Abstract
Aim
To determine the role of primary antifungal prophylaxis in the prevention of cryptococcal meningitis and all-cause mortality in advanced HIV infection
Materials & methods
This was a systematic review and meta-analysis of randomized trials and observational studies. Google Scholar™, PubMed and Embase databases were searched for relevant studies. Quality was assessed using different criteria, depending on study type. Publication bias was assessed and subgroup and sensitivity analyses were performed. When the results of the meta-analysis were homogeneous, the fixed-effects model was used; when the results of the meta-analysis were heterogenous, the random effects model was used.
Results
Primary prophylaxis prevented cryptococcal meningitis but did not confer protection against overall mortality, although there was evidence of a reduction in cryptococcal-specific mortality in resource-limited settings.
Conclusion
Primary antifungal prophylaxis should be recommended in patients with advanced HIV infection in resource-limited settings with a high incidence of cryptococcal meningitis.
Keywords: advanced HIV infection, all-cause mortality, cryptococcal meningitis, fluconazole, itraconazole, prevention of cryptococcal meningitis, primary antifungal prophylaxis, resource-limited settings
Cryptococcal meningitis is an often-fatal infectious disease caused by the fungus Cryptococcus neoformans. Cryptococcal meningitis primarily occurs in profoundly immunosuppressed patients, and it is one of the major opportunistic infections causing morbidity and mortality in patients with advanced HIV infection. The main portal of entry of C. neoformans infection to the body is thought to be via inhalation of the fungus, with most patients initially developing a subclinical pulmonary infection [1, 101]. At the time of hospitalization, most HIV-infected patients with C. neoformans infection have a CD4+ T lymphocyte (CD4 cell) count ≤100 cells/µl [2]. Patients mainly present to hospital following CNS involvement leading to meningoencephalitis. Death from HIV-associated cryptococcal meningoencephalitis remains common in resource-limited settings, accounting for 13–44% of all AIDS-related deaths in sub-Saharan Africa [3–5], even with the increased availability of antiretroviral therapy (ART). As a result, strategies to prevent morbidity and mortality from cryptococcal meningitis are required in such settings. Prevention of cryptococcal meningitis in the context of HIV-infection may take one of two forms; provision of antifungal medication with the sole aim of preventing infection in those without prior evidence of the disease (primary prophylaxis), or secondary antifungal prophylaxis, which involves provision of antifungal medication following diagnosis and treatment of cryptococcal meningitis in order to prevent relapse of infection [102]. Studies performed in most settings have lent support to the role of azoles as being effective in the primary prevention of cryptococcal infections [6], although most of these studies have not shown prophylaxis with azole drugs to confer a survival benefit.
Recommendations formulated by expert panels in industrialized countries do not support routine primary prophylaxis with azole drugs in HIV-infected individuals, given the comparatively low incidence of cryptococcal infection in such settings, the potential emergence of azole-resistant Candida species strains, possible problems with compliance and drug interactions, and cost [7]. Moreover, the sharp decline in the incidence of opportunistic infections resulting from the wider use of HAART has further tilted the balance away from primary prophylaxis in industrialized countries [8,9]. However, in resource-constrained settings, where both the incidence of cryptococcal meningitis and the associated case–fatality rate are high, strategies including pre-emptive treatment for latent infection or antifungal prophylaxis may have a measurable impact on overall mortality in a population with advanced HIV infection. These approaches are supported by findings from studies done in these settings. In two observational studies done in resource-limited settings, serum cryptococcal antigen (CRAG) testing was utilized for all patients with advanced HIV infection (i.e., CD4 lymphocyte counts below 100 cells/mm3) [10,11]. In one of the studies, all patients with positive cryptococcal antigenemia were started on ART and then given either fluconazole for 2–4 weeks or no antifungal treatment. Of the patients receiving no fluconazole, all died within 2 months of initiation of ART, while in the group receiving fluconazole, clinical cryptococcal meningitis developed in only three patients; survival was 71% at 30 months, and it was judged to be cost effective to screen for serum cryptococcal antigen and to provide antifungal treatment [11]. In the second study, approaches were compared: no antifungal medication; performing serum CRAG testing followed by provision of antifungal medication to CRAG-positive patients; and primary prevention with fluconazole, irrespective of CRAG test status [10]. Serum CRAG testing and prophylaxis was found to be the most economical means of preventing AIDS-associated cryptococcosis in patients with a CD4 cell count below 100 cells/mm3, over a short-term period. Treatment based on the results of CRAG testing was found to be cheaper but less effective than routine azole prophylaxis. In resource-constrained settings with a high prevalence of cryptococcal antigenemia, WHO currently recommends routine serum or plasma CRAG testing in ART-naive HIV-infected adults, and provision of pre-emptive antifungal treatment if the CRAG test is positive, in patients who have not yet begun receiving ART and who have CD4 cell counts below 100 cells/mm3 [102]. In a clinical trial in Uganda, HIV-infected patients with a CD4 cell count of 200 cells/mm3 and a negative test for cryptococcal antigenemia were recruited and randomized to placebo or primary antifungal prophylaxis. In this study, there was a reduction in cryptococcal meningitis-specific mortality in patients receiving antifungal prophylaxis [12]. Early ART is the best and most cost-effective strategy for preventing cryptococcal meningitis and associated mortality, as well as other HIV-associated opportunistic infections [103]. WHO recommends initiation of ART for HIV infection as soon as the CD4 cell count falls to less than 350 cells/mm3 [102]. However, given the fact that most HIV-infected patients present for treatment late in the course of HIV infection (when their CD4 cell counts are much less than 250 cells/mm3), and given the high mortality and morbidity associated with cryptococcal meningitis in resource-limited settings [2–4,13], primary antifungal prophylaxis in patients with advanced HIV infection may lead to a survival benefit. In this systematic review and metaanalysis, we examine the role of primary antifungal prophylaxis in the prevention of cryptococcal meningitis and its impact on all-cause mortality in those with advanced HIV infection.
Materials & methods
Study selection
Patient selection was based on the PICO criteria, which require defining the population of interest, the intervention group, the comparison group and the outcome(s) of interest. Our population of interest was adult patients with HIV infection. Our study intervention of interest was the use of an azole drug (e.g., fluconazole or itraconazole) for the prevention of fungal infections, and the comparison group of interest was use of placebo or no intervention. We assessed two outcomes: the primary outcome was the occurrence of cryptococcal meningitis and the secondary outcome was all-cause mortality.
Inclusion & exclusion criteria
We included all randomized trials and observational studies assessing the role of primary antifungal prophylaxis for the prevention of cryptococcal meningitis or fungal infections in patients with HIV-infection, with or without all-cause mortality data. The studies had to have been published in English in a peer-reviewed journal; there were no date restrictions. Primary antifungal prophylaxis for cryptococcal meningitis was defined as the provision of an antifungal medication (mainly azole drugs) with the aim of preventing cryptococcal meningitis in HIV/AIDS patients with negative serum CRAG test results. Participants in the studies had to have confirmed HIV infection and no prior treatment for cryptococcal meningitis. Studies comparing antifungal medication to placebo or to no treatment for the prevention of cryptococcal meningitis in HIV-infected patients were included. Studies were not included if study participants had a positive serum or cerebral spinal fluid CRAG test, a prior history of treatment for cryptococcal meningitis or were on secondary antifungal prophylaxis. In addition, studies of primary prophylaxis but with no comparison group were excluded, as were studies comparing different doses of antifungal medication for the prevention of cryptococcal meningitis.
Definition of exposure & outcomes
Exposure was defined as the use of an azole drug, such as fluconazole or itraconazole, in a patient with HIV infection for prevention of an index episode of cryptococcal meningitis, while the control groups either received placebo or no intervention. The outcomes of interest were defined as the occurrence of cryptococcal meningitis (primary outcome) and all-cause mortality (secondary outcome).
Research questions
For patients with advanced HIV infection, does primary antifungal prophylaxis reduce the risk of cryptococcal meningitis (primary outcome) or all-cause mortality (secondary outcome)?
Search strategy
Databases searched included: PubMed, Google Scholar™ and Embase. Reference sections of the identified studies were also examined in order to identify additional relevant studies. The search details are given in Box 1. The search was carried out between September 2012 and February 2013 with the help of a qualified librarian. All headings, as well as abstracts, were checked, and the articles satisfying the requirements for inclusion were taken up for review.
Data extraction
The required information was collected using a checklist and included details on the resources of the study region and year, study type, quality indicators, study population, sample size, antifungal medication given, frequency and dose of antifungal medication, CD4 cell count cutoff for inclusion in the study and details concerning the comparison group. Data for the primary and secondary outcomes were also extracted.
Systematic review
For the randomized trials, methodological quality was assessed by checking steps taken to ensure adequate randomization (i.e., methods for generating the allocation sequence and if the allocation concealment was adequate); whether blinding was used and steps taken to ensure it was adequate; method of analysis (i.e., intention-to-treat vs per-protocol analysis); and duration of follow-up. Concealment of allocation to treatment arms was graded by means of the standard Cochrane criteria as: adequate (A), unclear (B), not adequate (C) and if no allocation criteria were utilized (D) [6,14]. Methods of adequate allocation concealment include centralized or pharmacy-generated randomization; sequentially numbered or coded similar appearing containers that are distributed serially; use of a random number generator; and sequentially numbered, sealed, opaque envelopes [15]. Inadequate methods of allocation concealment include alternation of interventions on admission; interventions based on use of hospital record numbers; interventions based on date of enrollment or date of birth; and enrollment based on an open record of random digits. Trial quality was also assessed using the Jadad scale, an assessment tool graded from 0 to 5, with a higher score implying higher quality. On this scale, for each of the following: randomized, double blind, description of withdrawals and study dropouts, a point is awarded. An additional point each is given if blinding and randomization are considered appropriate or deducted if they are not considered appropriate. A study is of poor quality if the Jadad score is below three [16]. For the case—control studies, three principles of comparability were used: study base — ensure all comparisons are taken from the same reference population; control for confounding — that measurements of the effects of different levels of exposure on the outcome of interest are not altered by the effects of extraneous variables; and comparable accuracy — any inaccuracies in exposure assessment do not differ with regard to disease status [17]. Cohort study quality assessment was done by means of the Newcastle—Ottawa scale, which includes: selection criteria for the study participants, ascertainment of exposure and establishing evidence of the absence of the study outcome at the initiation of the study; comparability of the study arms on the basis of the design or analysis; and outcome measurement (if duration of follow-up was adequate for the outcome to occur) [104].
Data analysis
In order to compute the relative risk (RR) and 95% CI for the development of cryptococcal meningitis, data from the included studies were used by dividing the risk of cryptococcal meningitis, following provision of primary antifungal prophylaxis by the risk of cryptococcal meningitis given no prior primary antifungal prophylaxis. The odds ratio for case—control studies was used as an estimate of the risk ratio for these studies. We performed the same procedure for the secondary outcome of all-cause mortality, when the data were available. RRs above one implied a greater risk of cryptococcal meningitis or all-cause mortality in the different groups and those below one implied a reduced risk of the outcome of interest.
Meta-analysis
The summary RR estimates were calculated and combined by use of both the fixed effects inverse variance weighting approach [18] and the random effects approach [19], the latter of which takes into account heterogeneity in a study. Heterogeneity among the studies was explored by means of the general variance-based method [20]. If heterogeneity was demonstrated, CIs in the random effects model were adjusted and presented using the method of Shore et al., in order to account for between-study variance [21]. Calculation was done for the I2 statistic for each analysis as an estimation of the proportion of the variation in the effect estimate resulting from heterogeneity. Significant heterogeneity was deemed to be present if the χ2-test statistic divided by the degrees of freedom gave a value more than one, the p-value was <0.20, and the I2 result was more than 50%. Sensitivity analysis was performed to establish the impact of different study characteristics on the pooled estimates and to know how these characteristics, if any, led to a change in the summary effect estimate. Subgroup analysis was also performed by calculating the resulting estimates based on the type of study, dosing frequency of antifungal medication, type of antifungal medication, resources of the country where the study was conducted (i.e., whether it was or was not a resource-limited setting) and the CD4 lymphocyte count cutoff criteria used for enrollment in the study. Forest plots were created to assess heterogeneity. Bias due to publication was explored by plotting the logarithm of the RR for each study by its standard error [22] and using a funnel plot; a plot of the effect size against the precision. Assessment for publication bias was limited to outcomes with at least a total of ten studies. Publication bias was further explored by means of the Egger’s [23] and Begg’s tests [24]. If there was generally equal dispersion of effect sizes around the overall estimate of effect, then publication bias was deemed absent. Bias was considered to be present if the Beggs test p-value was less than 0.20. All statistical analysis and plots were performed by means of STATA software (Version 12.1; STATA corporation, College Stn, TX, USA). Results were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines, which consist of a checklist and a flow chart, comprising four phases, and reflect the requirements for provision of results for these types of studies [25].
Results
Use of the search terms in the search engines yielded 1148 publications from PubMed and 3015 publications from Embase. Seven records were identified from other sources. The search details are given in Box l. Of these records, 45 were screened. When these were searched for relevance to the topic, 23 articles were considered for inclusion in this analysis. Of these 23, 11 articles were excluded (Figure 1) [6,10,11,37–44]. The characteristics of the excluded articles are summarized in Table 1. Twelve studies satisfied the criteria for inclusion for the systematic review and meta-analysis [12,26–36]. Studies carried out in different states qualified for meta-analysis for the outcome of cryptococcal meningitis (six from the USA, one from Uganda, two from Italy, two from Thailand and one carried out in multiple countries) [28]. Studies from six states and the multinational study [28] were included in the meta-analysis for all-cause mortality as the outcome. The characteristics of the studies used in the meta-analysis are shown in Table 2. The 12 studies comprised five randomized trials, three cohort studies and four case–control studies. The studies took place between 1992 and 2011. The number of patients included in the meta-analysis for the primary outcome (cryptococcal meningitis) was 3734 patients. Of these 3734 patients, 2936 were in studies comparing patients who received fluconazole prophylaxis with placebo or no intervention, while 798 were in studies comparing patients who received itraconazole to those who received placebo.
Figure 1.
Flow diagram for the study selection process.
Table 1.
Studies excluded from the meta-analysis.
| Study (year) | Reason for exclusion | Ref. |
|---|---|---|
| Pradier et al. (1992) | Only abstract found | [37] |
| Caputi et al. (1993) | Only abstract found | [38] |
| Nelson et al. (1994) | Retrospective case series, evaluated role of fluconazole and itraconazole for the treatment and prophylaxis of cryptococcal meningitis |
[39] |
| Powderly et al. (1995) | Compared fluconazole to clotrimazole troches for prevention of fungal infections in HIV patients |
[40] |
| Havlir et al. (1998) | Compared weekly to daily fluconazole for prevention of fungal infections in patients with AIDS |
[41] |
| Nina et al. (1996) | Only looked at patients on fluconazole prophylaxis, no comparison group |
[42] |
| Bozzette et al. (1991) | Compared role of fluconazole to placebo for prophylaxis after treatment of cryptococcal meningitis |
[43] |
| Meyer et al. (2013) | Study assessed role of cryptococcal antigen testing and antifungal treatment for prevention of all-cause mortality, but did not assess for the prevention of cryptococcal meningitis and included patients with confirmed clinical cryptococcal meningitis |
[44] |
| Chang et al. (2005) | Cochrane review of role of primary antifungal prophylaxis for the prevention of cryptococcal meningitis |
[6] |
| Micol et al. (2010) | Used a hypothetical cohort of patients to establish the cost-effectiveness of primary prophylaxis of AIDS-associated cryptococcal meningitis |
[10] |
| Meya et al. (2010) | Study provided pre-emptive antifungal treatment to patients with advanced HIV infection and a positive cryptococcal antigen test, but did not assess for the prevention of cryptococcal meningitis |
[11] |
Table 2.
Studies included in the meta-analysis.
| Study (year) | Sample size (n) |
Type of study | Exposure/control | Antifungal dose | CD4 cell cutoff for enrollment |
Mortality data available |
Country | Ref. |
|---|---|---|---|---|---|---|---|---|
| Nightingale et al. (1992) | 666 | Cohort | Fluconazole/no intervention | 100 mg/day | <68/mm3 | No | USA | [33] |
| Ammassari et al. (1995) | 51 | Case–control | Fluconazole/no intervention | 100–150 mg/day | <50/mm3 | No | Italy | [34] |
| Quagliarello et al. (1995) | 90 | Case–control | Fluconazole/no intervention | Not given | <250/mm3 | No | USA | [32] |
| Newton et al. (1995) | 145 | Cohort | Fluconazole/no intervention | 100 mg/week | <100/mm3 | No | USA | [35] |
| Manfredi et al. (1997) | 90 | Cohort | Fluconazole/no intervention | 100 mg/third week | <200/mm3 | Yes | Italy | [36] |
| Kris et al. (1999) | 111 | Case-control | Fluconazole/no intervention | Not given | <250/mm3 | Yes | USA | [31] |
| McKinsey et al. (1999) | 295 | Randomized trial | Itraconazole/placebo | 200 mg/day | <150/mm3 | Yes | USA | [29] |
| Smith et al. (2001) | 374 | Randomized trial | Itraconazole/placebo | 200 mg/day | <300/mm3 | Yes | Multiple | [28] |
| Chariyalertsak et al. (2002) | 132 | Randomized trial | Itraconazole/placebo | 200 mg/day | <200/mm3 | Yes | Thailand | [27] |
| Chetchotisakd et al. (2004) | 90 | Randomized trial | Fluconazole/placebo | 400 mg/week | <100/mm3 | Yes | Thailand | [26] |
| Cantey et al. (2005) | 174 | Case-control | Fluconazole/none | Not given | Not given | No | USA | [30] |
| Parkes-Ratanshi et al. (2011) | 1519 | Randomized trial | Fluconazole/placebo | 200 mg three times/week | <200/mm3 | Yes | Uganda | [12] |
Study quality
All the randomized trials [12,26–29] were deemed to be of good quality, with the study by Parkes-Ratanshi et al. [12] being of the best quality (Table 3). One of the cohort studies [33] was of good quality, one [36] was of average quality and one [35], was of poor quality (Table 4). Of the case—control studies, three [31,32,34] were of good quality and one [30] was of average quality (Table 5).
Table 3.
Quality assessment of the randomized trials.
| Study (year) | Allocation concealment grade |
Mention of study as double-blind and randomized |
Was blinding and randomization adequate? |
Description of withdrawals and dropouts |
Jadad score |
Ref. |
|---|---|---|---|---|---|---|
| McKinsey et al. (1999) | A | Yes | Randomization process was adequate No mention of who was blinded to treatment allocation |
Study does not give information on losses to follow-up; only mentions those stopping treatment due to side effects |
3 | [29] |
| Smith et al. (2001) | A | Yes | Randomization process was adequate No mention of who was blinded to treatment allocation |
Adequate description of withdrawals and dropouts |
4 | [28] |
| Chariyalertsak et al. (2002) | A | Yes | Randomization adequate No mention of who was blinded to treatment allocation |
Adequate description of withdrawals and dropouts |
4 | [27] |
| Chetchotisakd et al. (2004) | B (no mention of method of sequence generation) |
Yes | No explanation of randomization No explanation of ways in which blinding was done |
No mention if any patients were lost to follow-up |
3 | [26] |
| Parkes-Ratanshi et al. (2011) | A | Yes | Yes | Adequate description of withdrawals and dropouts |
5 | [12] |
Table 4.
Quality assessment of the cohort studies.
| Study (year) | Selection | Comparability | Outcome | Ref. |
|---|---|---|---|---|
| Nightingale et al. (1992) | Exposed cohort representative of community from which cases arose Nonexposed cohort drawn from historical reference group Ascertainment of prior exposure obtained by checking a clinic database for both cases and controls Demonstration that outcome of interest was not present at the start of the study for both cases and controls |
The study included patients with CD4 cell counts <68 cells/mm3 and no prior fungal infection for both cases and controls Not mentioned if analysis was done to ensure comparability |
Monthly blood cultures for fungemia for all cases and controls Follow-up was 1 year for both groups, long enough for outcomes to occur All subjects were accounted for in the exposure and control groups |
[33] |
| Newton et al (1995) | Retrospective cohort study, cases and controls seen during the same period (July 1988 up to January 1993), somewhat representative of community Exposure ascertained by review of pharmacy and clinical records Could not demonstrate if outcome of interest was not present at the start of the study for both cases and controls |
Pharmacy and clinical records were reviewed to include only those with CD4 Cell counts <100 cells/mm3 and those who received/did not receive fluconazole for at least 3 months No control for confounding factors in the analysis |
Since data were collected without the study in mind, it is not clear if accurate data on the outcome were captured for all the patients Follow-up was long enough for outcome to occur |
[35] |
| Manfredi et al. (1997) | Exposed group was representative Nonexposed group drawn from historical reference group Ascertainment for prior exposure obtained by clinic database checking by two independent persons Not clear how it was established that there was no prior fungal infection |
Inclusion criteria were CD4 Cell counts <200 cells/mm3 and no prior systemic fungal disease or antifungal prophylaxis Not mentioned if analysis was performed to ensure comparability |
Assessment of outcome was not performed by independent blind assessment as patients had to be referred to the study hospital only on suspicion by primary care physicians Follow-up was adequate for outcome in both cases and controls No comment on losses to follow-up |
[36] |
Table 5.
Quality assessment of the case-control studies.
| Study (year) |
Same study base comparisons? |
Control for confounding | Comparable accuracy | Ref. |
|---|---|---|---|---|
| Canteyet al. (2005) | Not clear if cases and controls came from the same study base |
Matched on CD4 category Conditional logistic regression during analysis |
Not clear if the same tests were performed to establish diagnosis for the cases and controls Unable to collect complete data on the dose and duration of antifungal treatment prior to study recruitment |
[30] |
| Kris et al. (1999) | Cases and controls came from the same study base |
Matched based on year of enrollment and length of follow-up Inclusion required CD4 cell count <250 cells/mm3 for both cases and controls Conditional logistic regression was used for other confounders during analysis |
Diagnosis of CCM required positive tests for the cases Any control complaining of features similar to cryptococcal meningitis had lumbar puncture and tests on CSF performed to rule out fungal infection |
[31] |
| Quagliarello et al. (1995) |
Cases and controls came from the same study base |
Matched according to age, sex and time of lumbar puncture Inclusion required: CD4 <250 cells/mm3, lumbar puncture and CSF culture, follow-up of 6 months before lumbar puncture Conditional logistic regression was done to adjust for unmatched confounders |
Patient eligibility was determined without knowledge of drug history Similar tests were conducted for both cases and controls (CD4 cell count, lumbar puncture and CSF tests) |
[32] |
| Ammassariet al. (1995) | Cases and controls came from the same study base |
Matched according to presence or absence of AIDS-defining event, CD4 cell count, date of CD4 testing Inclusion required: documented HIV infection, follow-up of 1 year before matching Conditional logistic regression was used to adjust for unmatched confounders |
For cases, diagnosis of cryptococcal meningitis required positive CSF cultures and CRAG tests Exposure to fluconazole (dose, duration) for both cases and controls was established by checking prescriptions in the patient clinical records |
[34] |
CCM: Cryptococcal meningitis; CRAG: Cryptococcal antigen; CSF: Cerebrospinal fluid.
Meta-analysis
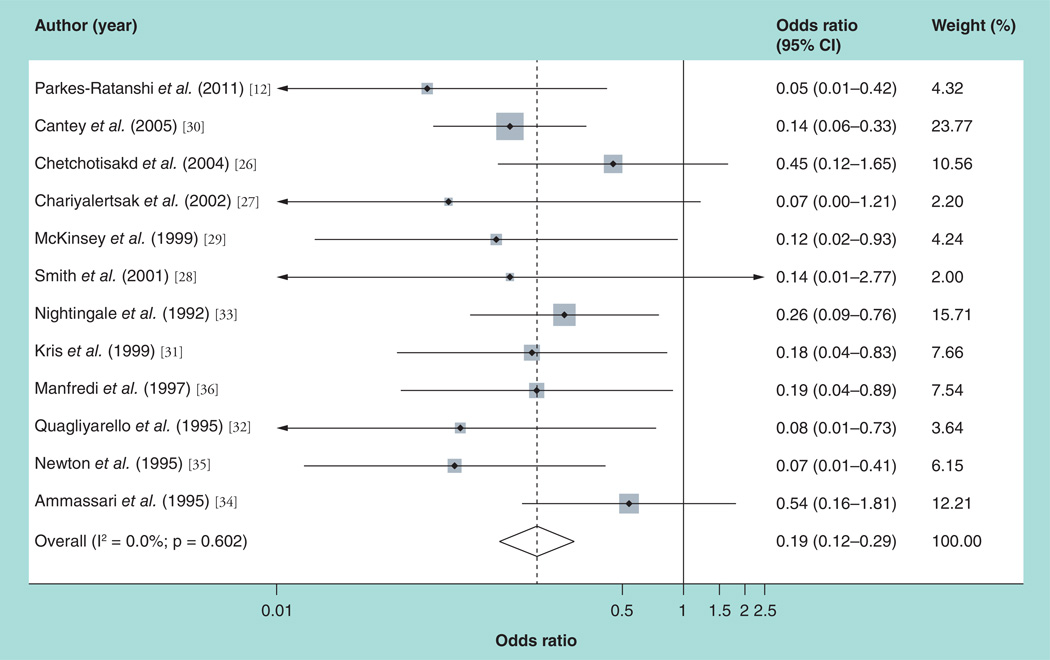
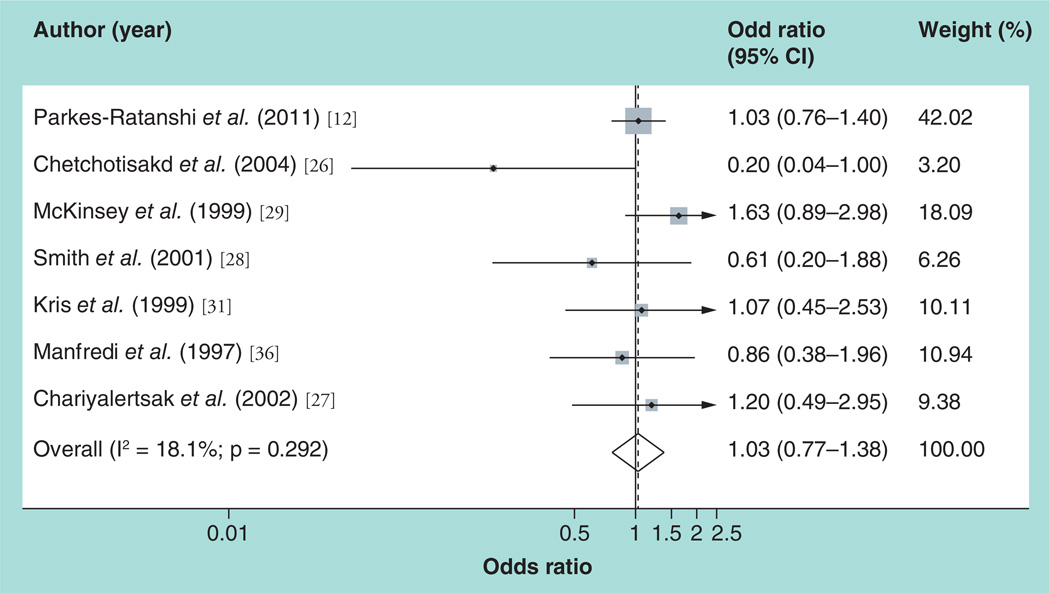
For the primary outcome of cryptococcal meningitis, the pooled estimate of the 12 studies was RR = 0.19 (p < 0.0001; fixed-effects 95% CI: 0.12–0.29) (Table 6). The forest plot in Figure 2 summarizes these findings and shows significant overlap between the studies. The I2 statistic implied homogeneity (0%) between the studies (χ2 = 9.22; degrees of freedom =11; p = 0.602), and so the fixed effects model was used. Subgroup analysis was done to explore the contribution of different study characteristics on the pooled outcome. Homogeneity was maintained when a meta-analysis was done for randomized trials, cohort studies, studies done in resource-rich settings, and studies based on the CD4 cutoff point of 100 cells/mm3. On subgroup analysis, heterogeneity was noted only when the meta-analysis was utilized for case–control studies, studies in resource-limited settings, studies comparing fluconazole to placebo and studies for which antifungal prophylaxis was given daily. In the sensitivity analysis, eliminating the study by Cantey et al. [30] (due to its large weight, it was considered to be a potential outlier) had no effect on the homogeneity; also, eliminating the study by Newton et al. [35] (as it was a poor-quality study) had no effect on the homogeneity. The results of these meta-analyses are shown in Table 6. For the secondary outcome (all-cause mortality), the random effects pooled estimate of the seven studies was RR = 1.03 (random effects shore-adjusted 95% CI: 0.81–1.35; p = 0.426). The forest plot in Figure 3 summarizes these findings. The I2 statistic did not imply heterogeneity (18.1%), but at χ2 = 7.33 (degrees of freedom = 6; p = 0.292), the random effects model with shore correction of the CIs was used. Subgroup analysis was utilized to assess the role of the various study characteristics in the observed heterogeneity, and heterogeneity was maintained when the azole drug regimen and resources of the study country were taken into consideration (Table 7). For studies conducted in resource-limited settings, the pooled measure of effect for the prevention of all-cause mortality was RR = 0.86 (random effects shore-adjusted 95% CI: 0.67–1.50; p = 0.666). Table 6 summarizes the results of all the meta-analyses regarding the outcome of all-cause mortality.
Table 6.
Summary of pooled measures of effect and subgroup analyses for antifungal prophylaxis prevention of all-cause mortality in the included studies.
| Subgroups analyzed | Total studies |
Participants (n) | Fixed-effects RR (95% CI) |
Random-effects RR (95% CI) |
Shore-adjusted 95% CI |
Heterogeneity χ2 (p-value) |
|---|---|---|---|---|---|---|
| All studies | 7 | 2608 | 1.04(0.83–1.31) | 1.03(0.77–1.38) | 0.81–1.35 | 7.33 (0.29) |
| Randomized trials | 5 | 2407 | 1.06(0.82–1.36) | 1.01 (0.65–1.56) | 0.76–1.48 | 7.10(0.13) |
| Randomized trials of itraconazole/placebo | 3 | 798 | 1.28(0.81–2.02) | 1.25(0.75–2.07) | 0.78–2.09 | 2.30(0.32) |
| Randomized trials of fluconazole/placebo | 2 | 1609 | 0.98(0.72–1.32) | 0.55(0.12–2.65) | 0.54–1.76 | 3.87(0.05) |
| All studies with fluconazole | 4 | 1810 | 0.97(0.74–1.27) | 0.91 (0.61–1.37) | 0.71–1.33 | 4.00(0.26) |
| Resource-limited settings studies | 3 | 1738 | 1.00(0.75–1.33) | 0.86(0.44–1.69) | 0.67–1.50 | 4.05(0.13) |
| Resource-rich settings studies | 4 | 870 | 1.14(0.76–1.69) | 1.14(0.76–1.69) | 0.76–1.69 | 3.00(0.39) |
RR: Relative risk
Figure 2.
Forest plot for the prevention of the outcome of cryptococcal meningitis.
Figure 3. Forest plot for the prevention of the outcome of all-cause mortality.
Weights are from random-effects anaysis.
Table 7.
Summary of pooled measures of effect and subgroup analyses for the prevention of cryptococcal meningitis.
| Subgroups analyzed | Total studies |
Participants (n) | Fixed-effects RR (95% CI) |
Random-effects RR (95% CI) |
Shore-adjusted 95% CI |
Heterogeneity χ2 (p-value) |
|---|---|---|---|---|---|---|
| All studies | 12 | 3734 | 0.19(0.12–0.29) | NA | 0.13–0.28 | 9.22(0.60) |
| Randomized trials | 5 | 2407 | 0.18(0.08–0.44) | NA | 0.08–0.43 | 3.81 (0.43) |
| Randomized trials of itraconazole/placebo | 3 | 798 | 0.11 (0.03–0.46) | NA | 0.07–0.16 | 0.13(0.94) |
| Randomized trials of fluconazole/placebo | 2 | 1609 | 0.24(0.08–0.73) | 0.18(0.02–1.41) | 0.04–1.59 | 2.91 (0.09) |
| All studies with fluconazole | 9 | 2936 | 0.20(0.13–0.31) | 0.20(0.13–0.32) | 0.13–0.32 | 8.46(0.39) |
| Studies of fluconazole/no intervention | 7 | 1327 | 0.19(0.12–0.31) | NA | 0.12–0.30 | 5.40(0.49) |
| Case-control studies | 4 | 426 | 0.2(0.11–0.37) | 0.2(0.10–0.42) | 0.10–0.42 | 3.91 (0.27) |
| Cohort studies | 3 | 901 | 0.18(0.08–0.4) | NA | 0.09–0.36 | 1.47(0.48) |
| Resource-limited settings studies | 3 | 1738 | 0.21 (0.07–0.58) | 0.16(0.04–0.71) | 0.05–0.81 | 3.55(0.17) |
| Resource-rich settings | 9 | 1996 | 0.19(0.12–0.30) | NA | 0.13–0.28 | 5.63 (0.69) |
| Studies with daily antifungal prophylaxis | 5 | 1515 | 0.27(0.13–0.55) | NA | 0.15–0.49 | 2.91 (0.57) |
| Studies with no daily antifungal prophylaxis | 4 | 1844 | 0.18(0.08–0.39) | 0.16(0.06–0.43) | 0.07–0.45 | 4.23 (0.24) |
| Eligibity cutoff CD4 cell count ≤100 cells/mm3 | 4 | 952 | 0.3(0.16–0.57) | 0.3(0.14–0.62) | 0.15–0.63 | 3.89(0.27) |
| Eligibility cutoff CD4 count >100 cells/mm3 | 7 | 2611 | 0.12(0.06–0.26) | NA | 0.09–0.18 | 1.45(0.96) |
| Cantey et al., 2005 [30] excluded | 11 | 3560 | 0.21 (0.13–0.34) | NA | 0.13–0.33 | 8.59(0.57) |
| Newton et al., 1995 [35] excluded | 11 | 3589 | 0.20(0.13–0.31) | NA | 0.14–0.30 | 8.00(0.63) |
NA: Not applicable; RR: Relative risk
Assessment for publication bias
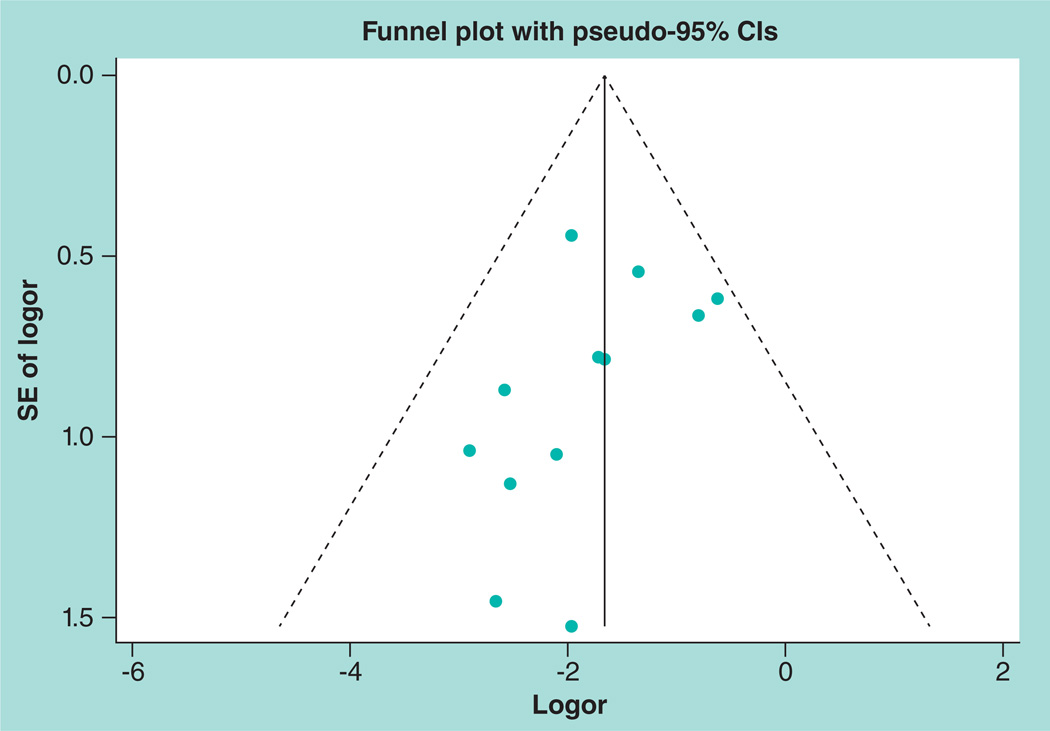
A funnel plot was drawn to explore the possibility of publication bias with regard to the studies included for the primary outcome of cryptococcal meningitis (Figure 4). It was suggestive of publication bias, as the large studies were not very near the aggregate measure of effect and, in addition, it was suggestive of small study bias because only small studies reporting the preventive role of the antifungal medication for cryptococcal meningitis were represented. In addition, Egger’s test, p = 0.161 showed evidence of publication bias, although the Begg’s test (the distance an observed value lies from the mean (z) = −1.23 and probability (Pr)>|z| = 0.217) was not suggestive of this. A funnel plot was not used to assess for the possibility of publication bias with regard to the secondary outcome of all-cause mortality as the total number of studies was not large enough to give meaningful interpretation of the plot.
Figure 4. Assessment of publication bias for studies reporting the preventative effect of primary antifungal prophylaxis on cryptococcal meningitis.
Logor: Logarithm of the odds ratio; SE: Standard error.
Discussion
The results presented here suggest that primary antifungal prophylaxis is effective in preventing cryptococcal meningitis in HIV-infected patients, but does not appear to have a protective effect on all-cause mortality in such patients.
These findings do not differ from those from an earlier study [6]. The results are unlikely to be due to chance, given the pooled p-values of p < 0.0001 (95% CI: 0.12–0.29) and RR = 0.19 for the prevention of cryptococcal meningitis. However, there is a possibility that publication bias is responsible for these findings, as small studies reporting no preventative role are under-represented, which could have led to bias of the pooled effect away from the null. For the secondary outcome of all-cause mortality, the random effects model pooled effect was RR = 0.95 (random effects shore-adjusted 95% CI: 0.76–1.38; p = 0.613). It was not possible to assess for publication bias as the total number of studies was less than ten. On subgroup analysis for studies in resource-limited settings for the impact of primary prophylaxis on all-cause mortality, the pooled RR was 0.86 (random effects shore-adjusted 95% CI: 0.67–1.50; p = 0.666). Although the CIs include one, the value of 0.84 is much closer to the lower CI value of 0.67. This might imply that the reduced cryptococcal-specific mortality associated with prophylaxis might have an impact on all-cause mortality in this setting. A high level of consistency in the direction of effect for the prevention of cryptococcal meningitis was seen for all the included studies and homogeneity was preserved when all of these studies were pooled. For the secondary outcome of all-cause mortality, primary azole prophylaxis provided no survival benefit. Exploration of heterogeneity in effect size using subgroup analyses showed that heterogeneity remained when: the type of study, azole drug used for prophylaxis or the resources of the country in which the study was done were taken into consideration. All the randomized trials were of high quality. The subgroup analyses of these trials enabled us to assess withdrawals in a similar way across all of the subpopulations and to establish factors that might have affected the presence or size of an effect of any of the azole drugs used. The observational studies were found to be of good quality apart from one [35]. Primary prophylaxis with cotrimoxazole in HIV-infection has been documented to be successful for the prevention of Toxoplasmosis gondii infection and Pneumocystis jirovecii pneumonia [45] and is also associated with an overall survival benefit in resource-limited settings, irrespective of ART use [46–48]. However, while most studies demonstrated that primary antifungal prophylaxis prevents cryptococcal meningitis, primary antifungal prophylaxis is not widely used as a result of the low incidence of cryptococcal meningitis in some settings, the fear of development of drug-resistant Candida species strains, and possible problems with compliance, drug interactions, and cost [7], as well as the finding of no overall survival benefit [6]. A decision to change treatment policies in resource-limited settings with a high incidence and mortality owing to cryptococcal meningitis so as to favor routine provision of primary antifungal prophylaxis to HIV-infected patients has considerable health, policy and cost implications, and needs to be supported by convincing data. Notably, some studies performed in resource-limited settings have demonstrated a survival benefit (i.e., a lower all-cause mortality rate) for patients receiving primary antifungal prophylaxis [10,27], unlike studies performed in resource-rich settings. A difference in the effect of primary antifungal prophylaxis on the all-cause mortality outcome is probably due to the fact that there is less access to HAART, and consequently the incidence of cryptococcal infection is higher in these settings in comparison with what is seen in industrialized countries such as the USA [6]. Studies to evaluate the economic benefits of primary antifungal prophylaxis in preventing mortality in resource-limited settings have also provided encouraging results [10,49]; the authors of one of these studies [10]; recommended primary prophylaxis for patients with CD4 cell counts of less than 50 cells/mm3, and a negative CRAG result, as well as antifungal treatment for patients with CD4 cell counts of at least 50 cells, but below 100 cells. Another study, performed in Uganda, involving only patients with negative CRAG tests and CD4 cell counts less than 200 cells/mm3 established reduced cryptococcal meningitis-specific mortality in patients receiving primary antifungal prophylaxis, and improved survival after initiating HAART [12]. These findings, along with those from the study on cost savings in Cambodia [10], suggest that the present WHO recommendation for pre-emptive antifungal treatment for patients with a CD4 cell count of <100 cells and a positive CRAG test [102] supported by findings from a study performed in Uganda [11], should be extended, to support provision of primary antifungal prophylaxis for patients with a negative CRAG test and a CD4 cell count less than 100 cells. This review has important limitations. Although we managed to contact some study authors, we were not able to contact others, and so it is possible that we lacked information on some studies; the review only included studies published in English and may have missed relevant studies published in other languages; the models used for the meta-analysis have their weaknesses (the fixed effects CI does not take into account variability between studies, while the random effects CIs are wide, as this model gives relatively large weights to small studies); only one randomized control trial showed a survival benefit following primary antifungal prophylaxis [27]; another randomized control trial only showed reduced cryptococcal-specific mortality [12]; one observational study done to assess cost savings showed a reduction in all-cause mortality [10], but observational studies are susceptible to selection bias, confounding, and information bias. The studies included provided primary antifungal prophylaxis at different dosages, frequency and duration and at different CD4 cell count cutoffs, suggesting that there is no consensus about these factors. Although some of the studies in resource-limited settings have stressed the importance of timely ART in addition to antifungal prophylaxis [12], they have not specified when this ART should be initiated in the context of primary antifungal prophylaxis. The benefits of successful primary prophylaxis against HIV-associated cryptococcal meningitis would translate to a reduced incidence of an infection that is associated with high mortality and morbidity, both in resource-rich settings (accounting for 10–25% of HIV/AIDS-associated mortality in these settings), where there is a low incidence [50] and in resource-limited settings (13–44% of HIV/AIDS-related mortality), where there is a high incidence of cryptococcal meningitis [3–5]. The high case—fatality associated with cryptococcal meningitis is thought to be due to the less than fully effective current antifungal drug regimens and to a failure to manage raised intracranial pressure [51,52], which is thought to result from impaired resorption of cerebrospinal fluid by the arachnoid villi [53]. The case—fatality proportion is even worse in resource-limited settings, where patients often present late for medical care, antifungal drugs may not be available, and the ability to provide intravenous amphotericin B and monitoring of its nephrotoxic side effects is often lacking [2–4,54]. In addition, although lumbar punctures have been found to be an effective therapeutic intervention for the control of increased intracranial pressure, there is a cultural bias against this procedure among patients or their attendants in some resource-limited settings [54].
Conclusion
Given these findings, patients in resource-limited settings with advanced HIV infection and a CD4 cell count <100 cells/mm3 and a negative CRAG test with delayed or limited access to ART should routinely receive primary antifungal prophylaxis. Primary antifungal prophylaxis in resource-limited settings will translate to a reduced incidence of cryptococcal meningitis and hence a reduction in mortality and morbidity associated with this disease, although perhaps not to a reduction in all-cause mortality.
Future perspective
Further studies are needed to establish: the optimal time of initiation of ART in relation to the antifungal prophylaxis; the duration of primary antifungal prophylaxis in this patient group, as well as the dosage and frequency; and the CD4 cell count at which primary antifungal prophylaxis can be safely be stopped.
Executive summary.
-
▪
HIV-associated cryptococcal meningitis causes substantial morbidity and mortality in resource-limited settings, even with increased availability of antiretroviral therapy.
-
▪
Primary antifungal prophylaxis may translate to a reduction in cryptoccocal-specific morbidity and mortality in resource-limited settings with limited and delayed antiretroviral drug access.
-
▪
Primary antifungal prophylaxis may not confer a benefit against all-cause mortality.
-
▪
Primary antifungal prophylaxis should be provided routinely to patients with advanced HIV-infection in resource-limited settings and a negative cryptococcal antigen test result.
Box 1. Search terms used for the studies.
Embase search
-
▪
2. #1 AND (‘cohort analysis’/de OR ‘controlled clinical trial’/de OR ‘controlled study’/de OR ‘double blind procedure’/de OR ‘human’/de OR ‘meta analysis’/de OR ‘prospective study’/de OR ‘randomized controlled trial’/de) AND (‘cryptococcosis’/de OR ‘human immunodeficiency virus infection’/de) AND (‘fluconazole’/de OR’itraconazole’/de) 1470 results
-
▪
1. ‘aids’/exp OR aids AND (‘fluconazole’/exp OR fluconazole) 3015 results
PubMed search
-
▪
“Acquired Immunodeficiency Syndrome”[MeSH] AND “ltraconazole”[MeSH] AND Clinical Trial[ptyp]
-
▪
“(“acquired immunodeficiency syndrome”[MeSH Terms] OR (“acquired”[All Fields] AND “immunodeficiency”[AII Fields] AND “syndrome”[All Fields]) OR “acquired immunodeficiency syndrome”[AII Fields] OR “aids”[All Fields]) AND (“fluconazole”[MeSH Terms] OR “fluconazole”[AII Fields]) Acquired Immunodeficiency Syndrome”[MeSH] AND “Fluconazole”[MeSH] 1148 results
Google Scholar™ search terms
-
▪
Keywords used included: acquired immunodeficiency syndrome, human immunodeficiency virus, prophylaxis, antifungal, azole, fluconazole, itraconazole, meningitis, cryptococcal, cryptococcus, cryptococcosis, chemoprevention, antifungal agents, randomized trials, case-control studies and cohort studies.
Acknowledgements
The authors would like to thank AH Smith and C Steinmus for guidance with the statistical analysis and K Andrews and A Nabasirye for help and guidance with the data search.
The authors would like to thank the Forgarty AIDS International Training and Research Program (grant number: 1-D43-TW0003) for providing all support to R Ssekitoleko while studying at the School of Public Health, University of California, Berkeley.
Footnotes
Author contributions
Concept development: R Ssekitoleko, MR Kamya and AL Reingold. Data collection: R Ssekitoleko. Supervision of the study: MR Kamya and AL Reingold. Data interpretation and revision of all the drafts: R Ssekitoleko, MR Kamya and AL Reingold. All authors read and approved the final manuscript.
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Chariyalertsak S, Sirisanthana T, Saengwonloey O, Nelson K. Clinical presentation and risk behaviors of patients with acquired immunodeficiency syndrome in Thailand, 1994–1998: regional variation and temporal trends. Clin. Infect. Dis. 2001;32:955–962. doi: 10.1086/319348. [DOI] [PubMed] [Google Scholar]
- 2.Tihana B, Thomas SH. Cryptococcal meningitis. Br. Med. Bull. 2004;72(1):99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- 3.French N, Gray K, Watrea C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS. 2002;16:1031–1038. doi: 10.1097/00002030-200205030-00009. [DOI] [PubMed] [Google Scholar]
- 4.Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 natural history cohort in Uganda. Int. J. Epidemiol. 1998;27:698–702. doi: 10.1093/ije/27.4.698. [DOI] [PubMed] [Google Scholar]
- 5.Casadevall A, Steenbergen JN, Nosanchuk JD. ‘Readymade’ virulence and ‘dual use’ virulence factors in pathogenic environmental fungi — the Cryptococcus neoformans paradigm. Curr. Opin. Microbiol. 2003;6:332–337. doi: 10.1016/s1369-5274(03)00082-1. [DOI] [PubMed] [Google Scholar]
- 6.Chang LW, Phipps WT, Kennedy GE, Rutherford GW. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database Syst. Rev. 2005;(3) doi: 10.1002/14651858.CD004773.pub2. CD004773. [DOI] [PubMed] [Google Scholar]
- 7.Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010;50(3):291–322. doi: 10.1086/649858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocroft A, Katlama C, Johnson AM, et al. AIDS across Europe, 1994-98: the EuroSIDA study. Lancet. 2000;356:291–296. doi: 10.1016/s0140-6736(00)02504-6. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs JA, Masur H. Prophylaxis against opportunistic infections in patients with human immunodeficiency virus infection. N. Engl. J. Med. 2000;342:1416–1429. doi: 10.1056/NEJM200005113421907. [DOI] [PubMed] [Google Scholar]
- 10. Micol R, Tajahmady A, Lortholary O, et al. Cost—effectiveness of primary prophylaxis of AIDS associated, cryptococcosis in Cambodia. PLoS ONE. 2010;5(11):e13856. doi: 10.1371/journal.pone.0013856. ▪▪ Study that assessed the cost—effectiveness of primary antifungal prophylaxis in advanced HIV infection.
- 11.Meya D, Manabe Y, Castelnuovo B, et al. Cost—effectiveness of serum cryptococcal antigen screening to prevent deaths among HIV-infected persons with a CD4+ cell count < or 100 cells/microL who start HIV therapy in resource-limited settings. Clin. Infect. Dis. 2010;51:448–455. doi: 10.1086/655143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parkes-Ratanshi R, Wakeham K, Levin J, et al. Primary prophylaxis of cryptococcal disease with fluconazole in HIV-positive Ugandan adults: a double-blind, randomised, placebo-controlled trial. Lancet. 2011;11(12):933–941. doi: 10.1016/S1473-3099(11)70245-6. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 13.Wanyenze RK, Kamya MR, Fatch R, et al. Missed opportunities for HIV testing and late stage diagnosis among HIV-infected patients in Uganda. PLoS ONE. 2011;6(7):e21794. doi: 10.1371/journal.pone.0021794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006] UK: John Wiley & Sons, Ltd; [Google Scholar]
- 15.Schulz KF, Grimes DA. Allocation concealment in randomized trials: defending against deciphering. Lancet. 2002;359(9306):614–618. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Caroll D, et al. Assessing the quality of reports on randomized clinical trials: is blinding necessary? Control. Clin. Trials. 1996;17(1):1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.Wacholder S, McLaughlin JK, Silverman DT, Mandel JS. Selection of controls in case control studies. Am. J. Epidemiol. 1992;135:1019–1028. doi: 10.1093/oxfordjournals.aje.a116396. [DOI] [PubMed] [Google Scholar]
- 18.Rothman KJ, Greenland S. Meta-analysis. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. PA, USA: Lippincott-Raven Publishers; 1998. pp. 643–673. [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Petitti D. Meta-Analysis, Decision Analysis and Cost—Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine. NY, USA: Oxford University Press; 1994. Statistical methods in metaanalysis; pp. 7.1–7.8. [Google Scholar]
- 21.Shore RE, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br. J. Ind. Med. 1993;50:971–997. doi: 10.1136/oem.50.11.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Light RJ, Pillemar DB. Summing Up: the Science of Reviewing Research. MA, USA: Havard University Press; 1984. Quantitative procedures; pp. 50–103. [Google Scholar]
- 23.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple graphical test. Br. Med. J. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Publication bias. In: Cooper H, Hedges LV, editors. Handbook of Research Synthesis. NY, USA: Russell Sage Foundation; 1994. pp. 399–409. [Google Scholar]
- 25.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double-blind, placebo-controlled trial of primary cryptococcal meningitis prophylaxis in HIV-infected patients with severe immune deficiency. HIV Med. 2004;5:140–143. doi: 10.1111/j.1468-1293.2004.00201.x. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 27. Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clin. Infect. Dis. 2002;34:277–284. doi: 10.1086/338154. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 28. Smith DE, Bell J, Johnson M, et al. A randomized, double-blind, placebo-controlled study of itraconazole capsules for the prevention of deep fungal infections in immunodeficient patients with HIV infection. HIV Med. 2001;2:78–83. doi: 10.1046/j.1468-1293.2001.00060.x. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 29. McKinsey DS, Wheat LJ, Cloud GA, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo controlled, double-blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clin. Infect. Dis. 1999;28:1049–1056. doi: 10.1086/514744. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 30. Cantey PT, Stephens DS, Rimland D. Prevention of cryptococcosis in HIV-infected patients with limited access to highly active antiretroviral therapy: evidence for primary azole prophylaxis. HIV Med. 2005;6:253–259. doi: 10.1111/j.1468-1293.2005.00289.x. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 31. Kris AK, Moore R, Chaisson R. Risk factors for cryptococcal meningitis in HIV-infected patients. AIDS Res. Hum. Retroviruses. 1999;7:625–631. doi: 10.1089/088922299310926. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 32. Quagliarello VJ, Viscoli C, Horwitz RI. Primary prevention of cryptococcal meningitis by fluconazole in HIV-infected patients. Lancet. 1995;345:548–552. doi: 10.1016/s0140-6736(95)90465-4. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 33. Nightingale SD, Cal SX, Peterson DM, et al. Primary prophylaxis with fluconazole against systemic fungal infections in HIV-positive patients. AIDS. 1992;6:191–194. doi: 10.1097/00002030-199202000-00008. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 34. Ammassari A, Linzalone A, Murri R, Marasca G, Morace G, Antinori A. Fluconazole for the primary prophylaxis of AIDS-associated cryptococcosis: a case—control study. Scand. J. Infect. Dis. 1995;27(3):235–237. doi: 10.3109/00365549509019015. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 35. Newton JA, Jr, Tasker SA, Bone WA, et al. Weekly fluconazole for the suppression of recurrent thrush in HIV-seropositive patients: impact on the incidence of disseminated cryptococcal infection. AIDS. 1995;9(11):1286–1287. doi: 10.1097/00002030-199511000-00012. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 36. Manfredi R, Antonio M, Olga C, et al. Fluconazole as prophylaxis against fungal infection in patients with advanced HIV infection. Arch. Intern. Med. 1997;157:64–69. ▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 37.Pradier C, Bernard E, Lubick CD, et al. Does fluconazole prevent cryptococcal meningitis in human immunodeficiency virus-infected patients? J Infect. Dis. 1992;165:787. doi: 10.1093/infdis/165.4.787. [DOI] [PubMed] [Google Scholar]
- 38.Caputi R, Marston B, Siegel B, Steinberg J. Use of azole drugs to prevent cryptococcal infection in patients with AIDS; Presented at: 33rd Inter-Science Conference on Antimicrobial Agents and Chemotherapy; 17–30 October 1993; New Orleans, LA, USA. [Google Scholar]
- 39.Nelson MR, Fisher M, Cartledge J, Rogers T, Gazzard BG. The role of azoles in the treatment and prophylaxis of cryptococcal disease in HIV infection. AIDS. 1994;8(5):651–654. doi: 10.1097/00002030-199405000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Powderly WG, Finkelstein D, Feinberg J, et al. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. N. Engl. J. Med. 1995;332:700–705. doi: 10.1056/NEJM199503163321102. [DOI] [PubMed] [Google Scholar]
- 41.Havlir DV, Dube MP, McCutchan JA, et al. Prophylaxis with weekly versus daily fluconazole for fungal infections in patients with AIDS. Clin. Infect. Dis. 1998;27:1369–1375. doi: 10.1086/515018. [DOI] [PubMed] [Google Scholar]
- 42.Nina S, Barnish M, Berman S, et al. Low dose fluconazole as primary prophylaxis for cryptococcal infection in AIDS patients with CD4 cell counts of less than 100/mm3; demonstration of efficacy in a prospective, multicentre trial. Clin. Infect. Dis. 1996;23:1282–1286. doi: 10.1093/clinids/23.6.1282. [DOI] [PubMed] [Google Scholar]
- 43.Bozzette SA, Larsen RA, Chiu J, et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N. Engl. J. Med. 1991;324(9):580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 44.Meyer A, Kendi CL, Penner CK, et al. The impact of routine cryptococcal antigen screening on survival among HIV-infected individuals with advanced immunosuppression in Kenya. Trop. Med. Int. Health. 2013;18(4):495–503. doi: 10.1111/tmi.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Centers for Disease Control and Prevention (CDC); National Institutes of Health; HIV Medicine Association of the Infectious Diseases Society of America. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm. Rep. 2009;58:1–207. [PubMed] [Google Scholar]
- 46.Anglaret X, Chene G, Attia A, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 47.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiktor SZ, Sassan-Morokro M, Grant AD, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’lvoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 49. Tuli K, Clark T, Ningsanond P, et al. Primary prophylaxis against fungal infections among persons living with AIDS in Thailand: a cost effectiveness analysis; Presented at: 10th Conference on Retroviruses and Opportunistic Infections; 10–14 February 2003; Boston, MA, USA. ▪▪ Study in which the ability of primary antifungal prophylaxis to prevent index fungal infection was investigated.
- 50.Lortholary O, Poizat G, Zeller V, et al. Long-term outcome of AIDS-associated cryptococcosis in the era of combination antiretroviral therapy. AIDS. 2006;20:2183–2191. doi: 10.1097/01.aids.0000252060.80704.68. [DOI] [PubMed] [Google Scholar]
- 51.van der Horst CM, Saag MS, Cloud GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 1997;331:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 52.Robinson PA, Bauer M, Leal ME, et al. Early mycological treatment failure in AIDS associated cryptococcal meningitis. Clin. Infect. Dis. 1999;28:82–92. doi: 10.1086/515074. [DOI] [PubMed] [Google Scholar]
- 53.Denning DW, Armstrong RW, Lewis BH, Stevens DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency. Am. J. Med. 1991;91:267–272. doi: 10.1016/0002-9343(91)90126-i. [DOI] [PubMed] [Google Scholar]
- 54.Kambugu A, Meya DB, Rhein J, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clin. Infect. Dis. 2008;46(11):1694–1701. doi: 10.1086/587667. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.CDC. [(Accessed 10 November 2012)];Fungal diseases – Cryptococcus neoformans cryptococcosis. www.cdc.gov/fungal/cryptococcosis-neoformans.
- 102.WHO. [(Accessed 10 November 2012)];Rapid advice: diagnosis, management and prevention of cryptococcal disease in HIV-infected adults, adolescents and children. 2011 www.who.int/hiv/pub/cryptococcal_disease2011/en. [PubMed]
- 103.WHO. [(Accessed 11 November 2012)];Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach: 2010 revision. www.who.int/hiv/pub/arv/adult2010/en/index.html. [PubMed]
- 104.Wells GA, Shea B, O’Connell Det al. [(Accessed 10 November 2012)];The Newcastle—Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.htm.