Abstract
Thiazolidinediones (TZDs) are potent insulin sensitizers that act through the nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) and are highly effective oral medications for type 2 diabetes. However, their unique benefits are shadowed by the risk for fluid retention, weight gain, bone loss and congestive heart failure. This raises the question as to whether it is possible to build a safer generation of PPARγ-specific drugs that evoke fewer side effects while preserving insulin-sensitizing potential. Recent studies that have supported the continuing physiologic and therapeutic relevance of the PPARγ pathway also provide opportunities to develop newer classes of molecules that reduce or eliminate adverse effects. This review highlights key advances in understanding PPARγ signaling in energy homeostasis and metabolic disease and also provides new explanations for adverse events linked to TZD-based therapy.
The PPARs are members of the nuclear receptor superfamily of ligand-inducible transcription factors1. In mammals, there are three PPARs: PPARα (also called NR1C1), PPARβ/δ (also called NR1C2) and PPARγ (also called NR1C3). By binding to PPAR-responsive regulatory elements as obligate heterodimers with retinoid X receptor (RXR), the PPARs control the expression of networks of genes involved in adipogenesis, lipid metabolism, inflammation and maintenance of metabolic homeostasis2. Similar to typical nuclear receptors, PPARs are comprised of distinct functional domains, including an N-terminal transactivation domain (AF1), a highly conserved DNA-binding domain (DBD) and a C-terminal ligand-binding domain (LBD) containing a ligand-dependent transactivation function (AF2)3. These domains are all potential targets for modulation of the PPAR signaling cascades. Although they are known as receptors for common dietary fats such as oleic, linoleic and linolenic acids, PPARs also bind and respond to diverse lipid metabolites, including prostaglandin J2, 8S-hydroxyeicosatetraenoic acid and a collection of oxidized phospholipids4–6. Ligand binding induces a conformational change in the receptor that allows for differential recruitment of cofactors and subsequent modulation of PPAR activity3.
Despite their many similarities, each PPAR isoform has unique functions in vivo, probably because of distinct tissue distributions, differential responses to distinct ligands and inherent differences in biochemical properties1,3. PPARα, the first PPAR to be identified, is expressed predominantly in the liver, heart and brown adipose tissue (BAT), where it is a major activator of fatty acid oxidation pathways and is the target of the hypolipidemic fibrate drugs1,3. Although PPARδ (also called PPARβ and commonly referred to as PPARδ/β) shares similar functions with PPARα, it is ubiquitously expressed and has a crucial role in fatty acid oxidation in key metabolic tissues such as skeletal muscle, liver and heart2,3. PPARγ is most highly expressed in white adipose tissue (WAT) and BAT, where it is a master regulator of adipogenesis as well as a potent modulator of whole-body lipid metabolism and insulin sensitivity1,7. Because of alternative splicing and differential promoter usage, PPARγ exists as two isoforms, PPARγ1 and PPARγ2, with the latter containing an additional 30 amino acids at its N terminus7,8. Whereas PPARγ1 is expressed in many tissues, the expression of PPARγ2 is restricted to adipose tissue under physiological conditions but can be induced in other tissues by a high-fat diet (HFD)8,9. Although all three PPARs are strongly implicated in the metabolic syndrome10, the aim of this review is to focus on PPARγ and highlight recent findings that have shed light onto this signaling pathway and renewed interest in its therapeutic potential for the treatment of type 2 diabetes.
Even though fatty acids and their derivatives can bind and activate PPARγ, the identification of specific endogenous PPARγ ligands has been difficult, and thus specific modes of action related to fatty acids and their metabolites have not been clearly defined4,6. In contrast, synthetic ligands, such as TZDs, are potent activators of PPARγ with robust insulin-sensitizing activities11. Consequences of highly effective oral medications used in the treatment of difficult-to-manage type 2 diabetes that chronically activate PPARγ include weight gain, fluid retention and osteoporosis11. Meta-analyses of clinical trials have implicated the TZD rosiglitazone (Avandia) in increasing the risk of congestive heart failure, myocardial infarction, cardiovascular disease and all-cause mortality12,13, leading to tightly restricted access in the United States and a recommendation for market withdrawal in Europe14 and several other jurisdictions. Pioglitazone (Actos), another TZD, does not seem to impart the same cardiovascular risks that rosiglitazone does. Indeed, a large placebo-controlled clinical trial indicated a modest reduction in major cardiovascular events in people with high-risk diabetes receiving pioglitazone over a 3-year period15. However, safety concerns have also been raised about pioglitazone in relation to congestive heart failure16 and bladder cancer17,18, the latter leading to safety warnings and drug withdrawal in parts of Europe12.
Accordingly, further understanding of how different TZDs trigger specific side effects, as well as the alternative routes of PPARγ activation, will potentially lead to new and improved therapies for type 2 diabetes. Recently, in part because of powerful new technologies (Box 1), much progress has been made in understanding the signaling, regulation and tissue-specific roles of PPARγ19–23. Many of these advances reveal new insights into the mechanisms underlying PPARγ-mediated insulin sensitization as well as its associated side effects, providing opportunities to develop newer classes of molecules that reduce or eliminate the adverse effects associated with TZDs.
BOX 1. Applying genome-wide analyses to study regulation of PPARγ signaling.
Recent advances in high-throughput technologies, such as next-generation sequencing methods, have allowed the investigation of genome-wide transcriptional regulation in an unbiased manner to provide abundant information regarding gene expression, cis-acting elements, trans-acting factors, epigenetic status and chromatin structure137. Such genomic studies of PPARγ have revealed the comprehensive binding-site distribution of PPARγ in adipocytes and macrophages, the colocalization frequency of PPARγ with other transcription factors, such as RXR-α, C/EBPs and PU.1, comparative histone modification profiling in adipocytes and macrophages and chromatin architecture changes during adipogenesis72,138–141. Notably, analysis of data obtained using chromatin immunoprecipitation combined with next-generation sequencing (ChIP-seq) in the context of human adipocytes shows that PPARγ binding sites are rarely in the promoter regions of genes, accounting for just 3% of genome-wide sites, with introns (45%) and intergenic enhancers (48%) comprising the majority of binding sites142. Interestingly, although promoter-localized PPARγ binding sites are rare, these genes are robustly TZD responsive142,143. Additionally, DNase I hypersensitive sites sequencing (DNase-seq) revealed that 33% of PPARγ target sites are present in an ‘accessible’ or open chromatin structure before DNA binding during adipogenesis, indicating a frequent (though not mandatory) cooperative action of PPARγ with early adipogenic transcription factors such as C/EBPs. Adding another layer of transcriptional control, cytosine hydroxymethylation, also participates in PPARγ enhancer function during adipogenesis144.
Collectively we know that PPARγ response networks are extremely complex and distinctly regulated in a cell type–specific manner. However, many questions remain to be answered to develop a more comprehensive understanding about PPARγ signaling. What are the co-regulators involved in determining cell-type specificity? How do changes in the epigenome and chromatin structure affect transcriptional outcomes? How do distal regulatory elements such as enhancers control the activity of promoters?
Although individual genomic approaches can provide highly informative and specific answers, a combination of high-throughput sequencing applications and data integration is necessary to comprehensively understand transcriptional events in an unbiased, genome-wide manner during complex biological processes. As a transcription factor, regulation of PPARγ signaling must be understood by its specific pattern of association with target DNA and, through this process, positive or negative regulation of proximal promoters. In addition, from a dynamic point of view, recruitment of tissue specific coactivators and co-repressors will need to be established to more fully understand the dynamics of chromatin modification and gene control.
New functions for PPARγ in adipose tissue
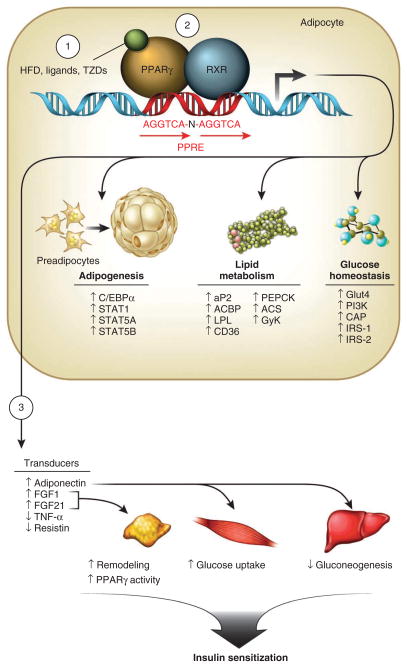
PPARγ was originally described as a factor induced during adipocyte differentiation7,24,25 and is best known for its role in regulating adipogenic and lipogenic pathways (Fig. 1). Generation of the PPARγ-null mouse, which is completely devoid of adipose tissue, firmly established PPARγ as a master regulator of adipocyte differentiation26. Recently a dynamic adipocyte progenitor population was identified within the WAT perivascular niche whose expression of PPARγ suggests it may play a part in adipocyte self renewal27,28. PPARγ is also required for mature adipocyte function, as revealed by the finding that adipocytes only survive for a few days after selective ablation of PPARγ in mature adipocytes of mice29,30.
Figure 1.
PPARγ has multiple roles in adipose tissue. HFD, ligands or TZDs (1) activate PPARγ-RXR functional heterodimers (2) and maintain metabolic homeostasis through direct regulation of genes harboring PPAR response elements (PPREs) involved in adipocyte differentiation, lipid metabolism and glucose homeostasis, as well as the expression of adipose secreted factors that act as transducers for PPARγ (3). C/EBPα, CCAAT/enhancer-binding protein α; STAT1, STAT5A and STAT5B, signal transducer and activator of transcription 1, 5A and 5B, respectively; aP2, fatty acid binding protein 2; ACBP, acyl-CoA–binding protein; LPL, lipoprotein lipase; CD36, cluster of differentiation 36; PEPCK, phosphoenolpyruvate carboxykinase; ACS, acyl-CoA synthetase; GyK, glycerol kinase; Glut4, glucose transporter 4; PI3K, phosphoinositide 3 kinase; IRS-1 and IRS-2, insulin receptor substrate 1 and 2, respectively.
In addition to its role in adipocyte differentiation and lipid metabolism, PPARγ is also crucial for controlling gene networks involved in glucose homeostasis, including increasing the expression of glucose transporter type 4 (Glut4) and c-Cbl–associated protein (CAP). Moreover, PPARγ controls the expression of numerous factors secreted from adipose tissue, such as adiponectin, resistin, leptin and tumor necrosis factor-α (TNF-α), which also influence insulin sensitivity31–34. Considering that these factors probably act through distinct signaling pathways and different, although overlapping, tissue targets, several different mechanisms may be involved in achieving the insulin-sensitizing effect. For example, some factors might act on inflammation (TNF-α), hepatic glucose output (adiponectin) or feeding behavior (leptin). Physiologically, these pathways may complement each other, and although pharmacologic increase of one of the pathways could be sufficient to achieve a therapeutic benefit, none of these factors has moved into advanced human trials. However, they clearly illustrate the promise for multiple (and potentially safe) routes for therapeutic intervention.
Consistent with its central role in adipogenesis and insulin sensitization, humans with dominant-negative mutations in a single allele of PPARG have partial lipodystrophy and insulin resistance35–37. The discovery that TZDs, which have known potent adipogenic and anti-diabetic effects, are agonists for PPARγ solidified the importance of PPARγ in insulin sensitization and prompted numerous and extensive studies on this nuclear receptor4,7,38. Thus, despite its many caveats, directly targeting PPARγ itself remains the ‘gold standard’ for treating metabolic disease.
The fibroblast growth factor connection
The ability of PPARγ to control the expression of adipose-secreted factors, such as adiponectin, led to a search for additional regulatory factors39 (Fig. 1). This resulted in the identification of two PPARγ-responsive members of the fibroblast growth factor family (FGF1 and FGF21), which act locally in adipose tissue to promote insulin sensitization in response to HFD22,23,39. FGF21 was originally found to be induced in the liver by PPARα in response to fasting to regulate carbohydrate and lipid metabolism. It was subsequently revealed that FGF21 is induced in WAT by both HFD and PPARγ agonists40. Unexpectedly, whereas hepatic FGF21 circulates as a hormone, adipose FGF21 does not circulate and instead acts in an autocrine fashion to locally transduce PPARγ signaling to enhance adipogenesis23. As they are unable to store fat, FGF21-knockout mice have reduced adiposity with decreased expression of PPARγ target genes in WAT23. Moreover, these mice are resistant to the insulin-sensitizing effects of TZDs as well as the associated weight gain and fluid retention, implicating FGF21 as an important mediator of the antidiabetic actions and negative side effects of TZDs. Notably, FGF21 knockout mice have increased sumoylation of PPARγ (discussed in more detail below), which reduces its transcriptional activity23. Taken together these findings reveal that during the fed state, PPARγ induces FGF21, which works locally in adipose tissue to amplify PPARγ activity and promote insulin sensitization.
Recently it was found that FGF1, the prototype of the FGF family of proteins, is also regulated by PPARγ and highly induced in visceral adipose tissue in response to HFD or treatment with TZDs22. Of three alternative gene promoters, only the FGF1A isoform shows diet or TZD inducibility22. Although FGF1 has been implicated in a diverse range of physiological processes, FGF1 knockout mice show no phenotype under standard laboratory conditions, which led to the long-held assumption that FGF1 was dispensable41. Strikingly, however, when placed on a HFD, FGF1 knockout mice develop a crippling, disabling ‘fat fibrosis’ that structurally restricts adipose expansion, resulting in an aggressive diabetic phenotype22. Moreover, after HFD withdrawal, the adipose tissue of these mice cannot properly contract, leading to marked fat necrosis and structural fragmentation of the fat pad. Collectively, these concurrent and severe pathologies indicate a key role for a PPARγ-FGF1 signaling axis in adaptive adipose remodeling to maintain metabolic homeostasis during cycles of feast and famine.
By working in an autocrine manner, a paracrine manner or both, FGF21 and FGF1 act locally in adipose tissue to mediate the physiological and pharmacological actions of PPARγ. Further studies are needed to elucidate the relative roles of these two FGFs and their regulation by PPARγ. Nevertheless, these newly identified PPARγ signaling pathways identify FGFs as important new mediators of the beneficial effects of TZDs and represent a paradigm shift in which signals such as HFD or drugs act on sensors such as PPARγ that in turn direct the production of locally acting factors (FGFs) to control energy homeostasis (Fig. 1). In the case of FGF21, pharmacological administration of this protein in mice results in marked bone loss, potentially limiting its use therapeutically42. A clear understanding of the two FGF signaling pathways, as well as other components of the PPARγ signaling cascade, will be crucial for dissociating the specific mechanisms responsible for improved insulin sensitization from those involved in causing the adverse side effects associated with TZDs.
PPARγ has tissue-specific effects
Adipose tissue is where PPARγ is most highly expressed and is the tissue with the most notable gene expression changes in response to treatment with PPARγ agonists43. Therefore, the insulin-sensitizing effects, as well as certain negative side effects, of TZDs are generally attributed to adipose-specific PPARγ activation. In adipose tissue, PPARγ upregulates genes involved in glucose uptake and also controls the expression of adipocyte-secreted factors, such as adiponectin, that communicate with other organs to affect whole-body insulin sensitivity1. Furthermore, as high concentrations of circulating fatty acids are positively correlated with insulin resistance, enhanced uptake and sequestration of fatty acids in adipose tissue by PPARγ activation are thought to ameliorate insulin resistance44. It has been postulated that the increased uptake of fatty acids and enhanced adipogenic capacity in WAT, elicited by PPARγ activation, are also responsible for TZD-associated weight gain. However, selective activation of PPARγ in adipocytes of mice is sufficient to cause whole-body insulin sensitization without an increase in weight. This finding raises the possibility that adipose tissue might be sufficient for the insulin-sensitizing effects of TZDs but not the side effects such as weight gain45. Conversely, the facts that PPARγ is expressed, albeit at lower levels, in a variety of nonadipose tissues and that TZDs improve insulin sensitivity in lipodystrophic (fatless) mice suggest that other tissues may also be direct targets and contribute to the insulin-sensitizing effects of TZDs, as well as the unwanted side effects46,47.
Experiments with tissue-specific knockouts of PPARγ have been crucial in helping dissect the relative contributions of PPARγ activity to insulin sensitization in different tissues. Mice with adipose-specific ablation of PPARγ show insulin resistance in adipose tissue and liver but not skeletal muscle30. In these mice, TZDs improved insulin sensitivity in the skeletal muscle and liver but not adipose tissue, indicating a direct action of TZDs in skeletal muscle and liver as well as adipose tissue30. In this regard, two groups independently examined mice with targeted ablation of PPARγ in skeletal muscle48,49. In one study with older mice, a lack of skeletal-muscle PPARγ resulted in severe insulin resistance, and the skeletal muscles of these mice were unresponsive to TZD treatment, indicating a role for skeletal-muscle PPARγ in the action of TZD48. In a second study, younger mice with targeted muscle PPARγ deficiency did not develop skeletal-muscle insulin resistance and remained responsive to TZD treatment49. Interestingly, these mice developed excess adiposity and hepatic insulin resistance, suggesting that the insulin-sensitizing effects of TZDs on muscle are indirect and age dependent and that skeletal-muscle PPARγ may have a role in the regulation of whole-body insulin sensitivity, perhaps through tissue crosstalk49. Although further investigation will help resolve the apparent age-dependent differences, it is clear that skeletal muscle plays a part in TZD-induced insulin sensitization. The liver is also a proposed site of TZD action; however, the effects of PPARγ agonism on the liver remain under debate, with some studies showing that it promotes hepatic steatosis through upregulation of genes involved in lipid uptake and storage43 and others showing that it prevents hepatic steatosis and fibrosis, possibility by sequestering fatty acids in adipose tissue and preventing hepatic stellate cell activation50–54. Treatment with PPARγ agonists also decreases the expression of genes involved in gluconeogenesis, and liver-specific disruption of PPARγ in mice results in increased adiposity, hyperlipidemia and insulin resistance, yet these mice remain responsive to TZD treatment55. However, on a lipodystrophic background, liver-specific ablation of PPARγ renders these mice resistant to TZD treatment, indicating that in the absence of adipose tissue, the liver becomes a major site of TZD action55. PPARγ is also expressed in pancreatic beta cells, where it induces the expression of key genes involved in glucose-stimulated insulin secretion (GSIS), and TZDs have been shown to enhance GSIS in insulin-resistant rodents and humans56–59. However, results from in vivo studies have been conflicting, with one study showing alterations in beta-cell mass but no change in glucose homeostasis in mice lacking PPARγ in their beta cells and a more recent study showing that loss of PPARγ in the whole pancreas results in hyperglycemia with impaired GSIS60,61. Further investigation will be required to determine the precise role of PPARγ in pancreatic beta cells and its contribution to mediating the insulin-sensitizing effects of TZDs. Taken together, these studies reveal that TZDs act through several key metabolic organs to exert their insulin-sensitizing effects.
PPARγ also has an important role in various immune cells, with most studies focusing on its role in antigen-presenting myeloid dendritic cells and macrophages62–65. In dendritic cells, PPARγ regulates lipid metabolism and transport as well as various processes, including antigen uptake, maturation, activation, migration, cytokine production and antigen presentation62,63. Macrophage PPARγ is implicated in anti-inflammation and lipid metabolism54,66–69, and mice lacking macrophage PPARγ are more prone to whole-body insulin resistance70,71. Furthermore, these mice have impaired maturation of anti-inflammatory ‘M2’ macrophages71. Reciprocally, the inflammatory gene network in wild-type proinflammatory ‘M1’ macrophages is potently inhibited by TZD treatment. In this regard, differential localization of PPARγ, distinct sets of co-regulators and specific epigenetic modifications collectively influence cell- and tissue-specific PPARγ functions72. Emerging technological advances provide new methods for identifying epigenomic processes and interrogating PPARγ in different tissues and cell types on a genome-wide scale that will help uncover the tissue-specific functions of PPARγ (Box 1). Notably, although anti-inflammatory CD4+ tissue-resident regulatory T (Treg) cells are widely distributed throughout the body, those in visceral adipose tissue uniquely express high levels of PPARγ. In contrast, Treg cells in other adipose depots are not PPARγ positive73. The relative numbers of Treg cells are markedly reduced in obese and insulin-resistant states. Furthermore, Treg cell–specific knockout of PPARγ reduces responsiveness to insulin sensitizers73. PPARγ also exerts an anti-inflammatory role in the artery wall, as revealed by studies in low-density lipoprotein receptor–null mice74. Much more work needs to be done to explore the role of PPARγ in Treg cells, macrophages and other types of immune cells to better elucidate the molecular connection between PPARγ, inflammation and lipid metabolism.
Deciphering the side effects of TZDs
In addition to the insulin-sensitizing benefits of TZDs, it is important to identify the tissues that contribute to their side effects, such as weight gain, fluid retention, bone loss and heart problems. Although TZD-induced weight gain has been attributed to PPARγ activation in adipose tissue, studies in mice and humans have suggested a central role for PPARγ in whole-body energy homeostasis45,75–77. Although PPARγ is known to have a neuroprotective and anti-inflammatory role in the central nervous system (CNS)78, two recent and independent reports indicated that activation of PPARγ in the brain, rather than in adipose tissue, contributes to TZD-induced weight gain20,21. Using combinations of pharmacological and genetic approaches in HFD-fed rodents, these studies showed that by controlling food intake and energy expenditure, the action of PPARγ in the CNS is required for the increased weight gain associated with TZDs20,21. Although both studies suggest that increased leptin sensitivity may mediate these metabolic effects, additional work will be required to define the specific neural targets and clarify the balance between central and peripheral PPARγ signaling in insulin sensitization. Nevertheless, these two studies strongly suggest that PPARγ action in the CNS contributes to TZD-induced weight gain, leading to the question of what the properties of non–brain penetrant TZDs are.
Although TZDs were shown to improve the pathogenesis of diabetic nephropathy79, fluid retention with associated edema remains a substantial side effect of TZDs11, and strategies designed to eliminate this aspect of PPARγ signaling would be extremely beneficial. Recent studies have revealed that TZDs contribute to this fluid retention and peripheral edema by altered sodium and water reabsorption in the distal collecting ducts of the kidney80,81. However, the precise mechanism by which TZDs exert this action remains under debate, as there have been divergent findings concerning the role of the epithelial sodium channel in this phenomenon82,83. Besides an impact on weight gain, reduced fluid retention may also lower the risk for adverse cardiovascular events, such as congestive heart failure. Although it is expressed at low levels in the heart, the expression of PPARγ is increased in the hearts of humans with metabolic syndrome84. Studies of PPARγ in the cardiomyocytes of mice have not resulted in a consensus. Mice with cardiomyocyte-specific knockout of PPARγ show cardiac hypertrophy, whereas mice overexpressing PPARγ in cardiomyocytes develop dilated cardiomyopathy with increased lipid and glycogen stores and disrupted mitochondria85,86. Although fluid retention may be the major contributor to the negative impact of TZDs on the heart, further studies are needed to clarify the role of PPARγ in this organ.
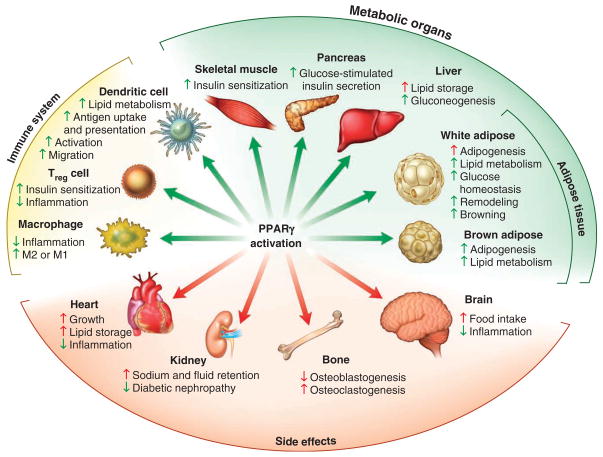
Another reported side effect of TZDs is a higher rate of fractures in human patients11. Consistent with this, TZDs cause bone loss in mice and rats by simultaneously decreasing bone formation (osteoblastogenesis) and increasing bone resorption (osteoclastogenesis)87. PPARγ has been shown to inhibit osteoblast differentiation and bone formation88. PPARγ-null embryonic stem cells do not differentiate into adipocytes and instead spontaneously differentiate into osteoblasts, whereas TZDs inhibit osteoblast differentiation and promote adipogenesis87,88. PPARγ promotes osteoclast differentiation, and deletion of PPARγ in mouse hematopoietic lineages results in osteoclast deficiency and resistance to TZD-stimulated bone resorption89. These findings indicate that TZD-induced bone loss is the result of bone cell–autonomous PPARγ action, which simultaneously inhibits osteoblastogenesis while enhancing osteoclastogenesis. Together these studies reveal that TZDs act on a variety of tissues to confer insulin sensitization as well as cause deleterious side effects (Fig. 2). These important findings open up new avenues of research to help direct the development of tissue-specific compounds that improve the differential between beneficial and adverse events.
Figure 2.
Known effects of PPARγ activation. Activation of PPARγ results in beneficial effects (green arrows) as well as adverse side effects (red arrows).
Post-translational modifications regulate PPARγ function
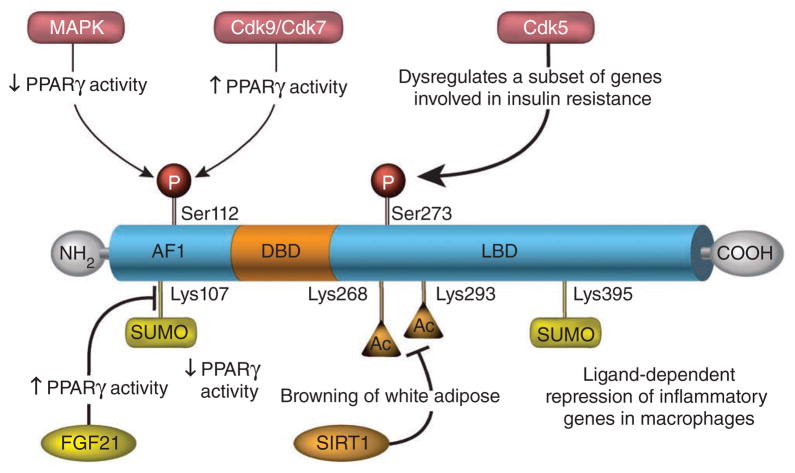
PPARγ is also regulated by post-translational modifications, including phosphorylation, acetylation, sumoylation and ubiquitination, each of which represents a potentially distinct feature that could be exploited for cell- or tissue-specific modulation of this molecule90,91 (Fig. 3). PPARγ is phosphorylated within the AF1 region by mitogen-activated protein kinases (MAPKs) (PPARγ2 at Ser112 and PPARγ1 at Ser82), which represses its transcriptional activity by inhibiting ligand binding and altering cofactor recruitment92–94. Notably, phosphorylation of the same site by the cyclin-dependent kinases Cdk7 and Cdk9 increases PPARγ activity95,96. Therefore, the phosphorylation of PPARγ at Ser112 can result in different transcriptional outcomes depending on the physiological context and the kinases involved. In vivo studies of this phosphorylation site have been discrepant, indicating that further investigation will be required to elucidate the function of this site and determine the relative roles of these two kinases in regulating PPARγ activity75,97,98.
Figure 3.
Post-translational modifications of PPARγ. Post-translational modifications of PPARγ influence both its transcriptional activity and its protein stability in a cell- and context-dependent manner. Ac, acetylation; P, phosphorylation; Cdk9/Cdk7, Cyclin-dependent kinases 9 and 7.
Recently it was found that PPARγ2 is phosphorylated within the LBD at Ser273 by Cdk5, a protein kinase that can be activated by various proinflammatory cytokines whose amounts are elevated in obesity99,100. Notably, phosphorylation of PPARγ by Cdk5 does not affect its adipogenic capacity but does alter the expression of a distinct group of genes that are aberrantly regulated in obesity101. Accordingly, Cdk5-mediated phosphorylation of PPARγ is increased in adipose tissue of HFD-fed mice and is inversely correlated with TZD-induced insulin sensitization in humans101. PPARγ ligands prevent Cdk5-mediated phosphorylation of PPARγ103. Interestingly, MRL24, a non-TZD compound with poor agonist activity but excellent antidiabetic effects, is very effective at blocking Cdk5-mediated phosphorylation of PPARγ, suggesting that it may be possible to create new PPARγ ligands that block Cdk5-mediated PPARγ phosphorylation yet lack classical agonism101. Recently a compound named SR1664 was identified as a PPARγ agonist that has no adipogenic action in vitro19. Studies in obese mouse models have shown that SR1664 has strong antidiabetic actions similar to those elicited by TZDs but without many of the unwanted side effects. Although the poor pharmacokinetic properties of SR1664 will probably preclude its use in humans, these findings provide hope that it is possible to develop a new class of highly targeted and effective drugs that preserve the strong antidiabetic efficacy of TZDs yet eliminate many of the unwanted side effects that occur due to classical agonism on PPARγ target genes19.
Some TZDs are also capable of inducing BAT-like features in WAT102. In contrast to WAT that stores energy in the form of triacylglycerol, BAT burns energy through uncoupled respiration in the mitochondria103. This, combined with recent evidence that adult humans have metabolically active BAT, led to intense interest in activating these cells therapeutically104. Although PPARγ has been known to promote BAT adipogenesis, the mechanism for the intrinsic ‘browning’ of WAT remains unclear105. However, it was recently reported that TZDs increase the half-life of PRDM16, a transcription factor that has been linked to BAT development and the browning of WAT106. Interestingly, only PPARγ full agonists, such as rosiglitazone, and not partial agonists, such as MRL24, have been reported to elicit browning106. This raises the question of whether browning is directly linked to the potency of PPARγ activation or whether browning- specific agonists can be identified. For example, compounds stabilizing PRDM16 and that lack full agonist activity may be promising for the treatment of obesity and diabetes. In this context, the recent evidence that deacetylation of PPARγ at Lys268 and Lys293 by the NAD-dependent deacetylase sirtuin 1 (SIRT1) promotes the recruitment of PRDM16 to PPARγ suggests a new pathway through which WAT browning could be induced90.
Another layer of control over PPARγ activity involves its sumoylation, the covalent attachment of small ubiquitin-like modifier (SUMO) peptides that typically leads to repression of transcription factors107. Sumoylation of PPARγ2 at Lys107 (or of PPARγ1 at Lys77) in the AF1 region blocks its transcriptional activity, possibly by promoting co-repressor recruitment108. The new findings with FGF21 knockout mice discussed above show that FGF21 increases PPARγ activity in adipocytes by preventing PPARγ sumoylation at Lys107, resulting in a feed-forward mechanism22. PPARγ is also sumoylated at Lys395, which in macrophages results in its recruitment to the promoters of inflammatory genes, where it is thought to inhibit transcription by preventing clearance of co-repressor complexes109. Additionally, PPARγ has been shown to be ubiquitinated, which is enhanced by ligand binding, as well as exposure of adipocytes to the cytokine interferon-γ110,111. Although the ubiquitin acceptor sites have yet to be identified, polyubiquitination and subsequent degradation by the proteosome is consistent with the short half-life (~2 h) of the PPARγ protein and represents another level of control over PPARγ activity110. In aggregate, these studies demonstrate the true potential to exploit ligand- and signaling-dependent post-translational modification to both better understand the nature of insulin sensitization and lead to the development of a mechanistically new class of drugs that regulate PPARγ.
Translating insights in PPARγ biology into the clinic
Although TZDs clearly have potent antidiabetic effects, it is now apparent that they are accompanied by a myriad of unwanted side effects ranging from bone fractures to heart disease. The majority of these side effects were initially unpredictable, mainly because of a lack of awareness about the complexity of PPARγ signaling. Recent findings have shed light on the intricacies of the PPARγ pathway and are helping dissociate the benefits from the adverse side effects of TZDs. This multitude of PPARγ-mediated signaling pathways, including its recently recognized role as an inducer of secreted factors such as FGF1 and FGF21, opens up new opportunities for drug development22,23. Evidence that FGF1 is required for insulin sensitization and that FGF21 delivery promotes insulin sensitization suggests two new biologic approaches to treat metabolic disease. Furthermore, it is now evident that the positive and negative effects of PPARγ action are segmented to different cell- and tissue-types and that tissue- targeted TZDs could be a future therapeutic strategy. In addition to its action in classic metabolic tissues, which was the initial focus of previous PPARγ research, the CNS is emerging as a potential new mediator of TZD-induced weight gain20,21. Therefore, compounds that avoid activating brain PPARγ may be promising. Adding further complexity, new post-translational modifications of PPARγ have been recognized, including its phosphorylation at Ser273 by Cdk5, which does not affect its adipogenic capacity but does alter the expression of a distinct group of genes that are abnormally regulated in obesity101. Compounds have already been developed that specifically block phosphorylation at this site and seem to be effective insulin sensitizers despite poor classical PPARγ agonist properties19.
These recent findings provide reason to believe that it will be possible to develop a new class of highly targeted and effective drugs that preserve the strong antidiabetic efficacy of TZDs yet eliminate many of the unwanted side effects. As previously discussed, this could be achieved in a variety of ways and may ultimately include a combination of approaches, including targeting specific PPARγ pathways, selectively agonizing or antagonizing PPARγ in certain tissues, altering specific post-translational modifications of PPARγ or inducing certain epigenetic modifications. Fortunately, many of these ideas are not without precedent. The development of ligands that selectively modulate a nuclear receptor has been demonstrated with selective estrogen receptor modulators, in which agonism for the estrogen receptor is achieved in one tissue while partial agonism or even antagonism occurs in another tissue112. For PPARγ, this has been termed the ‘selective PPARγ modulator’ concept, which is based on the idea that structurally distinct PPARγ ligands will result in unique receptor-ligand conformations with signature affinities for different co-regulators, thereby allowing discrete gene activation profiles within different cells and tissue types113. The relatively large ligand-binding pocket of PPARγ facilitates the binding of structurally diverse endogenous and synthetic ligands114–116. In addition to SR1664, several selective PPARγ modulators with minimal side effects have been identified113,116. In this regard it might also be possible to administer TZDs in conjunction with certain epigenetic modifiers, such as histone deacetylase inhibitors72,117. Alternatively, a new combinatorial approach that allows for peptide-mediated selective tissue targeting of nuclear receptors may be beneficial, as has recently been demonstrated for an estrogen receptor agonist118. We have seen from studies with Cdk5 that it is possible to design compounds that alter specific post-translational modifications of PPARγ and prevent unwanted side effects but retain antidiabetic potency. As mentioned above, meta-analyses of clinical trials have attributed increased cardiovascular risks to rosiglitazone but not pioglitazone. A possible explanation is the observation that pioglitazone has a favorable impact on circulating lipids, notably decreasing triglyceride concentrations in patients with type 2 diabetes, whereas rosiglitazone has the opposite effect119–121. Although the mechanism underlying this difference has not been elucidated fully, it may be explained at least in part by pioglitazone having more off-target effects, such as PPARα agonist activity122,123, although other differential actions have been reported, such as increased skeletal-muscle mitochondrial respiration124. This and other evidence suggest that there may be a role for mixed PPAR receptor targeting. In this regard, a number of such dual PPARα and PPARγ agonists have been developed123,125, but safety concerns surrounding heart failure and renal impairment have led to the withdrawal of some of these agents from clinical trials. Currently one compound, aleglitazar, an equipotent agonist of PPARα and PPARγ, is in a phase 3 trial in patients with recent acute coronary syndrome and type 2 diabetes123. Additionally, RXR-selective ligands (referred to as rexinoids) that selectively activate the RXR-PPARγ heterodimer may also be promising targets, especially because isotypes of RXR have been shown to be differentially regulated in pathological conditions such as obesity126–129. Alternatively, activating autophagy or specific lipases could be a strategy to generate bioactive lipid ligands for PPARγ130,131. It is also worth considering that both the benefits and adverse effects of TZDs could result from activation of non-PPARγ targets such as AMP-activated protein kinase (AMPK)132. Benefit may also be derived from changing the way we screen for PPARγ-targeted therapeutics from potent, long-lasting compounds to less specific and less stable compounds that allow for more rapid clearance and shorter duration of action. Along with appropriate dosing schedules, this strategy may lead to the development of therapies that act in a more circadian manner and mimic physiological fasting and feeding cycles. Nevertheless, because the US Food and Drug Administration already demands cardiovascular safety data for all classes of antidiabetic agents, it might be beneficial to target this clinical outcome upfront, either in the compound identification stage or during preclinical studies in nonhuman primates. A summary of clinical trial experience with TZDs has been tabulated in a recent review of this drug class74.
Despite the substantial progress that has been made on the subject, there is still much to be clarified regarding PPARγ signaling, and several important questions remain. Both FGF1 and FGF21 are regulated by PPARγ in adipose tissue, but why are they both there, what are their relative contributions, and do they have unique or overlapping functions with each other (as well as with other transducers of PPARγ-mediated insulin sensitization, such as adiponectin)? In this regard, crosstalk between PPARγ and various cytokines and transcription factors also exists and may act coordinately with these factors to promote insulin sensitization133. Also, how mechanistically can FGF21, which normally circulates as a hepatic hormone, act locally when expressed in adipose tissue? Further investigation into the composition of the extracellular matrix of adipose tissue will be important, especially when considering how to target these molecules therapeutically. In this regard, FGF21 has been administered to mice, pharmacologically producing strong antidiabetic effects but also causing bone loss35,40,42. Although the metabolic importance of the PPARγ-FGF1 signaling pathway was revealed through dietary manipulation in knockout mouse studies, it will be interesting to see whether FGF1 can itself serve as a pharmacologically independent regulator of glucose homeostasis and insulin resistance.
Tissue-specific knockouts of PPARγ in mice have been crucial in revealing that almost every tissue examined is implicated in some aspect of TZD-induced insulin sensitization. Although it is clear that adipose tissue is a central mediator of these insulin-sensitizing effects, the relative importance of other tissues in TZD-mediated insulin sensitization, as well as in potential tissue crosstalk, needs to be clarified, particularly in light of the observation that individual loss of PPARγ from a single cell or tissue type can effect whole-body insulin sensitization. One feature may be that loss of PPARγ could predispose an animal to chronic inflammation, which may promote insulin resistance. In reality, the field has not carefully explored whether these deficiencies can be rescued by increasing the amounts of a different sensitizer or through the use of another small molecule (such as glucocorticoids, LXR or PPARδ agonists) known to suppress macrophage inflammation. There are many fruitful areas here for future study. Another intriguing aspect of many recent studies is their association with HFD, which seems to be required to elicit many of the PPARγ phenotypes. It is possible that one or several fatty acid ligands produced by HFD feeding may mediate components of these effects. If this is the case, it will be important to determine from a nutritional point of view whether such natural molecules could selectively trigger different PPARγ signaling, including the regulation of FGFs, the activation of PPARγ in the brain or the phosphorylation of PPARγ by Cdk5. In this regard, PPARγ has been shown to have distinct transcriptional activities in normal, nonpathological conditions as compared to in obese or diabetic states129,134–136. There are also important questions regarding post-translational modifications of PPARγ. For example, how does phosphorylation of PPARγ by Cdk5 alter the expression of a distinct subset of genes, and does it affect co-regulator recruitment? Does this phosphorylation occur in other tissues, and if so, is it also induced by HFD? Additionally, although various post-translational modifications of PPARγ have been identified, it is not clear what reverses this process. Factors such as phosphatases are probably just as crucial in modulating PPARγ activity. Lastly, we must also keep in mind that much of our knowledge of PPARγ signaling is based on data in rodent models, which will not always translate directly to humans.
We have highlighted just a few of the questions that have been sparked by recent findings. Many intriguing avenues of PPARγ research have been opened and hold the potential to ultimately lead to newer classes of more selective molecules. As we move forward with the development of the next generation of PPARγ-targeted therapeutics, we must focus on strategies that retain the ‘good’ potent insulin-sensitizing effects of current PPARγ-specific drugs and simultaneously reduce or eliminate the ‘bad’ associated side effects. Recent findings have provided insight into the mechanisms underlying the insulin-sensitizing effects of PPARγ as well as its adverse effects, suggesting new approaches to drug design. As discussed here, these new strategies will probably involve changing the way we screen for compounds, focusing on downstream effectors of PPARγ-mediated insulin sensitization, targeting specific post-translational modifications of PPARγ and selectively agonizing or antagonizing PPARγ in specific tissues. A combination of these approaches will aid in the development of safer yet highly efficacious molecules and provide hope for a promising future of PPARγ-targeted therapeutics.
Acknowledgments
We thank R. Yu, C.D. De Magalhaes Filho and S. Sarshar for useful discussions and L. Ong and C. Brondos for administrative assistance. R.M.E. is an Investigator of the Howard Hughes Medical Institute at the Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by US National Institutes of Health grants to R.M.E. (DK057978, DK090962, HL088093, HL105278 and ES010337), the Glenn Foundation for Medical Research, the Leona M. and Harry B. Helmsley Charitable Trust, Ipsen/Biomeasure, The Ellison Medical Foundation and the Howard Hughes Medical Institute. C.L. and M.D. are funded by grants from the National Health and Medical Research Council of Australia Project Grants 512354, 632886 and 1043199. M.A. is supported by an F32 Ruth L. Kirschstein National Research Service Award (National Institute of Diabetes and Digestive and Kidney Diseases). N.H. is supported by the Pioneer Fund. We apologize for those references we could not include due to space limitations.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–361. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 2.Barish GD, Narkar VA, Evans RM. PPARδ: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poulsen L, Siersbaek M, Mandrup S. PPARs: fatty acid sensors controlling metabolism. Semin Cell Dev Biol. 2012;23:631–639. doi: 10.1016/j.semcdb.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Forman BM, et al. 15-Deoxy-δ 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPARγ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 5.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forman BM, Chen J, Evans RM. The peroxisome proliferator-activated receptors: ligands and activators. Ann NY Acad Sci. 1996;804:266–275. doi: 10.1111/j.1749-6632.1996.tb18621.x. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- 8.Medina-Gomez G, et al. PPARγ2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007;3:e64. doi: 10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saraf N, Sharma PK, Mondal SC, Garg VK, Singh AK. Role of PPARγ2 transcription factor in thiazolidinedione-induced insulin sensitization. J Pharm Pharmacol. 2012;64:161–171. doi: 10.1111/j.2042-7158.2011.01366.x. [DOI] [PubMed] [Google Scholar]
- 10.Vacca M, Degirolamo C, Mariani-Costantini R, Palasciano G, Moschetta A. Lipid-sensing nuclear receptors in the pathophysiology and treatment of the metabolic syndrome. Wiley Interdiscip Rev Syst Biol Med. 2011;3:562–587. doi: 10.1002/wsbm.137. [DOI] [PubMed] [Google Scholar]
- 11.Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- 12.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 13.Graham DJ, et al. Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. J Am Med Assoc. 2010;304:411–418. doi: 10.1001/jama.2010.920. [DOI] [PubMed] [Google Scholar]
- 14.European Medicines Agency. European Medicines Agency recommends suspension of Avandia, Avandamet and Avaglim. 2010 〈 http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/09/news_detail_001119.jsp〉.
- 15.Wilcox R, Kupfer S, Erdmann E. Effects of pioglitazone on major adverse cardiovascular events in high-risk patients with type 2 diabetes: results from PROspective pioglitAzone Clinical Trial In macro Vascular Events (PROactive 10) Am Heart J. 2008;155:712–717. doi: 10.1016/j.ahj.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 16.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. J Am Med Assoc. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 17.Azoulay L, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case-control study. Br Med J. 2012;344:e3645. doi: 10.1136/bmj.e3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann A, et al. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55:1953–1962. doi: 10.1007/s00125-012-2538-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi JH, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. doi: 10.1038/nature10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan KK, et al. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu M, et al. Brain PPAR-γ promotes obesity and is required for the insulin-sensitizing effect of thiazolidinediones. Nat Med. 2011;17:618–622. doi: 10.1038/nm.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonker JW, et al. A PPARγ-FGF1 axis is required for adaptive adipose remodelling and metabolic homeostasis. Nature. 2012;485:391–394. doi: 10.1038/nature10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutchak PA, et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;148:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 25.Brun RP, et al. Differential activation of adipogenesis by multiple PPAR isoforms. Genes Dev. 1996;10:974–984. doi: 10.1101/gad.10.8.974. [DOI] [PubMed] [Google Scholar]
- 26.Barak Y, et al. PPARγ is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- 27.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 2011;14:116–122. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai T, et al. Peroxisome proliferator-activated receptor γ is required in mature white and brown adipocytes for their survival in the mouse. Proc Natl Acad Sci USA. 2004;101:4543–4547. doi: 10.1073/pnas.0400356101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He W, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717. doi: 10.1073/pnas.2536828100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollenberg AN, et al. Functional antagonism between CCAAT/enhancer binding protein-α and peroxisome proliferator-activated receptor-γ on the leptin promoter. J Biol Chem. 1997;272:5283–5290. doi: 10.1074/jbc.272.8.5283. [DOI] [PubMed] [Google Scholar]
- 32.Iwaki M, et al. Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52:1655–1663. doi: 10.2337/diabetes.52.7.1655. [DOI] [PubMed] [Google Scholar]
- 33.Hofmann C, et al. Altered gene expression for tumor necrosis factor-α and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134:264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- 34.Tomaru T, Steger DJ, Lefterova MI, Schupp M, Lazar MA. Adipocyte-specific expression of murine resistin is mediated by synergism between peroxisome proliferator-activated receptor γ and CCAAT/enhancer-binding proteins. J Biol Chem. 2009;284:6116–6125. doi: 10.1074/jbc.M808407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-γ gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- 36.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- 37.Savage DB, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-γ. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- 38.Lehmann JM, et al. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor γ (PPARγ) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 39.Sun K, Scherer PE. The PPARγ-FGF1 axis: an unexpected mediator of adipose tissue homeostasis. Cell Res. 2012;22:1416–1418. doi: 10.1038/cr.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kliewer SA, Mangelsdorf DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S–257S. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller DL, Ortega S, Bashayan O, Basch R, Basilico C. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20:2260–2268. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei W, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci USA. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Way JM, et al. Comprehensive messenger ribonucleic acid profiling reveals that peroxisome proliferator-activated receptor γ activation has coordinate effects on gene expression in multiple insulin-sensitive tissues. Endocrinology. 2001;142:1269–1277. doi: 10.1210/endo.142.3.8037. [DOI] [PubMed] [Google Scholar]
- 44.Ahmadian M, Duncan RE, Sul HS. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol Metab. 2009;20:424–428. doi: 10.1016/j.tem.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugii S, et al. PPARγ activation in adipocytes is sufficient for systemic insulin sensitization. Proc Natl Acad Sci USA. 2009;106:22504–22509. doi: 10.1073/pnas.0912487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burant CF, et al. Troglitazone action is independent of adipose tissue. J Clin Invest. 1997;100:2900–2908. doi: 10.1172/JCI119839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JK, et al. Differential effects of rosiglitazone on skeletal muscle and liver insulin resistance in A-ZIP/F-1 fatless mice. Diabetes. 2003;52:1311–1318. doi: 10.2337/diabetes.52.6.1311. [DOI] [PubMed] [Google Scholar]
- 48.Hevener AL, et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med. 2003;9:1491–1497. doi: 10.1038/nm956. [DOI] [PubMed] [Google Scholar]
- 49.Norris AW, et al. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nan YM, et al. Rosiglitazone prevents nutritional fibrosis and steatohepatitis in mice. Scand J Gastroenterol. 2009;44:358–365. doi: 10.1080/00365520802530861. [DOI] [PubMed] [Google Scholar]
- 51.Mayerson AB, et al. The effects of rosiglitazone on insulin sensitivity, lipolysis, and hepatic and skeletal muscle triglyceride content in patients with type 2 diabetes. Diabetes. 2002;51:797–802. doi: 10.2337/diabetes.51.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia. 2012;55:885–904. doi: 10.1007/s00125-011-2446-4. [DOI] [PubMed] [Google Scholar]
- 53.Kallwitz ER, McLachlan A, Cotler SJ. Role of peroxisome proliferators-activated receptors in the pathogenesis and treatment of nonalcoholic fatty liver disease. World J Gastroenterol. 2008;14:22–28. doi: 10.3748/wjg.14.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lutchman G, et al. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424–429. doi: 10.1002/hep.21661. [DOI] [PubMed] [Google Scholar]
- 55.Gavrilova O, et al. Liver peroxisome proliferator-activated receptor γ contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 56.Berkowitz K, et al. Effect of troglitazone on insulin sensitivity and pancreatic beta-cell function in women at high risk for NIDDM. Diabetes. 1996;45:1572–1579. doi: 10.2337/diab.45.11.1572. [DOI] [PubMed] [Google Scholar]
- 57.Higa M, et al. Troglitazone prevents mitochondrial alterations, beta cell destruction, and diabetes in obese prediabetic rats. Proc Natl Acad Sci USA. 1999;96:11513–11518. doi: 10.1073/pnas.96.20.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim HI, et al. Peroxisomal proliferator-activated receptor-γ upregulates glucokinase gene expression in beta-cells. Diabetes. 2002;51:676–685. doi: 10.2337/diabetes.51.3.676. [DOI] [PubMed] [Google Scholar]
- 59.Kim HI, et al. Identification and functional characterization of the peroxisomal proliferator response element in rat GLUT2 promoter. Diabetes. 2000;49:1517–1524. doi: 10.2337/diabetes.49.9.1517. [DOI] [PubMed] [Google Scholar]
- 60.Rosen ED, et al. Targeted elimination of peroxisome proliferator-activated receptor γ in beta cells leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol. 2003;23:7222–7229. doi: 10.1128/MCB.23.20.7222-7229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gupta D, et al. In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor γ response element in the mouse pdx-1 promoter. J Biol Chem. 2008;283:32462–32470. doi: 10.1074/jbc.M801813200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Szatmari I, Rajnavolgyi E, Nagy L. PPARγ, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann NY Acad Sci. 2006;1088:207–218. doi: 10.1196/annals.1366.013. [DOI] [PubMed] [Google Scholar]
- 63.Széles L, Torocsik D, Nagy L. PPARγ in immunity and inflammation: cell types and diseases. Biochim Biophys Acta. 2007;1771:1014–1030. doi: 10.1016/j.bbalip.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Szanto A, et al. STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ-regulated gene expression in macrophages and dendritic cells. Immunity. 2010;33:699–712. doi: 10.1016/j.immuni.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. 5PPARγ promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 66.Wahli W, Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends Endocrinol Metab. 2012;23:351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 67.Lee CH, Evans RM. Peroxisome proliferator-activated receptor-γ in macrophage lipid homeostasis. Trends Endocrinol Metab. 2002;13:331–335. doi: 10.1016/s1043-2760(02)00668-9. [DOI] [PubMed] [Google Scholar]
- 68.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARγ. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 69.Chawla A, et al. PPAR-γ dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 70.Hevener AL, et al. Macrophage PPARγ is required for normal skeletal muscle and hepatic insulin sensitivity and full antidiabetic effects of thiazolidinediones. J Clin Invest. 2007;117:1658–1669. doi: 10.1172/JCI31561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Odegaard JI, et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sugii S, Evans RM. Epigenetic codes of PPARγ in metabolic disease. FEBS Lett. 2011;585:2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cipolletta D, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–543. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cariou B, Charbonnel B, Staels B. Thiazolidinediones and PPARγ agonists: time for a reassessment. Trends Endocrinol Metab. 2012;23:205–215. doi: 10.1016/j.tem.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 75.Larsen PJ, et al. Differential influences of peroxisome proliferator-activated receptors γ and -α on food intake and energy homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- 76.Cecil JE, Watt P, Palmer CN, Hetherington M. Energy balance and food intake: the role of PPARγ gene polymorphisms. Physiol Behav. 2006;88:227–233. doi: 10.1016/j.physbeh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 77.Sarruf DA, et al. Expression of peroxisome proliferator-activated receptor-γ in key neuronal subsets regulating glucose metabolism and energy homeostasis. Endocrinology. 2009;150:707–712. doi: 10.1210/en.2008-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen YC, et al. Peroxisome proliferator-activated receptor γ (PPAR-γ) and neurodegenerative disorders. Mol Neurobiol. 2012;46:114–124. doi: 10.1007/s12035-012-8259-8. [DOI] [PubMed] [Google Scholar]
- 79.Sarafidis PA, Georgianos PI, Lasaridis AN. PPAR-γ agonism for cardiovascular and renal protection. Cardiovasc Ther. 2011;29:377–384. doi: 10.1111/j.1755-5922.2010.00222.x. [DOI] [PubMed] [Google Scholar]
- 80.Zhang H, et al. Collecting duct-specific deletion of peroxisome proliferator-activated receptor γ blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA. 2005;102:9406–9411. doi: 10.1073/pnas.0501744102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuba K, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borsting E, Cheng VP, Glass CK, Vallon V, Cunard R. Peroxisome proliferator-activated receptor-γ agonists repress epithelial sodium channel expression in the kidney. Am J Physiol Renal Physiol. 2012;302:F540–F551. doi: 10.1152/ajprenal.00306.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guan Y, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 84.Marfella R, et al. Thiazolidinediones may contribute to the intramyocardial lipid accumulation in diabetic myocardium: effects on cardiac function. Heart. 2009;95:1020–1022. doi: 10.1136/hrt.2009.165969. [DOI] [PubMed] [Google Scholar]
- 85.Son NH, et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-γ both induce cardiac hypertrophy in mice. Circ Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 87.Wei W, Wan Y. Thiazolidinediones on PPARγ: the roles in bone remodeling. PPAR Res. 2011;2011:867180. doi: 10.1155/2011/867180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Akune T, et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J Clin Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wan Y, Chong LW, Evans RM. PPAR-γ regulates osteoclastogenesis in mice. Nat Med. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- 90.Qiang L, et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150:620–632. doi: 10.1016/j.cell.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Beekum O, Fleskens V, Kalkhoven E. Posttranslational modifications of PPAR-γ: fine-tuning the metabolic master regulator. Obesity (Silver Spring) 2009;17:213–219. doi: 10.1038/oby.2008.473. [DOI] [PubMed] [Google Scholar]
- 92.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARγ. Science. 1996;274:2100–2103. doi: 10.1126/science.274.5295.2100. [DOI] [PubMed] [Google Scholar]
- 93.Camp HS, Tafuri SR. Regulation of peroxisome proliferator-activated receptor γ activity by mitogen-activated protein kinase. J Biol Chem. 1997;272:10811–10816. doi: 10.1074/jbc.272.16.10811. [DOI] [PubMed] [Google Scholar]
- 94.Zhang B, et al. Insulin- and mitogen-activated protein kinase–mediated phosphorylation and activation of peroxisome proliferator-activated receptor γ. J Biol Chem. 1996;271:31771–31774. doi: 10.1074/jbc.271.50.31771. [DOI] [PubMed] [Google Scholar]
- 95.Iankova I, et al. Peroxisome proliferator-activated receptor γ recruits the positive transcription elongation factor b complex to activate transcription and promote adipogenesis. Mol Endocrinol. 2006;20:1494–1505. doi: 10.1210/me.2005-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Compe E, et al. Dysregulation of the peroxisome proliferator-activated receptor target genes by XPD mutations. Mol Cell Biol. 2005;25:6065–6076. doi: 10.1128/MCB.25.14.6065-6076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ristow M, Muller-Wieland D, Pfeiffer A, Krone W, Kahn CR. Obesity associated with a mutation in a genetic regulator of adipocyte differentiation. N Engl J Med. 1998;339:953–959. doi: 10.1056/NEJM199810013391403. [DOI] [PubMed] [Google Scholar]
- 98.Rangwala SM, et al. Genetic modulation of PPARγ phosphorylation regulates insulin sensitivity. Dev Cell. 2003;5:657–663. doi: 10.1016/s1534-5807(03)00274-0. [DOI] [PubMed] [Google Scholar]
- 99.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- 100.Utreras E, et al. Tumor necrosis factor-α regulates cyclin-dependent kinase 5 activity during pain signaling through transcriptional activation of p35. J Biol Chem. 2009;284:2275–2284. doi: 10.1074/jbc.M805052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi JH, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466:451–456. doi: 10.1038/nature09291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petrovic N, et al. Chronic peroxisome proliferator-activated receptor γ (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 104.Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11:268–272. doi: 10.1016/j.cmet.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 105.Nedergaard J, Petrovic N, Lindgren EM, Jacobsson A, Cannon B. PPARγ in the control of brown adipocyte differentiation. Biochim Biophys Acta. 2005;1740:293–304. doi: 10.1016/j.bbadis.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 106.Ohno H, Shinoda K, Spiegelman BM, Kajimura S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 2012;15:395–404. doi: 10.1016/j.cmet.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harmon GS, Lam MT, Glass CK. PPARs and lipid ligands in inflammation and metabolism. Chem Rev. 2011;111:6321–6340. doi: 10.1021/cr2001355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shimizu M, Yamashita D, Yamaguchi T, Hirose F, Osumi T. Aspects of the regulatory mechanisms of PPAR functions: analysis of a bidirectional response element and regulation by sumoylation. Mol Cell Biochem. 2006;286:33–42. doi: 10.1007/s11010-005-9052-z. [DOI] [PubMed] [Google Scholar]
- 109.Pascual G, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hauser S, et al. Degradation of the peroxisome proliferator-activated receptor γ is linked to ligand-dependent activation. J Biol Chem. 2000;275:18527–18533. doi: 10.1074/jbc.M001297200. [DOI] [PubMed] [Google Scholar]
- 111.Waite KJ, Floyd ZE, Arbour-Reily P, Stephens JM. Interferon-γ–induced regulation of peroxisome proliferator-activated receptor γ and STATs in adipocytes. J Biol Chem. 2001;276:7062–7068. doi: 10.1074/jbc.M007894200. [DOI] [PubMed] [Google Scholar]
- 112.Lewis JS, Jordan VC. Selective estrogen receptor modulators (SERMs): mechanisms of anticarcinogenesis and drug resistance. Mutat Res. 2005;591:247–263. doi: 10.1016/j.mrfmmm.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 113.Rangwala SM, Lazar MA. The dawn of the SPPARMs? Sci STKE. 2002;2002:pe9. doi: 10.1126/stke.2002.121.pe9. [DOI] [PubMed] [Google Scholar]
- 114.Hughes TS, et al. Ligand and receptor dynamics contribute to the mechanism of graded PPARγ agonism. Structure. 2012;20:139–150. doi: 10.1016/j.str.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chandra V, et al. Structure of the intact PPAR-γ–RXR–nuclear receptor complex on DNA. Nature. 2008;456:350–356. doi: 10.1038/nature07413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Higgins LS, Depaoli AM. Selective peroxisome proliferator-activated receptor γ (PPARγ) modulation as a strategy for safer therapeutic PPARγ activation. Am J Clin Nutr. 2010;91:267S–272S. doi: 10.3945/ajcn.2009.28449E. [DOI] [PubMed] [Google Scholar]
- 117.Tavera-Mendoza LE, et al. Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. Proc Natl Acad Sci USA. 2008;105:8250–8255. doi: 10.1073/pnas.0709279105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Finan B, et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat Med. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chappuis B, et al. Differential effect of pioglitazone (PGZ) and rosiglitazone (RGZ) on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab Res Rev. 2007;23:392–399. doi: 10.1002/dmrr.715. [DOI] [PubMed] [Google Scholar]
- 120.Deeg MA, et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2007;30:2458–2464. doi: 10.2337/dc06-1903. [DOI] [PubMed] [Google Scholar]
- 121.Goldberg RB, et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care. 2005;28:1547–1554. doi: 10.2337/diacare.28.7.1547. [DOI] [PubMed] [Google Scholar]
- 122.Sakamoto J, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 123.Rosenson RS, Wright RS, Farkouh M, Plutzky J. Modulating peroxisome proliferator-activated receptors for therapeutic benefit? Biology, clinical experience, and future prospects. Am Heart J. 2012;164:672–680. doi: 10.1016/j.ahj.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rabøl R, et al. Opposite effects of pioglitazone and rosiglitazone on mitochondrial respiration in skeletal muscle of patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:806–814. doi: 10.1111/j.1463-1326.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 125.Balakumar P, Rose M, Ganti SS, Krishan P, Singh M. PPAR dual agonists: are they opening Pandora’s Box? Pharmacol Res. 2007;56:91–98. doi: 10.1016/j.phrs.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 126.Mukherjee R, et al. Sensitization of diabetic and obese mice to insulin by retinoid X receptor agonists. Nature. 1997;386:407–410. doi: 10.1038/386407a0. [DOI] [PubMed] [Google Scholar]
- 127.Sugawara A, et al. Characterization of mouse retinoid X receptor (RXR)-β gene promoter: negative regulation by tumor necrosis factor (TNF)-α. Endocrinology. 1998;139:3030–3033. doi: 10.1210/endo.139.6.6130. [DOI] [PubMed] [Google Scholar]
- 128.Singh AB, Guleria RS, Nizamutdinova IT, Baker KM, Pan J. High glucose-induced repression of RAR/RXR in cardiomyocytes is mediated through oxidative stress/JNK signaling. J Cell Physiol. 2012;227:2632–2644. doi: 10.1002/jcp.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lefebvre B, et al. Proteasomal degradation of retinoid X receptor α reprograms transcriptional activity of PPARγ in obese mice and humans. J Clin Invest. 2010;120:1454–1468. doi: 10.1172/JCI38606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zechner R, et al. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hardie DG. AMPK: a key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond) 2008;32 (suppl 4):S7–S12. doi: 10.1038/ijo.2008.116. [DOI] [PubMed] [Google Scholar]
- 133.Takada I, Kouzmenko AP, Kato S. PPAR-γ signaling crosstalk in mesenchymal stem cells. PPAR Res. 2010;2010:341671. doi: 10.1155/2010/341671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yu JG, et al. The effect of thiazolidinediones on plasma adiponectin levels in normal, obese, and type 2 diabetic subjects. Diabetes. 2002;51:2968–2974. doi: 10.2337/diabetes.51.10.2968. [DOI] [PubMed] [Google Scholar]
- 135.Edvardsson U, et al. Rosiglitazone (BRL49653), a PPARγ-selective agonist, causes peroxisome proliferator-like liver effects in obese mice. J Lipid Res. 1999;40:1177–1184. [PubMed] [Google Scholar]
- 136.Sanchez JC, et al. Effect of rosiglitazone on the differential expression of obesity and insulin resistance associated proteins in lep/lep mice. Proteomics. 2003;3:1500–1520. doi: 10.1002/pmic.200300484. [DOI] [PubMed] [Google Scholar]
- 137.Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nat Rev Genet. 2010;11:476–486. doi: 10.1038/nrg2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lefterova MI, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008;22:2941–2952. doi: 10.1101/gad.1709008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lefterova MI, et al. Cell-specific determinants of peroxisome proliferator-activated receptor γ function in adipocytes and macrophages. Mol Cell Biol. 2010;30:2078–2089. doi: 10.1128/MCB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siersbæk R, et al. Extensive chromatin remodelling and establishment of transcription factor ‘hotspots’ during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hamza MS, et al. De-novo identification of PPARγ/RXR binding sites and direct targets during adipogenesis. PLoS ONE. 2009;4:e4907. doi: 10.1371/journal.pone.0004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schmidt SF, et al. Cross species comparison of C/EBPα and PPARγ profiles in mouse and human adipocytes reveals interdependent retention of binding sites. BMC Genomics. 2011;12:152. doi: 10.1186/1471-2164-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schmidt SF, Jorgensen M, Sandelin A, Mandrup S. Cross-species ChIP- seq studies provide insights into regulatory strategies of PPARγ in adipocytes. Transcription. 2012;3:19–24. doi: 10.4161/trns.3.1.19302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sérandour AA, et al. Dynamic hydroxymethylation of deoxyribonucleic acid marks differentiation-associated enhancers. Nucleic Acids Res. 2012;40:8255–8265. doi: 10.1093/nar/gks595. [DOI] [PMC free article] [PubMed] [Google Scholar]