Abstract
The class Ia ribonucleotide reductase (RNR) from Escherichia coli (Ec) employs a free-radical mechanism, which involves bidirectional translocation of a radical equivalent or “hole” over a distance of ∼35 Å from the stable diferric/tyrosyl-radical (Y122•) cofactor in the β subunit to cysteine 439 (C439) in the active site of the α subunit. This long-range, inter-subunit electron transfer occurs by a multi-step “hopping” mechanism via formation of transient amino acid radicals along a specific pathway and is thought to be conformationally gated and coupled to local proton transfers. Whereas constituent amino acids of the hopping pathway have been identified, details of the proton-transfer steps and conformational gating within the β sununit have remained obscure; specific proton couples have been proposed, but no direct evidence has been provided. In the key first step, the reduction of Y122• by the first residue in the hopping pathway, a water ligand to Fe1 of the diferric cluster was suggested to donate a proton to yield the neutral Y122. Here we show that forward radical translocation is associated with perturbation of the Mössbauer spectrum of the diferric cluster, especially the quadrupole doublet associated with Fe1. Density functional theory (DFT) calculations verify the consistency of the experimentally observed perturbation with that expected for deprotonation of the Fe1-coordinated water ligand. The results thus provide the first evidence that the diiron cluster of this prototypical class Ia RNR functions not only in its well-known role as generator of the enzyme's essential Y122•, but also directly in catalysis.
Introduction
Ribonucleotide reductases (RNRs) catalyze the reduction of ribonucleotides to deoxyribonucleotides in all organisms, thereby providing and controlling the only de novo pathway to the four precursors required for DNA replication and repair.1,2 RNRs use a free-radical mechanism, in which a transient cysteine thiyl radical (C•) in the active site of the enzyme initiates substrate reduction by abstraction of a hydrogen atom (H•) from C3′.1,3-9 In class Ia RNRs, such as the RNR from aerobically growing Escherichia coil (Ec), a stable tyrosyl radical (Y122• in the Ec ortholog) in close proximity to a μ-oxo-(FeIII)2 cluster in the β subunit10-14 reversibly generates the transient C• in the active site of the enzyme's α subunit in the functional α2β2 complex.5-7,15,16 A model of the complex, constructed by computer docking of the structures of the individual subunits7 and subsequently validated by electron-electron double resonance spectroscopic experiments,17-19 suggests a distance of ∼35 Å between Y122• in β and the H•-abstracting C439 in α. Electron transfer (ET) between C439 and Y122 by a single tunneling step over that distance would be far too slow to account for the enzyme's turnover rate (2-10 s-1).15,20 Instead, this long-range inter-subunit ET is mediated by a chain of strictly conserved aromatic amino acids, which form transient radicals in a “hopping” mechanism (Scheme 1A).7,15,21-28 Direct detection of these pathway radicals in the wild-type enzyme has been hampered by a preceding rate-limiting conformational change within the α2β2 complex.20 This slow conformational change, which occurs upon binding of substrate and allosteric effector to α and allows for translocation of the radical from its resting location on β-Y122 to where it functions in catalysis on α-C439, masks the subsequent, fast chemical events.20 Substitution of pathway tyrosines by unnatural amino acids with altered redox properties led to the first detection of pathway radicals and provided the most direct evidence that these residues are redox-active “stepping stones” in the long-range ET (Scheme 1A).21-26 The individual ET hopping steps in the overall 35-Å hole-translocation process are thought to be coupled to multiple short-range proton transfer steps (i.e., proton-coupled ET or PCET), which effectively tune the thermodynamics of the component steps for efficiency and reversibility of the overall process.15,29 The coupling of ET to proton transfer (PT) steps could therefore permit radical translocation to be controlled by the PT steps, which could, due to their more stringent distance and orientation requirements, be controlled by the conformation of the protein: engagement of a proton-transfer pathway upon substrate binding could be the basis for the conformational gating in RNR.
Scheme 1.
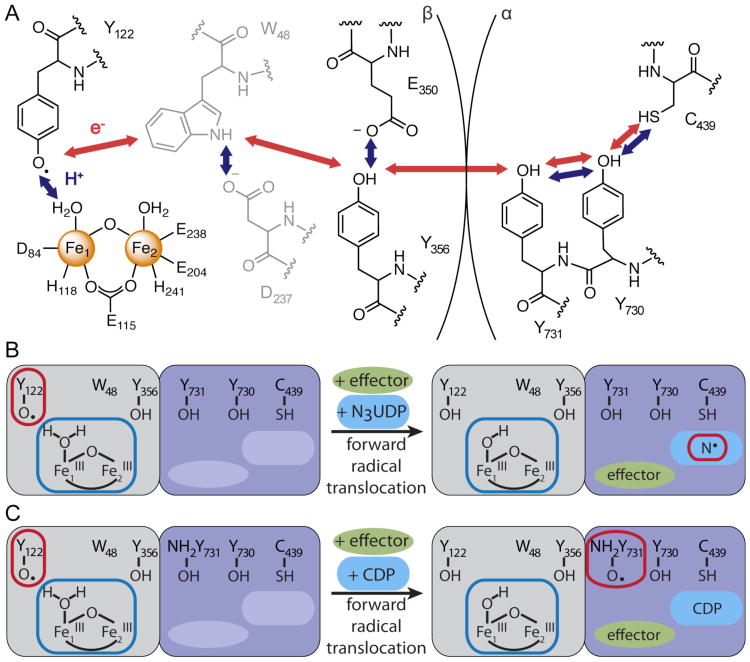
Proposed radical translocation pathway (A) and schematic representation of the two approaches used to trap the forward radical translocation product in Ec RNR (B) and (C). In (A) W48 and D237 are shown in gray because there is currently no direct evidence for involvement of W48 in radical translocation. Trapping of the forward radical translocation product induced by N3UDP in the wild-type α2β2 complex is shown in (B), and by CDP in a complex with an α variant containing the radical-stabilizing unnatural amino acid 3-aminotyrosine (NH2-Y) at residue α-Y731 (C). EPR- and Mössbauer-observable species are illustrated in (B) and (C) by red and blue outlines, respectively.
Whereas the radical-hopping nature of the process and the identities of the mediating residues have (with the exception of tryptophan 48 in β, which may or may not be a mediator) been established, much less is known about the details of the proton transfers. It has been proposed that PT proceeds orthogonally to ET in the β subunit and collinearly in α.15,29 A recent electron-nuclear double resonance (ENDOR) spectroscopic study provided evidence for hydrogen bonds among the three ET pathway residues of α in the active α2β2 complex, consistent with collinear PCET.30 Within the β subunit, specific proton coupling partners have been proposed (Scheme 1A), but little experimental evidence has been provided.27,31 Specifically, in the first step of forward (β-Y122• → α-C439) radical translocation, the neutral Y122• is reduced to the neutral Y122 by β-Y356 (or perhaps β-W48) in the pathway, and it has been suggested that the proton required to maintain neutrality of the Y122 side chain is delivered orthogonally to ET by the water ligand on the iron ion in site 1 (Fe1).15,32 In this study, we have trapped the cofactor in β in its product state of the forward radical translocation process by using either the radical-trapping substrate analog 2′-azido-2′-deoxyuridine 5′-diphosphate (N3UDP) with the wild-type enzyme or a natural substrate (CDP) with a variant of the α-subunit containing the radical-stabilizing unnatural amino acid 3-aminotyrosine (NH2-Y) at the subunit-interfacial pathway residue α-Y731 (Scheme 1B and C). We show that the Mössbauer spectrum of the (FeIII)2 cluster, especially the quadrupole splitting parameter (ΔEQ) associated with Fe1, changes while the oxidation state of the cluster remains unchanged and that this spectral perturbation is specific to the form of the enzyme that has engaged in forward radical translocation. The nature of the observed perturbation – a ∼ 0.5 mm/s diminution in |ΔEQ| of Fe1 with much smaller changes to |ΔEQ| of Fe2 and the isomer shifts (δ) of both sites – agrees remarkably well with the effect predicted by simple DFT calculations for removal of a proton from the Fe1-OH2 ligand. The results provide the first direct evidence that the diiron cluster of the prototypical class Ia RNR from Ec not only serves its well-known role as generator of the Y122•11,15,33 but also actively participates in the enzyme's catalytic cycle.
Results and Discussion
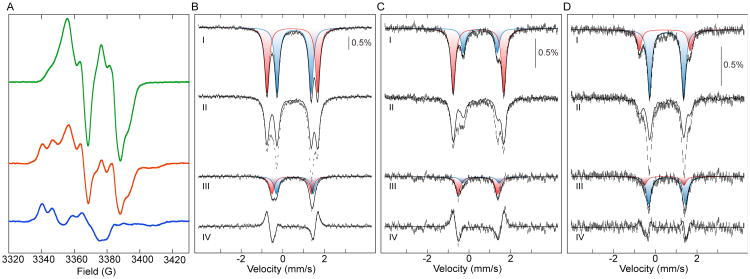
To initiate forward radical translocation and trap the enzyme in the product state of this step, the α and β subunits were incubated in the presence of the positive allosteric effector, thymidine triphosphate (TTP), with the substrate analog N3UDP, which brings about the irreversible reduction of Y122• in β along with the formation of a meta-stable, nucleotide-based, nitrogen-centered radical (N•) in the active site of α (Scheme 1B).34-36 Conversion of β-Y122• to the N• was confirmed by comparison of the X-band EPR spectrum at 14 K of the reaction sample (Fig. 1 A, red) to that of a control sample from which N3UDP was omitted (Fig. 1 A, green). Subtraction of the features of the unreacted β-Y122• from the spectrum of the N3UDP-treated sample yields the spectrum of the N• (Fig. 1A).36,37 Its intensity accounts for ∼36% of the Y122• originally present in the control (− N3UDP) sample. Conversions of ≤ 50% have generally been observed in such experiments with Ec RNR and have been attributed to the facile reaction of only one αβ pair in the α2β2 hetero-tetramer (“half-of-sites reactivity”), a property thought to be intrinsic to the enzyme. 17,23-26
Figure 1.
EPR and Mössbauer spectra of Ec RNR before and after N3UDP-induced trapping of the product of forward radical translocation. (A) EPR spectra of uniformly 57Fe labeled α2β2 with TTP and in the absence (green) or presence (red) of N3UDP. Subtraction of the features of the unreacted Y122• (green) from the spectrum of the N3UDP-treated sample (red) yields the spectrum of the N• (blue). 4.2-K/53-mT Mössbauer spectra of uniformly 57Fe labeled (B), site 1 57Fe enriched (C) and site 2 57Fe enriched (D) α2β2 with TTP and in the absence (I) or presence (II) of N3UDP. The solid lines in I are simulations of the two quadrupole doublets (red and blue) of the resting (FeIII)2 cluster with parameters quoted in the text and the sum of the two doublets (black). The solid line in II is the spectrum from I scaled appropriately to remove the contribution of the unreacted cofactor, and subtraction of this contribution from II reveals the spectrum of the product of the forward radical translocation (III), which can be simulated with two quadrupole doublets (red and blue; parameters quoted in the text). The sum of the two quadrupole doublet simulations is shown as a black line. The spectrum in IV is the total difference of I-II and the solid line is a simulation of the difference spectrum as a sum of the four quadrupole-doublet components.
The changes to the (FeIII)2 cluster in β upon this N3UDP-induced forward radical translocation were monitored by Mössbauer spectroscopy on two samples identical to the aforementioned EPR samples. The 4.2-K/53-mT Mössbauer spectrum of the control sample in the absence of N3UDP [i.e., of the resting (FeIII)2 cluster]38-40 consists of two resolved quadrupole doublets with parameters [isomer shift, δ1, of 0.46 mm/s and quadrupole splitting parameter, ΔEQ1, of 2.43 mm/s (Fig. 1B,I, red), and δ2 = 0.54 mm/s, ΔEQ2 = 1.63 mm/s (Fig. 1B, I, blue)] nearly identical to those reported for active β alone and previously assigned to Fe1 and Fe2, respectively.11,39,41-42 Incubation with N3UDP changes the Mössbauer spectrum significantly, and the spectrum exhibits new features (Fig. 1B, II, and clearly apparent in the difference spectrum, IV). Removal of the contribution of the unreacted (FeIII)2 cluster by subtraction of the appropriately scaled spectrum of the control (− N3UDP) sample (solid line) resolves the new spectrum of the radical translocation product (Fig. 1B, III). The new features account for ∼41% conversion of the active cofactor, which agrees well with the conversion of Y122• to N• determined by EPR (see also Table S2). The derived spectrum of the radical translocation product can be analyzed as two symmetrical quadrupole doublets with equal intensity, equal line width, and parameters δ1 = 0.43 mm/s, ΔEQ1 = 1.89 mm/s, and δ2 = 0.57 mm/s, ΔEQ2 = 1.76 mm/s (Fig. 1B, III, red and blue, respectively; Table S1).43 The modest changes to the isomer shifts of both sites and the fact that the spectrum still comprises quadrupole doublet features indicative of an integer-electron-spin ground state imply that no change in oxidation state of the (FeIII)2 cluster is effected by the N3UDP treatment,44 as expected from previous studies employing this compound.34-36 In addition, the large magnitudes of the ΔEQ-values further suggest that the oxo bridge remains intact.45
The spectra of control samples containing β, effector, either CDP or N3UDP, and an α variant having the hopping pathway disabled by substitution of the subunit-interfacial Y731 in α with F lack these new features and are essentially identical to the spectrum of the sample with wild-type α and β before reaction with N3UDP (Fig. S2). The spectrum of an additional control sample, in which the Y122• in β was reduced in the absence of α with hydroxyurea (HU) to yield the inactive (FeIII)2/Y122 met form (Fig. S2),46 is also very similar to that of the active form. These results imply that the observed perturbation to the spectrum of the (FeIII)2 cluster in the wild-type enzyme caused by N3UDP is related to radical translocation and not to either the absence of Y122• per se or nucleotide binding events.
The two quadrupole doublets that make up the spectrum of the radical translocation product were unambiguously assigned to Fe1 and Fe2 by site-selective labeling of β with 57Fe and 56Fe. The two sites in Ec β have different affinities for FeII, and this property can be exploited to obtain β with the Mössbauer active 57Fe enriched in one or the other site.41 The spectrum of a sample of the complex prepared with β subunit having site 1 enriched with 57Fe can be simulated with the same parameters used for spectra of the uniformly 57Fe labeled samples above, and the relative intensities of the two quadrupole doublets indicate that ∼73% of the 57Fe resides in site 1 and ∼27% in site 2 (Fig. 1C, I, red and blue). Treatment of this sample with N3UDP yielded a β-Y122• to N• conversion of ∼38%, as determined by EPR spectroscopy (Fig. S3, Table S2), similar to that achieved with uniformly 57Fe-labeled complex. The Mössbauer spectrum of the radical translocation product, obtained after subtraction of the unreacted component (Fig. 1C, II and III), constitutes 38% of the active cofactor, in agreement with the EPR quantification. It can be simulated with the same parameters used for the spectrum of the product in the uniformly labeled complex (Fig. 1C, III, red and blue). Owing to the site-selective labeling, the two quadrupole doublets have different intensities and can therefore be unambiguously assigned to Fe1 (red) and Fe2 (blue). Samples with 57Fe enriched in site 2 confirm this assignment (Fig. 1D). These samples contain ∼24% of the 57Fe in site 1 and ∼76% in site 2 (Fig. 1D, I, red and blue). Reaction with N3UDP resulted in conversion of ∼47% of initial β-Y122• to N•, as quantified by EPR (Fig. S3, Table S2), and produced the Mössbauer spectrum shown in Fig. 1D, II. The Mössbauer spectrum of the radical translocation product, obtained after removal of the unreacted component (Fig. 1D, III) and accounting for 52% conversion of the active cofactor, can again be simulated with the same parameters used for the spectrum of the product with the uniformly and site-1-enriched complexes (Fig. 1D, III, red and blue). Assignment of the two quadrupole doublets to Fe1 (red) and Fe2 (blue) shows that the spectrum of Fe1, the site with the water proposed to be the proton donor, changes much more (|ΔEQ| decreases by 0.5 mm/s) than that of Fe2 upon N3UDP-induced trapping of the radical translocation product.
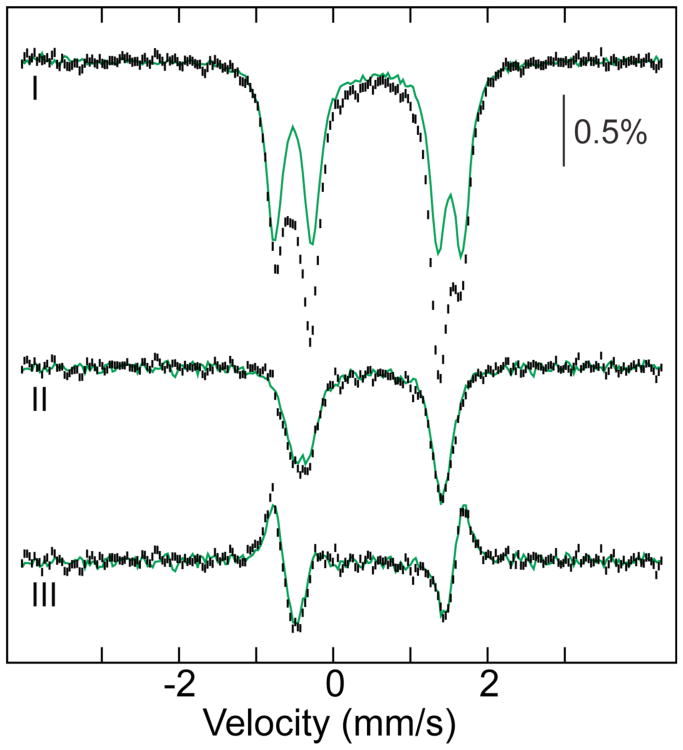
In addition to the use of the radical-trapping substrate analog, N3UDP, the product of forward radical translocation can also be trapped by using an α variant that contains an unnatural amino acid incorporated into the hopping pathway (Scheme IC). The radical produced from 3-aminotyrosine (NH2Y•) has a reduction potential estimated to be ∼190 mV less than that of Y•, causing the radical to reside on this unnatural residue during catalysis by Ec RNR variants.23,47 The variant α protein having NH2Y in place of Y731 is capable of nucleotide reduction, and, upon its incubation with the β subunit, the effector ATP and the CDP substrate, a NH2Y• accumulates to ∼50% of the total spin in the sample.23 The dependence on the presence of nucleotides and β, and the ability of this enzyme to catalyze deoxynucleotide production, show that the NH2Y• forms by gated radical translocation in the functional holoenzyme complex. Translocation of the radical from β-Y122• to α-NH2Y731 is expected to be accompanied by the same change to the (FeIII)2 cluster observed above in the wild-type complex upon reaction with N3UDP. Indeed, the experimental Mössbauer spectrum after reaction of β with α-Y731NH2Y, ATP, and CDP and the product spectrum (45% conversion) obtained after removal of the unreacted component (Fig. 2) are almost identical to the spectra of the radical translocation product trapped by N3UDP (Fig. 1B). The observation of the same perturbed quadrupole-doublet spectrum in samples in which the Y122•-reduced β was trapped by either N3UDP or the α-Y731NH2Y variant, but not in samples prepared either with a pathway-disabled α or by reduction of Y122• by HU in the absence of α, strongly suggests that the change to the (FeIII)2 cluster is associated specifically with functional translocation of the radical from Y122• into the hopping pathway.
Figure 2.
4.2-K/53-mT Mössbauer spectra of the product of forward radical translocation trapped in the (Y731NH2Y-α)2β2 complex. (I) Sample prepared with β, Y731NH2Y-α, ATP and CDP. The green line is the scaled control spectrum of β that had been incubated with pathway-blocked Y731F-α, ATP and CDP, and subtraction of this spectral contribution reveals the spectrum of the product of forward radical translocation (II). The green line overlaid in II is the spectrum of the radical translocation product induced with N3UDP from Fig.1B, III, and is shown to illustrate the near identity of the perturbed spectra resolved by the two different trapping approaches. The spectrum in III is the total difference of the spectrum of (Y731F-α)2β2 in the presence of ATP and CDP subtracted from I, and the green line is the total difference spectrum from the α2β2 ±N3UDP experiment in Fig.1B, IV.
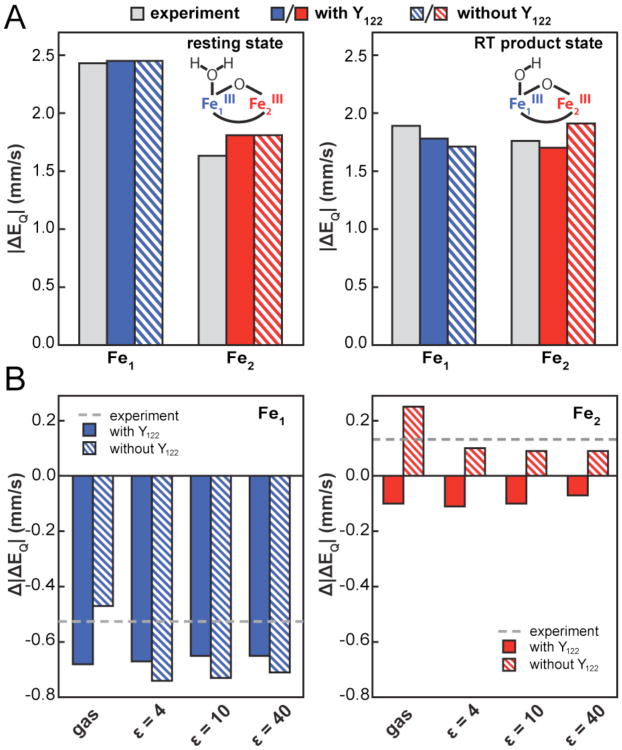
To evaluate whether the change to the Mössbauer spectrum of the diferric cluster observed upon radical translocation is consistent with the proposed deprotonation of the Fe1-OH2 (Scheme 1B and C), we performed a series of DFT calculations. Remarkably, the calculations, performed by the broken-symmetry DFT methodology,48 predict the same qualitative change to the Mössbauer parameters upon removal of a proton from the Fe1-OH2 [0.47-0.74 mm/s decrease in |ΔEQ| for Fe1, a much smaller (≤ 0.25 mm/s) increase or decrease in |ΔEQ| of Fe2, and almost no change (0.04 mm/s) to the isomer shift of either site; Fig. 3 and Table S3] as is observed experimentally upon radical translocation. The calculations were performed by starting from the published high-resolution structure of the Ec β protein,49 which is purportedly of the met form. All first-sphere (ligand) residues and non-protein oxygen ligands [an HO(H) ligand to each iron and the oxo bridge] were included in the models (Fig. S4). Two sets of models were considered. One set includes both the diferric cluster and the radical tyrosine (Y122) in either the resting state of the cofactor (Fe1-OH2/Y•) or the postulated radical-translocation-product state (Fe1-OH/Y). A second set includes the diferric cluster in either its resting (Fe1-OH2) or radical translocation (Fe1-OH) state but omits the tyrosine (Fig. S4). To limit the number of atoms while still preventing ligand motions that would be precluded by their attachment to the protein backbone from occurring during geometry optimization, the approach used by Roos and Siegbahn was adopted.50 With these geometric constraints, none of the calculated models diverged markedly from the experimental structure during optimization (Fig. S4). In addition to calculations in the gas phase, the effect of the protein environment on the calculated Mössbauer parameters was evaluated using the COSMO solvation model51,52 with various dielectric constants (ε) of 4, 10, and 40. Calculations using ε = 4 reproduce the experimental parameters remarkably well, to within ± 0.18 mm/s for the models that include Y122 (Fig. 3A). Moreover, all the calculations, whether in the gas phase or with the COSMO solvent model and with Y122 included or omitted, reproduce the essential features of the experimental spectral perturbation, giving a relatively large decrease in |ΔEQ| and much smaller change in δ for site 1and small changes in |ΔEQ| and δ for site 2 (Fig. 3B and Table S3). The results imply that the observed effect arises directly from the change in the charge of the Fe1 HO(H) ligand rather than some interaction of the cluster with the Y122/Y122•. Consistent with this conclusion and the experimental Mössbauer spectra, parameters calculated for a model having the reduced, neutral Y122 and the Fe1-OH2 ligand (corresponding to the met form of the protein) do not deviate significantly from those calculated for the active state (with Y122• and Fe1-OH2). These DFT calculations thus establish that the change to the Mössbauer spectrum associated with radical translocation is consistent with the proposed donation of a proton to Y122 by the Fe1–OH2 in the first PCET step.
Figure 3.
Comparison of the experimental quadrupole splitting (ΔEQ) parameters for the resting and radical-transolcation-product states of the cofactor to the values calculated by DFT. (A) Absolute values of ΔEQ from experiment (gray bars) and the DFT calculations using a dielectric constant of ε = 4 for models of either the full cofactor including Y122 (solid colored bars) or just the diferric cluster without Y122 (striped colored bars). The blue bars correspond to Fe1 and the red bars to Fe2. (B) The changes in the absolute values of ΔEQ associated with the radical-translocation/deprotonation event either from the experiments (dashed gray lines) or from the calculations on the models including (solid bars) or omitting (striped bars) Y122. The color coding is the same as in panel A.
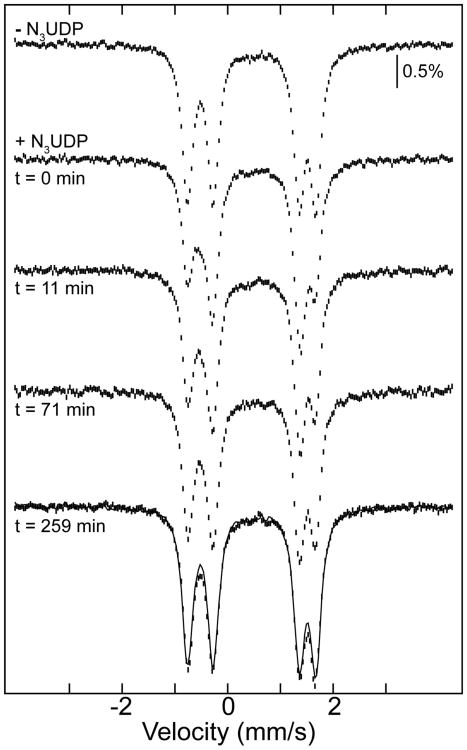
We anticipated that the Fe1–OH radical translocation product would be meta-stable and eventually undergo protonation (with the ultimate source being bulk solvent) to generate the Fe1–OH2 species of the stable met form (Fe1-OH2/Y). To test this notion, the radical translocation product trapped with N3UDP was thawed and incubated on ice to permit decay of the N• and enable subsequent adjustments of the protein complex and the (FeIII)2 cluster. Periodically, the sample was re-frozen for acquisition of its Mössbauer spectrum. Spectra acquired after total incubation times of 10 to 260 minutes demonstrate the return of the spectrum of the resting (presumably Fe1–OH2) form of the cluster (Fig. 4). This result further confirms that the observed perturbation to the (FeIII)2 cluster upon reaction with N3UDP or Y731NH2Y-α and CDP is specific to the complex actively engaged in radical translocation and catalysis. The regeneration is relatively slow (t1/2 of ∼60 min at ∼ 0 °C), consistent with the hypothesis that it reflects the slow diffusion of an extra proton from bulk solvent to the Fe1–OH in the protein interior to convert it to Fe1–OH2 of the resting met form (perhaps subsequent to decay of the N• and disengagement of β from α with a t1/2 of 23 min at 25 °C 53). That this proton transfer from bulk solvent would be slow is supported by studies in which the kinetics of electrochemical reduction of the β subunit in the presence of a facile ET mediator (methyl viologen) were monitored. These experiments revealed that the (FeIII)2 center in the met form can be reduced relatively rapidly (within seconds), whereas reduction of the active (FeIII)2/Y122• form of the protein proceeds with the initial fast reduction of Y122• followed by much slower reduction of the (FeIII)2 cluster.40 The observations suggest that the cluster in the met form and the one generated from the active (FeIII)2/Y122• cofactor upon rapid Y122• reduction are somehow different. A reasonable explanation is that the cluster after fast Y122• reduction has the same number of protons as the radical translocation product, possessing the Fe1–OH generated by transfer of a proton from the Fe–OH2 to Y122 (Scheme 1B and C), whereas the stable met form produced by HU reduction of Y122• in the absence of α has the cluster in the Fe1–OH2 form. The absence of this proton in the initial Y122•-reduced β and presence in the stable met form would make the charge of the buried cluster different by one unit, altering the electrostatics of the site and potentially causing the difference in reduction kinetics of the two forms.
Figure 4.
Disappearance of the spectrum of the N3UDP-trapped radical translocation product and return of the spectrum of the resting (FeIII)2 cluster upon prolonged incubation on ice. 4.2-K/53-mT Mössbauer spectra of α2β2 with TTP and in the absence or presence of N3UDP. The +N3UDP sample was thawed, incubated on ice, and periodically re-frozen after total incubation times of 10 to 260 minutes for re-acquisition of its Mössbauer spectrum. The spectrum at t = 260 minutes is almost identical to the spectrum of α2β2 with TTP in the absence N3UDP (overlaid as a solid line).
Conclusion
The diiron cluster in class Ia Ec RNR has long been known to generate the Y122• in the initial activation of the β subunit by reaction of its (FeII)2 form with O2.11,15,33 Our results now strongly suggest that the (FeIII)2 cluster also actively functions in the catalytic cycle, specifically during translocation of the oxidizing equivalent or hole from its resting position on Y122• in β to the nucleotide reduction site in α. Reduction of Y122• upon forward radical translocation requires transfer of a proton to yield a neutral Y122, and, upon reverse radical translocation and reoxidation to Y122•, the proton should be returned. The Mössbauer-detected change to the cluster seen upon use of either the substrate analog or the α variant is almost certainly associated specifically with propagation of the radical into the hopping pathway, because the perturbation (1) is observed when the radical on Y122 translocates in a functionally relevant reaction into α, but not when it is reduced in a nonfunctional context by HU, (2) relaxes upon decay of the N• in α, and (3) is not observed in a complex having the hopping pathway blocked by the α-Y731F substitution. The nature of the spectral perturbation (significant decrease in |ΔEQ| of site 1 and much smaller changes to the other three parameters) implies that the oxidation state of the cluster does not change and matches that predicted by DFT calculations for removal of a proton from the Fe1-OH2 ligand. Our data are thus consistent with the previous suggestion that this water ligand serves as the proton-coupling partner to Y122 for radical translocation (Scheme 1).15,32 The reduction of Y122• constitutes the first step of forward radical translocation and might, therefore, be a key step in the gating of the process by the protein. If one or more protein side chain is required to mediate this proton transfer (e.g., the nearby D84 carboxylate ligand), a conformational change in the α2β2 complex occurring upon substrate binding could engage this proton-transfer pathway and thereby open the gate to reduction of Y122• by either W48 or Y356 in the initial step of the long-distance radical translocation.
Methods
Materials, protein production and purification, reconstitution of RNR-β with 57Fe and/or 56Fe, and activity assays are described in the Supporting Information.
Reactions with N3UDP
The reaction was carried out in a final volume of 0.6 mL and contained 0.3 mM α2 (or 0.29 mM Y731F-α2), 0.3 mM β2 (0.27 mM β2 in the case of uniformly 57Fe labeled β and 0.29 mM β2 in the case of Y731F-α2), 0.8 mM TTP, 1 mM N3UDP, 15 mM MgSO4, 1 mM EDTA, and 1 mM DTT in HEPES buffer. The reaction was initiated by the addition of N3UDP and β2 and allowed to proceed at RT (21 ± 2 °C) for a total reaction time of 2.5 min. Aliquots (0.3 mL) were transferred to Mössbauer and EPR sample cells and frozen in liquid N2.
Reaction of Y731NH2Y-α2 with substrate
Pre-reduced Y731NH2Y-α2 and 57Fe reconstituted β in assay buffer were concentrated to 0.3 mL, and mixed with an aliquot (0.05 mL) of ATP and CDP in assay buffer. The final reaction solution (0.35 mL) contained 0.25 mM Y731NH2Y-α2, 0.25 mM β2, 3 mM ATP and 1 mM CDP and was incubated at RT (22°C) for a total reaction time of 20 s and frozen in liquid N2.
EPR spectroscopy and analysis
EPR spectra were recorded on a Bruker ESP300 spectrometer equipped with a ER 041 MR Microwave Bridge and a Bruker 4102ST TE102 X-band resonator. Spectrometer configuration and data acquisition was performed by an external PC via GPIB interface using EWWIN 6.1 software from Scientific Software Services. Spectra were acquired at a temperature of 14.0 ± 1 K, a microwave power of 8 μW a microwave frequency of 9.45 GHz, a modulation amplitude of 3 G, a modulation frequency of 100 kHz, a receiver gain of 5 × 104, and a conversion time of 0.029 s. Four scans were averaged for the spectra acquired from 2,000 to 4,000 G. For spectra collected over the narrower field range from 3,180 to 3,580 G, a modulation amplitude of 1.0 G was used, and two scans were averaged.
The total electron spin concentration in each sample was determined by integrating its EPR absorption spectrum and comparing the integrated area to that of the spectrum of a standard containing 1.025 mM CuSO4, 2 M NaClO4, and 0.01 M HCl.54 The first derivative spectra recorded from 2,000 to 4,000 gauss G were integrated and the baselines were corrected by using a linear function. The second integral was formed and integrated areas were corrected for differences in g-values, as previously described.55
Samples treated with N3UDP contain a mixture of Y122• and the nitrogen-centered radical (N•). Removal of the features of the Y122• by subtraction of the appropriately scaled spectrum of the control (– N3UDP) sample yields the spectrum of the N•. Quantification of the subspectra of Y122• (scaled control spectrum) and N• in the samples was accomplished by integrating the two subspectra, applying a linear baseline correction, and forming the second integral to determine the area. These areas were used to calculate the percent area of the two subspectra, which were then multiplied by the measured total spin concentration of the samples to determine the concentration of each radical. The values are reported in Table S2. The concentrations of the Y122• and N• in the N3UDP treated samples can also be calculated using the scaling factor of the Y122• control spectrum that was subtracted. This scaling factor was multiplied by the spin concentration of the control sample to determine the concentration of Y122• in the sample. This was then subtracted from the concentration of the reacted sample to determine the remaining spin concentration, which was attributed to N•. The values obtained by this procedure agree well with the values using the double integral of the two subspectra reported in Table S2.
Mössbauer spectroscopy and analysis
Mössbauer spectra were recorded at a temperature of 4.2 K and an externally applied magnetic field of 53 mT oriented parallel to the γ-beam on a SVT-400 spectrometer from WEB Research (Edina, MN). Data analysis was performed using WMOSS (WEB Research, Edina, MN). Isomer shifts are quoted relative to the centroid of a spectrum of a metallic foil of α-Fe at room temperature.
Parameters of the spectrum of the active α2β2 complex before reaction
The Mössbauer spectrum of the active α2β2 complex consists of two symmetrical quadrupole doublets of equal intensity. The spectrum was fitted by two quadrupole doublets with the linewidths (Γ) of the doublets constrained to be the same. The resulting parameters are cited in the main text and in Table S1.
Analysis of the spectrum of the α2β2 complex after reaction with N3UDP
Removal of the contribution of the unreacted (FeIII)2 cluster (71% of total Fe of the uniformly 57Fe labeled sample) from the spectrum of the sample reacted with N3UDP resolves the spectrum of the radical translocation product. The subtraction was guided by visual inspection of the resolved left line at -0.76 mm/s and by the objective of making the resulting spectrum symmetric. The spectrum of the radical translocation product was then analyzed as two symmetrical quadrupole doublets of equal intensity and linewidth. Two sets of physically meaningful parameters (left-right solution and inner-outer solution, Table S1) fit the data equally well and cannot be distinguished owing to the 1:1 ratio of the two quadrupole doublets.
The dependence of the Mössbauer parameters obtained for the radical translocation product on the fraction of the spectrum of unreacted complex subtracted from the experimental spectrum was evaluated. We found that δ and ΔEQ vary by only 0.001-0.02 mm/s, which is within the intrinsic uncertainty limit for the parameters (0.02 mm/s), when reference spectra for the radical translocation product corresponding to 29 ± 3% of total Fe (71 ± 3% of the experimental spectrum attributed to the unreacted cluster and removed in the subtraction) were analyzed.
Analysis of the spectra with site selective 57Fe labeled β
To determine the occupancies of 57Fe in sites 1 and 2 in the site-selectively labeled βs, Mössbauer spectra of the 57Fe1 and 57Fe2 enriched β (1.3 mM β2) used in all the experiments were recorded. The spectra were simulated by fixing the parameters to those obtained from analysis of the spectrum of the uniformly 57Fe labeled β in the sample of the α2β2 complex and allowing only the areas of the two doublets to vary. The relative areas for the two doublets correspond to the relative amounts of 57Fe in sites 1 and 2, respectively. These occupancies were kept constant for all subsequent analyses of the spectra of reaction samples.
Subtractions of the unreacted component (scaled spectra of α2β2 before reaction) from the spectra after reaction with N3UDP to obtain the spectrum of the radical translocation products were carried out as described above for the uniformly 57Fe labeled samples. The product spectrum was simulated with the same parameters as used for the spectrum of the product in the uniformly labeled complex but using the areas for the two quadrupole doublets determined above. To evaluate the robustness of the percentage of intensity assigned to the unreacted complex, theoretical spectra for the two quadrupole doublets of the radical translocation product (using the quoted parameters) were subtracted in appropriate fractions from product spectra generated by subtraction of different amounts of the unreacted component.
The spectra of the site-selectively labeled samples allow unambiguous assignment of the two quadrupole doublets to Fe1 (red) and Fe2 (blue). In addition, they should, in principle, allow differentiation between the two possible sets of parameters (left-right solution and inner-outer solution, Table S1). However, the parameters of the two solutions are very similar, and we cannot make this assignment with confidence. While the two low-energy lines are at least partially resolved, both solutions (inner-outer and left-right) assign the -0.52 mm/s and -0.31 mm/s lines to the quadrupole doublets associated with Fe1 and Fe2, respectively. For the high-energy lines, the two lines are nearly at the same position (1.37 mm/s and 1.45 mm/s). The small difference in line position of only of 0.08 mm/s precludes a distinction between the two solutions, because variations in the linewidth influence the simulations to a greater extent than the choice of inner-outer versus left-right parameters.
DFT calculations
All geometry optimizations were performed using the GAUSSIAN 0956 revision C.01 package. The initial geometry guess was based on the crystal structure of Ec RNR β, which is purportedly the diferric met form (PDB 1MXR49). First shell residues around the irons and Y122 were cut at the α carbon. To mimic the strain imposed by the protein environment, the α carbon and two out of the three adjacent hydrogen atoms were frozen in all calculations, similarly to the procedures described by Roos and Siegbahn.32,50 Four different models were explored: the active resting state (Fe1-OH2/Y•) and the predicted radical translocation product (Fe1-OH/Y) of the cofactor, both with and without Y122 to account for the tyrosine's influence on the Mössbauer parameters. In addition, the met form (Fe1-OH2/Y) of the enzyme was modeled with Y122 and calculated for reference. Calculations were performed in the gas phase as well as in three distinct dielectric environments implemented via a conductor-like screening model (COSMO)51 in the polarizable continuum model (PCM)52 framework, termed as C-PCM, with ε = 4, 10 and 40. Optimizations were performed within the unrestricted DFT formalism with the three-parameter Becke–Lee–Yang–Parr (B3LYP) hybrid functional.57,58 The antiferromagnetic coupling between the irons was achieved using broken-symmetry methods based on the ones developed by Noodleman et al.48 Pople's 6-31g basis set59 was used on all atoms except the irons, which were represented with the 6-311+g* basis set. Calculations of spectroscopic parameters were performed using the optimized geometries with the unrestricted DFT formalism using the ORCA60 2.9.1 package with the B3LYP functional and the 6-311g* basis set on all atoms. In the case of the iron atoms, a diffuse function was added to the basis set (6-311+g*). The broken symmetry state was realized via the “flipspin” feature implemented in ORCA. All ORCA calculations utilized the resolution of the identity Coulomb density fitting approximation61 with the chain of spheres exchange (RIJCOSX).62 The 57Fe isomer shifts (δ) were calculated from the electron density at the iron nucleus (ϱ(0)) using linear correlation response theory.63,64 Calibration of ϱ(0) values was performed by correlating DFT calculated ϱ(0) values of iron complexes using the geometries presented by Römelt et al. with the experimental isomer shifts of these complexes.65 The linear fit yielded the equation δ = 0.0963 – 0.3857 ϱ(0) – 11616.5) with δ in mm/s and ϱ(0) in au-3, and RMS = 0.9899 (Fig. S5).
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (GM-55365 to JMB and CK) and (GM-29595 to JS) and a postdoctoral fellowship of the Swiss National Science Foundation to BW.
Footnotes
Supporting Information. Materials, protein production and purification, reconstitution of RNR-β with 57Fe and/or 56Fe, and activity assays. Alternative solution to fit the Mössbauer spectra of the N3UDP-trapped radical translocation product (Fig. S1). Mössbauer spectra of control samples lacking the stable radical translocation product (Fig. S2). EPR spectra of site-selective labeled Ec RNR before and after N3UDP-induced trapping of the product of forward radical translocation (Fig. S3). DFT optimized structures using COSMO with a dielectric constant of ε = 4 (Fig. S4). Calibration of electron densities at the iron ϱ(0) (Fig. S5). Parameters used to simulate the Mössbauer spectra (Table S1). Quantification of the different diiron and radical species in the samples (Table S2). DFT calculated quadrupole splittings (ΔEQ), isomer shifts (δ), asymmetry parameters (η), and Fe1-Fe2 distances for the different diferric cofactor states (Table S3). Coordinates for optimized geometries of the investigated models calculated via COSMO with ε = 4. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Stubbe J, van der Donk WA. Chem Rev. 1998;98:705. doi: 10.1021/cr980059c. [DOI] [PubMed] [Google Scholar]
- 2.Nordlund P, Reichard P. Annu Rev Biochem. 2006;75:681. doi: 10.1146/annurev.biochem.75.103004.142443. [DOI] [PubMed] [Google Scholar]
- 3.Stubbe J, Ackles DJ. Biol Chem. 1980;255:8027. [PubMed] [Google Scholar]
- 4.Licht S, Gerfen GJ, Stubbe J. Science. 1996;271:477. doi: 10.1126/science.271.5248.477. [DOI] [PubMed] [Google Scholar]
- 5.Mao SS, Yu GX, Chalfoun D, Stubbe J. Biochemistry. 1992;31:9752. doi: 10.1021/bi00155a031. [DOI] [PubMed] [Google Scholar]
- 6.Stubbe J, Licht S, Gerfen G, Silva D, Booker S. In: In Vitamin B12and B12-proteins. Kräutler B, Arigoni D, Golding BT, editors. Weinheim: Wiley-VCH; 1998. p. 321. [Google Scholar]
- 7.Uhlin U, Eklund H. Nature. 1994;370:533. doi: 10.1038/370533a0. [DOI] [PubMed] [Google Scholar]
- 8.Sintchak MD, Arjara G, Kellogg BA, Stubbe J, Drennan CL. Nature Structural Biology. 2002;9:293. doi: 10.1038/nsb774. [DOI] [PubMed] [Google Scholar]
- 9.Logan DT, Andersson J, Sjöberg BM, Nordlund P. Science. 1999;283:1499. doi: 10.1126/science.283.5407.1499. [DOI] [PubMed] [Google Scholar]
- 10.Ehrenberg A, Reichard P. J Biol Chem. 1972;247:3485. [PubMed] [Google Scholar]
- 11.Atkin CL, Thelander L, Reichard P, Lang G. J Biol Chem. 1973;248:7464. [PubMed] [Google Scholar]
- 12.Sjöberg BM, Reichard P, Gräslund A, Ehrenberg A. J Biol Chem. 1977;252:536. [PubMed] [Google Scholar]
- 13.Sjöberg BM, Reichard P, Gräslund A, Ehrenberg A. J Biol Chem. 1978;253:6863. [PubMed] [Google Scholar]
- 14.Larsson A, Sjöberg BM. EMBO J. 1986;5:2037. doi: 10.1002/j.1460-2075.1986.tb04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stubbe J, Nocera DG, Yee CS, Chang MCY. Chem Rev. 2003;103:2167. doi: 10.1021/cr020421u. [DOI] [PubMed] [Google Scholar]
- 16.Ando N, Brignole EJ, Zimanyi CM, Funk MA, Yokoyama K, Asturias FJ, Stubbe J, Drennan CL. Proc Natl Acad Sci U S A. 2011;108:21046. doi: 10.1073/pnas.1112715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bennati M, Robblee JH, Mugnaini V, Stubbe J, Freed JH, Borbat P. J Am Chem Soc. 2005;127:15014. doi: 10.1021/ja054991y. [DOI] [PubMed] [Google Scholar]
- 18.Bennati M, Weber A, Antonic J, L D, Perlstein, Robblee J, Stubbe J. J Am Chem Soc. 2003;125:14988. doi: 10.1021/ja0362095. [DOI] [PubMed] [Google Scholar]
- 19.Seyedsayamdost MR, Chan CT, Mugnaini V, Stubbe J, Bennati M. J Am Chem Soc. 2007;129:15748. doi: 10.1021/ja076459b. [DOI] [PubMed] [Google Scholar]
- 20.Ge J, Yu G, Ator MA, Stubbe J. Biochemistry. 2003;42:10071. doi: 10.1021/bi034374r. [DOI] [PubMed] [Google Scholar]
- 21.Seyedsayamdost MR, Yee CS, Reece SY, Nocera DG, Stubbe J. J Am Chem Soc. 2006;128:1562. doi: 10.1021/ja055927j. [DOI] [PubMed] [Google Scholar]
- 22.Seyedsayamdost MR, Stubbe J. J Am Chem Soc. 2007;129:2226. doi: 10.1021/ja0685607. [DOI] [PubMed] [Google Scholar]
- 23.Seyedsayamdost MR, Xie J, Chan CT, Schultz PG, Stubbe J. J Am Chem Soc. 2007;129:15060. doi: 10.1021/ja076043y. [DOI] [PubMed] [Google Scholar]
- 24.Seyedsayamdost MR, Stubbe J. J Am Chem Soc. 2006;128:2522. doi: 10.1021/ja057776q. [DOI] [PubMed] [Google Scholar]
- 25.Yokoyama K, Uhlin U, Stubbe J. J Am Chem Soc. 2010;132:15368. doi: 10.1021/ja1069344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokoyama K, Smith AA, Corzilius B, Griffin RG, Stubbe J. J Am Chem Soc. 2011;133:18420. doi: 10.1021/ja207455k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Climent I, Sjöberg BM, Huang CY. Biochemistry. 1992;31:4801. doi: 10.1021/bi00135a009. [DOI] [PubMed] [Google Scholar]
- 28.Ekberg M, Sahlin M, Eriksson M, Sjöberg BM. J Biol Chem. 1996;271:20655. doi: 10.1074/jbc.271.34.20655. [DOI] [PubMed] [Google Scholar]
- 29.Reece SY, Hodgkiss JM, Stubbe J, Nocera DG. Philos Trans Royal Soc, B. 2006;361:1351. doi: 10.1098/rstb.2006.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Argirevic T, Riplinger C, Stubbe J, Neese F, Bennati M. J Am Chem Soc. 2012;134:17661. doi: 10.1021/ja3071682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ekberg M, Pötsch S, Sandin E, Thunnissen M, Nordlund P, Sahlin M, Sjöberg BM. J Biol Chem. 1998;273:21003. doi: 10.1074/jbc.273.33.21003. [DOI] [PubMed] [Google Scholar]
- 32.Siegbahn PEM, Eriksson L, Himo F, Pavlov M. J Phys Chem B. 1998;102:10622. [Google Scholar]
- 33.Bollinger JM, Jr, Edmondson DE, Huynh BH, Filley J, Norton JR, Stubbe J. Science. 1991;253:292. doi: 10.1126/science.1650033. [DOI] [PubMed] [Google Scholar]
- 34.Thelander L, Larsson B, Hobbs J, Eckstein F. J Biol Chem. 1976;251:1398. [PubMed] [Google Scholar]
- 35.Salowe SP, Ator MA, Stubbe J. Biochemistry. 1987;26:3408. doi: 10.1021/bi00386a024. [DOI] [PubMed] [Google Scholar]
- 36.Fritscher J, Artin E, Wnuk S, Bar G, Robblee JH, Kacprzak S, Kaupp M, Griffin RG, Bennati M, Stubbe J. J Am Chem Soc. 2005;127:7729. doi: 10.1021/ja043111x. [DOI] [PubMed] [Google Scholar]
- 37.van der Donk WA, Stubbe J, Gerfen GJ, Bellew BF, Griffin RG. J Am Chem Soc. 1995;117:8908. [Google Scholar]
- 38.Preparations of Ec β generally contain more (FeIII)2 clusters than Y122•. Metal analysis and Y• quantification indicate that our preparation here contains 71% of active (FeIII)2/Y122• β and 29% of the reduced, inactive (FeIII)2/Y122 (commonly referred to as “met”) form, consistent with published results. Details of this quantification are provided in Table S2 of the Supporting Information. All % conversions determined by analysis of the Mössbauer spectra are reported here relative to the active (FeIII)2/Y122• form rather than the total (FeIII)2 cluster.
- 39.Lynch JB, Juarez-Garcia C, Münck E, Que L., Jr J Biol Chem. 1989;264:8091. [PubMed] [Google Scholar]
- 40.Miller MA, Gobena FT, Kauffmann K, Münck E, Que L, Jr, Stankovich MT. J Am Chem Soc. 1999;121:1096. [Google Scholar]
- 41.Bollinger JM, Jr, Chen S, Parkin SE, Mangravite LM, Ley BA, Edmondson DE, Huynh BH. J Am Chem Soc. 1997;119:5976. [Google Scholar]
- 42.Han WG, Sandala GM, Giammona DA, Bashford D, Noodleman L. Dalton Trans. 2011;40:11164. doi: 10.1039/c1dt10950b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.A second solution with δ1 = 0.47 mm/s, ΔEQ1 = 1.97 mm/s and δ2 = 0.53 mm/s, ΔEQ2 = 1.68 mm/s) fits the data equally well. Experiments with preparations of β in which the Mössbauer active 57Fe was enriched in Fe1 or Fe2 (described below), showed that the parameters quoted in the main manuscript fit the data slightly better. However, we cannot determine with certainty which solution is the correct one (Figure S1).
- 44.Münck E. In: Physical Methods in Bioinorganic Chemistry. Que L Jr, editor. University Science Books; Sausalito, CA: 2000. p. 287. [Google Scholar]
- 45.Kurtz DM. Chem Rev. 1990;90:585. [Google Scholar]
- 46.Karlsson M, Sahlin M, Sjöberg BM. J Biol Chem. 1992;267:12622. [PubMed] [Google Scholar]
- 47.DeFelippis MR, Murthy CP, Broitman F, Weinraub D, Faraggi M, Klapper MH. J Phys Chem. 1991;95:3416. [Google Scholar]
- 48.Noodleman L. J Chem Phys. 1981;74:5737. [Google Scholar]
- 49.Högbom M, Galander M, Andersson M, Kolberg M, Hofbauer W, Lassmann G, Nordlund P, Lendzian F. Proc Natl Acad Sci U S A. 2003;100:3209. doi: 10.1073/pnas.0536684100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos K, Siegbahn PE. J Biol Inorg Chem. 2011;16:553. doi: 10.1007/s00775-011-0755-1. [DOI] [PubMed] [Google Scholar]
- 51.Klamt A, Schuurmann G. J Chem Soc, Perkin Trans. 1993;2:799. [Google Scholar]
- 52.Cossi M, Rega N, Scalmani G, Barone V. J Comput Chem. 2003;24:669. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]
- 53.Salowe S, Bollinger JM, Jr, Ator M, Stubbe J, McCracken J, Peisach J, Samano MC, Robins MJ. Biochemistry. 1993;32:12749. doi: 10.1021/bi00210a026. [DOI] [PubMed] [Google Scholar]
- 54.Malmström BG, Reinhammar B, Vänngård T. Biochim Biophys Acta. 1970;205:48. doi: 10.1016/0005-2728(70)90060-5. [DOI] [PubMed] [Google Scholar]
- 55.Aasa R, Vänngård T. J Magn Reson. 1975;19:308. [Google Scholar]
- 56.Frisch MJ, T GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision C 01. Gaussian, Inc.; Wallingford CT: 2009. [Google Scholar]
- 57.Becke AD. J Chem Phys. 1993;98:5648. [Google Scholar]
- 58.Lee C, Yang W, Parr RG. Phys Rev B: Condens Matter. 1988;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 59.Krishnan R, Binkley JS, Seeger R, Pople JA. J Chem Phys. 1980;72:650. [Google Scholar]
- 60.Neese F. WIREs Comput Mol Sci. 2012;2:73. [Google Scholar]
- 61.Neese F. J Comput Chem. 2003;24:1740. doi: 10.1002/jcc.10318. [DOI] [PubMed] [Google Scholar]
- 62.Neese F, Wennmohs F, Hansen A, Becker U. Chem Phys. 2009;356:98. [Google Scholar]
- 63.Filatov M. J Chem Phys. 2007;127:084101. doi: 10.1063/1.2761879. [DOI] [PubMed] [Google Scholar]
- 64.Sinnecker S, Slep LD, Bill E, Neese F. Inorg Chem. 2005;44:2245. doi: 10.1021/ic048609e. [DOI] [PubMed] [Google Scholar]
- 65.Römelt M, Ye S, Neese F. Inorg Chem. 2009;48:784. doi: 10.1021/ic801535v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.