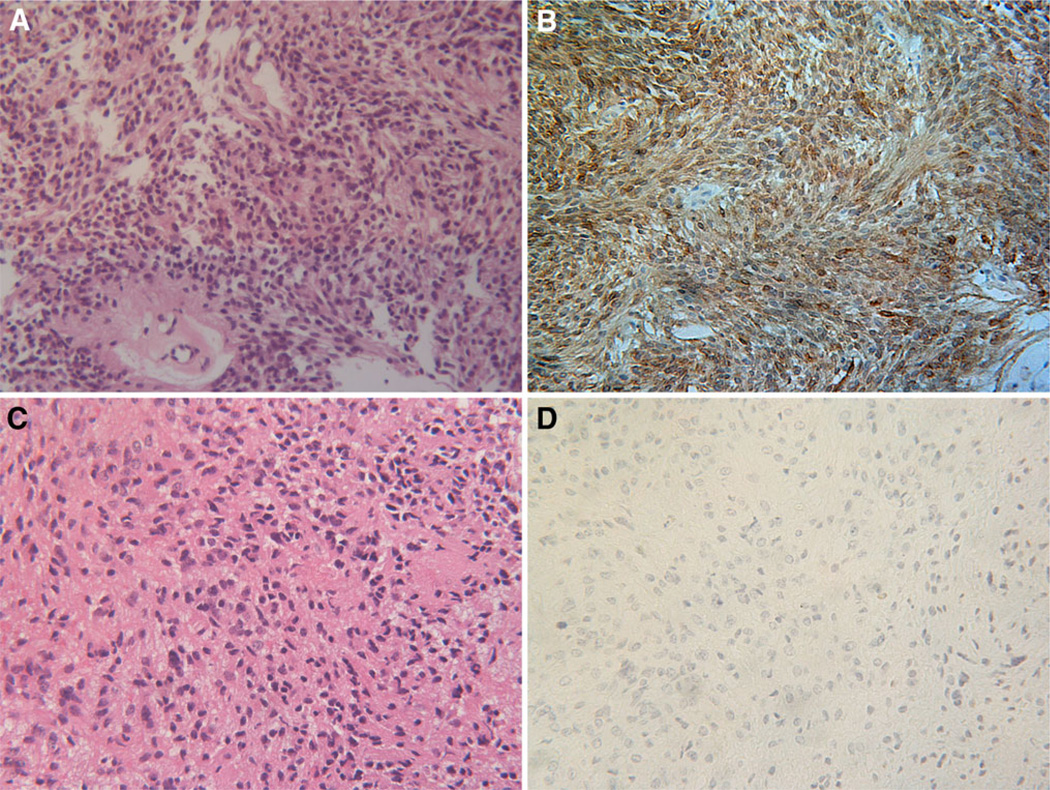
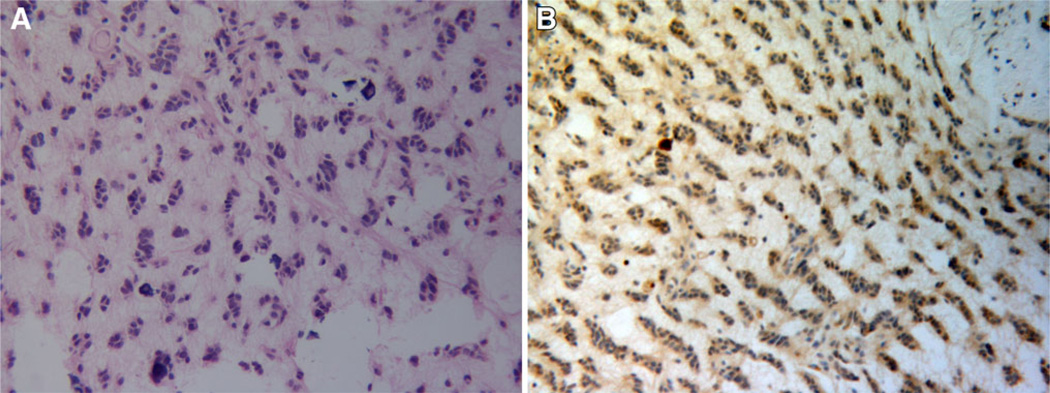
WHO grade II intramedullary oligodendroglioma is rarely diagnosed and one case was identified within the cohort. A characteristic “Indian file” pattern of cellular infiltration was observed in the pathological sample (Fig. 2a). Areas of calcification were also seen. PDGFRα immunoreactivity was seen to highlight the linear bands of tumor cells within an a cellular background (Fig. 2b).
Fig. 2.
Spinal cord oligodendroglioma (case 27). H&E staining shows a moderately cellular neoplasm within a mucinous background. The tumor cells exhibit a palisading growth pattern and are aligned in linear bands (a). The majority of tumor cells show PDGFRα immunoreactivity (brown) (b). Magnification in all panels 200×
Pilocytic astrocytoma
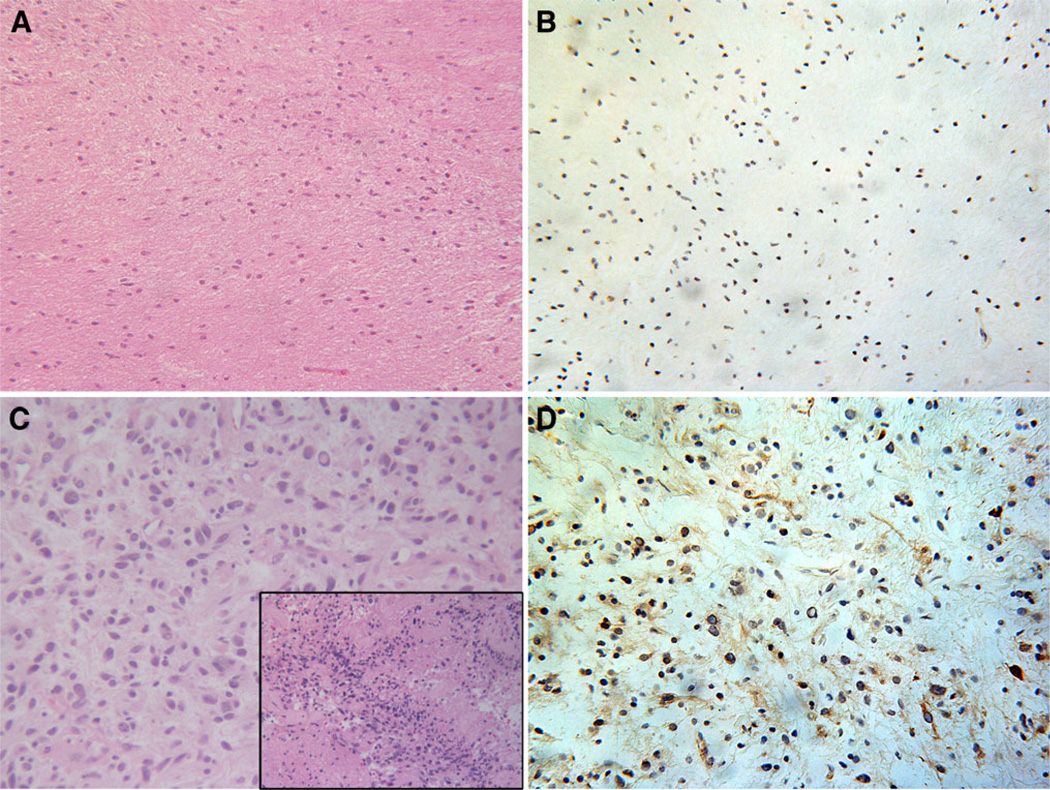
Both a WHO grade I (Fig. 3a–b) and an anaplastic WHO grade III (Fig. 3c–d) pilocytic astrocytoma were among the cohort of tumors samples in this series. The anaplastic pilocytic astrocytoma exhibited areas with distinct features of malignant progression (Fig. 3c, inset) with other areas showing lower grade histology. The grade I pilocytic astrocytoma was PDGFRα negative (Fig. 3b) while the anaplastic pilocytic astrocytoma was immunopositive for PDGFRα (Fig. 3d).
Fig. 3.
WHO grade I pilocytic astrocytoma (case 28) and anaplastic pilocytic astrocytoma of the spinal cord (case 29). H&E stained section from a WHO grade I pilocytic astrocytoma shows a low cellularity glial neoplasm within an eosinophilic background (a). No PDGFRα immunoreactivity is demonstrated within this tumor (b). In contrast, H&E section from a WHO grade III anaplastic pilocytic astrocytoma demonstrates a more cellular and pleomorphic tumor with areas of incipient necrosis (inset) (c). Immunoperoxidase staining demonstrates PDGFRα immunoreactivity (brown) within this tumor (d). Magnification in all panels 200X
Astrocytoma and glioblastoma
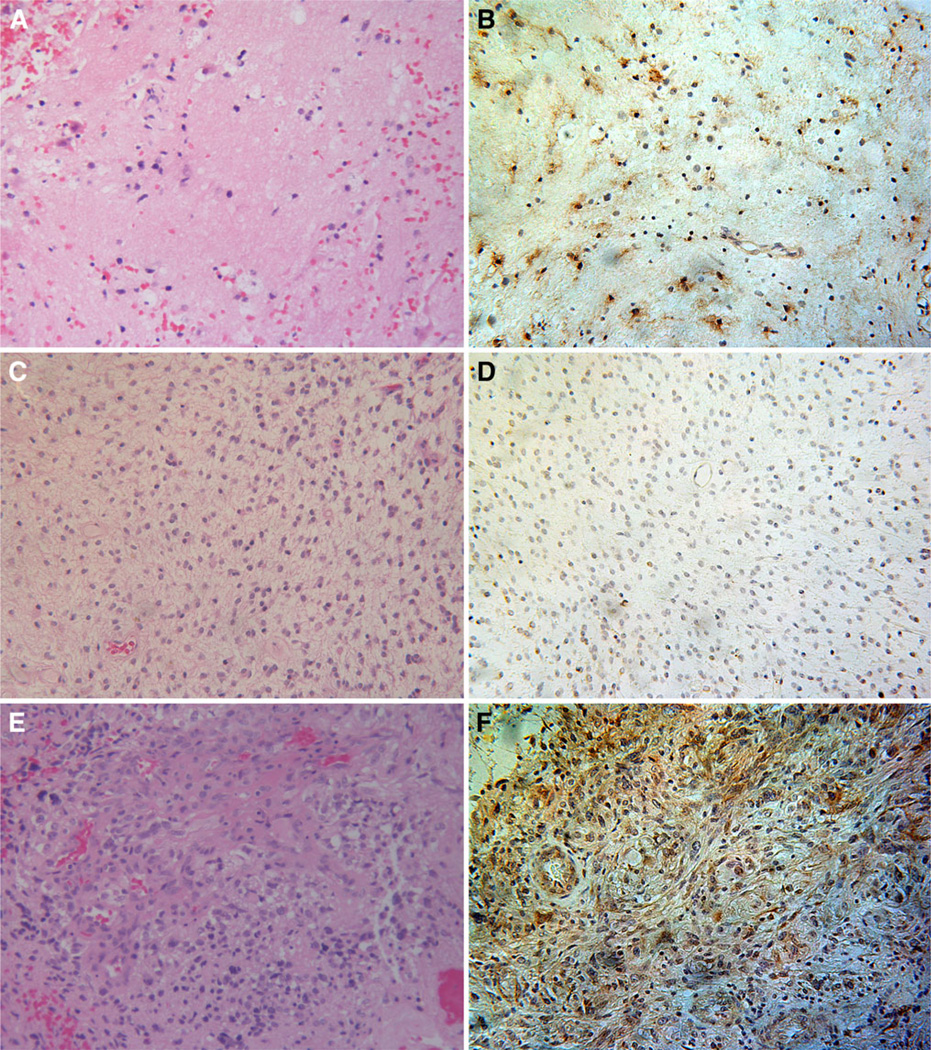
PDGFRα was expressed in 2 of 3 WHO grade II spinal cord astrocytomas and in the 1 spinal cord glioblastoma case analyzed (Fig. 4). The low cell density of the astrocytoma shown in (Fig. 4b) illustrates cytoplasmic and cell membrane PDGFRα immunoreactivity that highlights the boundaries between individual cells. Conversely, cellular borders are obscured within the glioblastoma due to the high density of neoplastic cells and dysplastic vasculature (Fig. 4f).
Fig. 4.
WHO grade II astrocytoma and glioblastoma (WHO grade IV) of the spinal cord. H&E section from a WHO grade II astrocytoma (case 30) demonstrates a low density of infiltrative glial cells within the spinal cord white matter (a). A subset of tumor cells distributed throughout the specimen show PDGFRα immunoreactivity (brown) (b). H&E section from another sample (case 32) of WHO grade II astrocytoma (c) with negative PDGFRα staining (d) is shown for comparison. A high-grade lesion with prominent glomeruloid vascular proliferation, hemorrhage, and necrosis consistent with a diagnosis of glioblastoma is demonstrated on H&E staining from case 33 of this series (e). The majority of cells in this tumor stain strongly positive for PDGFRα (brown) (f). Magnification in all panels 200X
Discussion
Abnormal PDGF signaling is well recognized to be an important mechanism of gliomagenesis in the brain [18, 19]. However, relatively little is known about the role of PDGF and its receptors in spinal gliomagenesis. In this study we present the largest and most comprehensive series of human spinal cord gliomas surveyed for PDGFRα expression to date. Using immnohistochemical analysis we demonstrate that a significant subset of spinal cord gliomas express receptors for the growth factor PDGF and show that these receptors localize to tumor cell membranes and cytoplasm. Approximately 30% of all tumors and 20% of ependymomas—the largest subset of gliomas included in this study—were immunopositive for PDGFRα. This is in contrast to the relatively high rate of PDGFRα immunopositivity (5 of 7 tumors) seen in a small series of spinal ependymomas by Barton et al. [5]. Within each diagnostic category the cohort sizes were too small to make statistical inferences, however, it is important to note that PDGFRα expression was seen in all major categories of glioma found in the spinal cord. These results have implications regarding the identification of the cell types that give rise to spinal cord gliomas, determining the mechanism of spinal cord glioma development, and delineating the potential therapeutic options for patients with these tumors.
A number of animal and human studies suggest that autocrine and paracrine signaling through the PDGF cell surface receptor is responsible for the initiation and progression of brain gliomas [7, 9, 10, 20–25]. Expression profiling of human cerebral gliomas indicates that PDGF and its receptors (PDGFR) are often co-expressed [7, 20]. In vitro studies have shown that PDGF is a powerful mitogen for glioma cells and that small molecule inhibitors of PDGF will block glioma cell proliferation and survival [26, 27]. Animal studies have shown that infecting glial progenitors in the subventricular zone (SVZ), subcortical white matter, or brainstem with PDGF-expressing retroviruses will induce the formation of gliomas [22, 28, 29]. Furthermore, viruses that express higher levels of PDGF drive cerebral gliomas to form faster, more consistently, and with more malignant features, suggesting that PDGF-driven gliomagenesis is a dose dependent phenomena [30].
Thus far, evidence primarily from studies in animal models implicates PDGF receptor signaling in spinal cord gliomas as well [1–3]. This study provides the first evidence from human spinal cord glioma tissue that PDGF receptor expression may play an important role in the development of at least a subset of spinal cord gliomas. Interestingly, the human brain contains a population of cycling PDGFRα-expressing progenitor cells thought capable of initiating glioma formation in the presence of PDGF [31–33]. These glial progenitors are thought to possess an inherent capacity to proliferate massively in response to PDGF stimulation enabling even genetically normal progenitors to become tumorigenic if exposed to sufficient levels of PDGF [31]. Confirmation that this is also true in the human spinal cord awaits the results of future studies.
While maximal, safe resection is the initial treatment strategy utilized for most low grade gliomas of the spinal cord, adjuvant therapy may be useful in the management of residual, recurrent, or high-grade tumors. However, there is little evidence that current adjuvant therapies for spinal cord gliomas are effective [34–36]. A better strategy in treating these patients may be the rational use of targeted chemotherapies that block specific signal transduction pathways in tumor cells such as those downstream of the PDGF receptor. Though the promising pre-clinical results from studies evaluating PDGF receptor blockade with agents such as imatinib, sunitinib, and sorafenib in cerebral gliomas have yet to convincingly show clinical benefit, this represents an active area of research [11–17]. This strategy has not been explored in the treatment of spinal cord gliomas save for a single case report of partial remission of a PDGFR-expressing spinal ependymoma with use of imatinib [4]. As nearly a third of spinal cord gliomas in this series were shown to express PDGFRα, initiating therapies that target this receptor may be helpful in a significant subset of patients.
Limitations of this study stem from the small cohort sizes, especially for the non-ependymal tumors, from the qualitative immunohistochemical approach used for expression analysis, and from the inherent heterogeneity within gliomas. As primary spinal cord tumors are rare being 10–15 times less common than intracranial tumors [37] the accumulation of larger cohorts in future studies may require grouping of samples from multiple institutions. Our use of immunohistochemical analysis was low-cost, technically straightforward, and proved very reliable for screening the small pieces of tissue typical of spinal cord tumor biopsies. The primacy of neurological function preservation during spinal cord tumor biopsies means that extensive analysis of intratumoral expression variability may only be possible on autopsy samples.
Conclusion
PDGFRα is expressed in a subset of intramedullary spinal cord gliomas including ependymoma, oligodendroglioma, pilocytic astrocytoma, astrocytoma, and glioblastoma. This finding supports previous studies which suggest that PDGF signaling can play an important role in spinal cord gliomagenesis. Validation of these results in larger cohorts using quantitative expression analysis will be important in future studies. The prognostic significance of PDGFRα expression in spinal cord gliomas as well as its implications for the effectiveness of targeted therapies remains to be determined.
Contributor Information
Jason A. Ellis, Email: jae2109@columbia.edu, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA.
Peter Canoll, Department of Pathology and Cell Biology, Columbia University Medical Center, New York, USA.
Paul C. McCormick, II, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA.
Neil A. Feldstein, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Richard C. Anderson, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Peter D. Angevine, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Michael G. Kaiser, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Paul C. McCormick, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Jeffrey N. Bruce, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
Alfred T. Ogden, Department of Neurological Surgery, Neurological Institute of New York, Columbia University Medical Center, 710 West 168th Street, New York, NY 10032, USA
References
- 1.Hitoshi Y, Harris BT, Liu H, Popko B, Israel MA. Spinal glioma: platelet-derived growth factor B-mediated oncogenesis in the spinal cord. Cancer Res. 2008;68:8507–8515. doi: 10.1158/0008-5472.CAN-08-1063. [DOI] [PubMed] [Google Scholar]
- 2.Ellis J, Castelli M, Canoll P, Bruce J, Ogden A. Adult white matter glial progenitors as cells of origin for intramedullary spinal cord tumors: evidence from a novel animal model. [Accessed 2 Aug 2011];AANS/CNS Section on Disorders of the Spine and Peripheral Nerves Abstract. 2010 http://univ.cns.org/EducationalTools.htm. [Google Scholar]
- 3.Ellis JA, Castelli M, Bruce JN, Canoll P, Ogden AT. Retroviral delivery of PDGF to spinal cord progenitor cells drives the formation of intramedullary gliomas. Neurosurgery. 2011 doi: 10.1227/NEU.0b013e31822ce963. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhrai N, Neophytou P, Dieckmann K, Nemeth A, Prayer D, Hainfellner J, Marosi C. Recurrent spinal ependymoma showing partial remission under Imatimib. Acta Neurochir (Wien) 2004;146:1255–1258. doi: 10.1007/s00701-004-0374-5. [DOI] [PubMed] [Google Scholar]
- 5.Barton VN, Donson AM, Kleinschmidt-DeMasters BK, Birks DK, Handler MH, Foreman NK. Unique molecular characteristics of pediatric myxopapillary ependymoma. Brain Pathol. 2010;20(3):560–570. doi: 10.1111/j.1750-3639.2009.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinho O, Longatto-Filho A, Lambros MB, Martins A, Pinheiro C, Silva A, Pardal F, Amorim J, Mackay A, Milanezi F, Tamber N, Fenwick K, Ashworth A, Reis-Filho JS, Lopes JM, Reis RM. Expression, mutation and copy number analysis of platelet-derived growth factor receptor A (PDGFRA) and its ligand PDGFA in gliomas. Br J Cancer. 2009;101:973–982. doi: 10.1038/sj.bjc.6605225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hermanson M, Funa K, Hartman M, Claesson-Welsh L, Heldin CH, Westermark B, Nister M. Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res. 1992;52:3213–3219. [PubMed] [Google Scholar]
- 8.Haberler C, Gelpi E, Marosi C, Rossler K, Birner P, Budka H, Hainfellner JA. Immunohistochemical analysis of plateletderived growth factor receptor-alpha, -beta, c-kit, c-abl, and Arg proteins in glioblastoma: possible implications for patient selection for imatinib mesylate therapy. J Neurooncol. 2006;76:105–109. doi: 10.1007/s11060-005-4570-9. [DOI] [PubMed] [Google Scholar]
- 9.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS One. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razis E, Selviaridis P, Labropoulos S, Norris JL, Zhu MJ, Song DD, Kalebic T, Torrens M, Kalogera-Fountzila A, Karkavelas G, Karanastasi S, Fletcher JA, Fountzilas G. Phase II study of neoadjuvant imatinib in glioblastoma: evaluation of clinical and molecular effects of the treatment. Clin Cancer Res. 2009;15:6258–6266. doi: 10.1158/1078-0432.CCR-08-1867. [DOI] [PubMed] [Google Scholar]
- 12.Baruchel S, Sharp JR, Bartels U, Hukin J, Odame I, Portwine C, Strother D, Fryer C, Halton J, Egorin MJ, Reis RM, Martinho O, Stempak D, Hawkins C, Gammon J, Bouffet E. A Canadian paediatric brain tumour consortium (CPBTC) phase II molecularly targeted study of imatinib in recurrent and refractory paediatric central nervous system tumours. Eur J Cancer. 2009;45:2352–2359. doi: 10.1016/j.ejca.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Raymond E, Brandes AA, Dittrich C, Fumoleau P, Coudert B, Clement PM, Frenay M, Rampling R, Stupp R, Kros JM, Heinrich MC, Gorlia T, Lacombe D, van den Bent MJ. Phase II study of imatinib in patients with recurrent gliomas of various histologies: a European Organisation for Research and Treatment of Cancer Brain Tumor Group Study. J Clin Oncol. 2008;26:4659–4665. doi: 10.1200/JCO.2008.16.9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, Abrey LE, Raizer J, Cloughesy TF, Fink K, Gilbert M, Chang S, Junck L, Schiff D, Lieberman F, Fine HA, Mehta M, Robins HI, DeAngelis LM, Groves MD, Puduvalli VK, Levin V, Conrad C, Maher EA, Aldape K, Hayes M, Letvak L, Egorin MJ, Capdeville R, Kaplan R, Murgo AJ, Stiles C, Prados MD. Phase I/II study of imatinib mesylate for recurrent malignant gliomas: North American Brain Tumor Consortium Study 99–08. Clin Cancer Res. 2006;12:4899–4907. doi: 10.1158/1078-0432.CCR-06-0773. [DOI] [PubMed] [Google Scholar]
- 15.Reardon DA, Dresemann G, Taillibert S, Campone M, van den Bent M, Clement P, Blomquist E, Gordower L, Schultz H, Raizer J, Hau P, Easaw J, Gil M, Tonn J, Gijtenbeek A, Schlegel U, Bergstrom P, Green S, Weir A, Nikolova Z. Multicentre phase II studies evaluating imatinib plus hydroxyurea in patients with progressive glioblastoma. Br J Cancer. 2009;101:1995–2004. doi: 10.1038/sj.bjc.6605411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, Duerinck J, Tynninen O, Nupponen N, Michotte A, De Greve J. Phase II study of sunitinib malate in patients with recurrent high-grade glioma. J Neurooncol. 2011;103(3):491–501. doi: 10.1007/s11060-010-0402-7. [DOI] [PubMed] [Google Scholar]
- 17.Hainsworth JD, Ervin T, Friedman E, Priego V, Murphy PB, Clark BL, Lamar RE. Concurrent radiotherapy and temozolomide followed by temozolomide and sorafenib in the first-line treatment of patients with glioblastoma multiforme. Cancer. 2010;116:3663–3669. doi: 10.1002/cncr.25275. [DOI] [PubMed] [Google Scholar]
- 18.Calzolari F, Malatesta P. Recent insights into PDGFinduced gliomagenesis. Brain Pathol. 2010;20(3):527–538. doi: 10.1111/j.1750-3639.2009.00335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shih AH, Holland EC. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006;232:139–147. doi: 10.1016/j.canlet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Westermark B, Heldin CH, Nister M. Platelet-derived growth factor in human glioma. Glia. 1995;15:257–263. doi: 10.1002/glia.440150307. [DOI] [PubMed] [Google Scholar]
- 21.Di Rocco F, Carroll RS, Zhang J, Black PM. Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery. 1998;42:341–346. doi: 10.1097/00006123-199802000-00080. [DOI] [PubMed] [Google Scholar]
- 22.Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozawa T, Brennan CW, Wang L, Squatrito M, Sasayama T, Nakada M, Huse JT, Pedraza A, Utsuki S, Yasui Y, Tandon A, Fomchenko EI, Oka H, Levine RL, Fujii K, Ladanyi M, Holland EC. PDGFRA gene rearrangements are frequent genetic events in PDGFRA-amplified glioblastomas. Genes Dev. 2010;24:2205–2218. doi: 10.1101/gad.1972310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uhrbom L, Hesselager G, Nister M, Westermark B. Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res. 1998;58:5275–5279. [PubMed] [Google Scholar]
- 26.Servidei T, Riccardi A, Sanguinetti M, Dominici C, Riccardi R. Increased sensitivity to the platelet-derived growth factor (PDGF) receptor inhibitor STI571 in chemoresistant glioma cells is associated with enhanced PDGF-BB-mediated signaling and STI571-induced Akt inactivation. J Cell Physiol. 2006;208:220–228. doi: 10.1002/jcp.20659. [DOI] [PubMed] [Google Scholar]
- 27.Oude Weernink PA, Verheul E, Kerkhof E, van Veelen CW, Rijksen G. Inhibitors of protein tyrosine phosphorylation reduce the proliferation of two human glioma cell lines. Neurosurgery. 1996;38:108–113. doi: 10.1097/00006123-199601000-00026. Discussion 113–104. [DOI] [PubMed] [Google Scholar]
- 28.Assanah MC, Bruce JN, Suzuki SO, Chen A, Goldman JE, Canoll P. PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia. 2009;57:1835–1847. doi: 10.1002/glia.20895. [DOI] [PubMed] [Google Scholar]
- 29.Masui K, Suzuki SO, Torisu R, Goldman JE, Canoll P, Iwaki T. Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia. 2010;58:1050–1065. doi: 10.1002/glia.20986. [DOI] [PubMed] [Google Scholar]
- 30.Shih AH, Dai C, Hu X, Rosenblum MK, Koutcher JA, Holland EC. Dose-dependent effects of platelet-derived growth factor-B on glial tumorigenesis. Cancer Res. 2004;64:4783–4789. doi: 10.1158/0008-5472.CAN-03-3831. [DOI] [PubMed] [Google Scholar]
- 31.Canoll P, Goldman JE. The interface between glial progenitors and gliomas. Acta Neuropathol. 2008;116:465–477. doi: 10.1007/s00401-008-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoshan Y, Nishiyama A, Chang A, Mork S, Barnett GH, Cowell JK, Trapp BD, Staugaitis SM. Expression of oligodendrocyte progenitor cell antigens by gliomas: implications for the histogenesis of brain tumors. Proc Natl Acad Sci USA. 1999;96:10361–10366. doi: 10.1073/pnas.96.18.10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Staugaitis SM, Trapp BD. NG2-positive glia in the human central nervous system. Neuron Glia Biol. 2009;5:35–44. doi: 10.1017/S1740925X09990342. [DOI] [PubMed] [Google Scholar]
- 34.Balmaceda C. Chemotherapy for intramedullary spinal cord tumors. J Neurooncol. 2000;47:293–307. doi: 10.1023/a:1006499313482. [DOI] [PubMed] [Google Scholar]
- 35.Harrop JS, Ganju A, Groff M, Bilsky M. Primary intra-medullary tumors of the spinal cord. Spine (Phila Pa 1976) 2009;34:S69–S77. doi: 10.1097/BRS.0b013e3181b95c6f. [DOI] [PubMed] [Google Scholar]
- 36.Isaacson SR. Radiation therapy and the management of intramedullary spinal cord tumors. J Neurooncol. 2000;47:231–238. doi: 10.1023/a:1006470523052. [DOI] [PubMed] [Google Scholar]
- 37.Chamberlain MC, Tredway TL. Adult primary intradural spinal cord tumors: a review. Curr Neurol Neurosci Rep. 2011;11(3):320–328. doi: 10.1007/s11910-011-0190-2. [DOI] [PubMed] [Google Scholar]