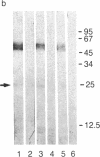
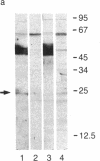
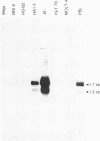
Abstract
CD2 (T11, sheep erythrocyte receptor) is a surface antigen of the human T-lymphocyte lineage. cDNA clones encoding CD2 have been isolated by using the purified, denatured CD2 to raise a rat antiserum. Positive clones were recognized in a phage lambda gt11 expression library prepared from the human leukemia T-cell line J6. The DNA sequence contained an open reading frame encoding 360 amino acids. The N-terminal 24 amino acids were characteristic of a signal peptide and were followed by a region that matched all 25 residues of the CD2 N terminus previously determined by amino acid sequencing. The predicted amino acid sequence is consistent with that of a transmembrane glycoprotein containing three potential N-glycosylation sites on the N-terminal side of a 26-amino acid hydrophobic segment. There is a large cytoplasmic domain of 125 amino acids that is rich in proline and in basic residues. RNA blot-hybridization analysis demonstrated hybridization only in those T cells that were positive for surface CD2 antigen. There are limited regions of sequence similarity to members of the immunoglobulin supergene family.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berget S. M. Are U4 small nuclear ribonucleoproteins involved in polyadenylation? Nature. 1984 May 10;309(5964):179–182. doi: 10.1038/309179a0. [DOI] [PubMed] [Google Scholar]
- Brottier P., Boumsell L., Gelin C., Bernard A. T cell activation via CD2 [T, gp50] molecules: accessory cells are required to trigger T cell activation via CD2-D66 plus CD2-9.6/T11(1) epitopes. J Immunol. 1985 Sep;135(3):1624–1631. [PubMed] [Google Scholar]
- Classon B. J., Tsagaratos J., McKenzie I. F., Walker I. D. Partial primary structure of the T4 antigens of mouse and sheep: assignment of intrachain disulfide bonds. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4499–4503. doi: 10.1073/pnas.83.12.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holter W., Fischer G. F., Majdic O., Stockinger H., Knapp W. T cell stimulation via the erythrocyte receptor. Synergism between monoclonal antibodies and phorbol myristate acetate without changes of free cytoplasmic Ca++ levels. J Exp Med. 1986 Mar 1;163(3):654–664. doi: 10.1084/jem.163.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Hünig T. R. The ligand of the erythrocyte receptor of T lymphocytes: expression on white blood cells and possible involvement in T cell activation. J Immunol. 1986 Mar 15;136(6):2103–2108. [PubMed] [Google Scholar]
- Hünig T. The cell surface molecule recognized by the erythrocyte receptor of T lymphocytes. Identification and partial characterization using a monoclonal antibody. J Exp Med. 1985 Sep 1;162(3):890–901. doi: 10.1084/jem.162.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Gagnon J., Barclay A. N., Williams A. F. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985 Oct;4(10):2539–2545. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Krissansen G. W., Owen M. J., Verbi W., Crumpton M. J. Primary structure of the T3 gamma subunit of the T3/T cell antigen receptor complex deduced from cDNA sequences: evolution of the T3 gamma and delta subunits. EMBO J. 1986 Aug;5(8):1799–1808. doi: 10.1002/j.1460-2075.1986.tb04429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Larsson E. L., Andersson J., Coutinho A. Functional consequences of sheep red blood cell rosetting for human T cells: gain of reactivity to mitogenic factors. Eur J Immunol. 1978 Oct;8(10):693–696. doi: 10.1002/eji.1830081005. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Littman D. R., Godfrey M., Maddon D. E., Chess L., Axel R. The isolation and nucleotide sequence of a cDNA encoding the T cell surface protein T4: a new member of the immunoglobulin gene family. Cell. 1985 Aug;42(1):93–104. doi: 10.1016/s0092-8674(85)80105-7. [DOI] [PubMed] [Google Scholar]
- Martin P. J., Longton G., Ledbetter J. A., Newman W., Braun M. P., Beatty P. G., Hansen J. A. Identification and functional characterization of two distinct epitopes on the human T cell surface protein Tp50. J Immunol. 1983 Jul;131(1):180–185. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Milanese C., Richardson N. E., Reinherz E. L. Identification of a T helper cell-derived lymphokine that activates resting T lymphocytes. Science. 1986 Mar 7;231(4742):1118–1122. doi: 10.1126/science.2935936. [DOI] [PubMed] [Google Scholar]
- Palacios R., Martinez-Maza O. Is the E receptor on human T lymphocytes a "negative signal receptor"? J Immunol. 1982 Dec;129(6):2479–2485. [PubMed] [Google Scholar]
- Reed J. C., Tadmori W., Kamoun M., Koretzky G., Nowell P. C. Suppression of interleukin 2 receptor acquisition by monoclonal antibodies recognizing the 50 KD protein associated with the sheep erythrocyte receptor on human T lymphocytes. J Immunol. 1985 Mar;134(3):1631–1639. [PubMed] [Google Scholar]
- Robb R. J., Terhorst C., Strominger J. L. Sequence of the COOH-terminal hydrophilic region of histocompatibility antigens HLA-A2 and HLA-B7. J Biol Chem. 1978 Aug 10;253(15):5319–5324. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. R., Thornton J. M. Recognition of super-secondary structure in proteins. J Mol Biol. 1984 Mar 15;173(4):487–512. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J., Goossens J., Decock W., Kung P., Goldstein G. Suppression of human T-cell mitogenesis and E-rosette formation by the monoclonal antibody OKT11A. Immunology. 1981 Dec;44(4):865–871. [PMC free article] [PubMed] [Google Scholar]
- Verbi W., Greaves M. F., Schneider C., Koubek K., Janossy G., Stein H., Kung P., Goldstein G. Monoclonal antibodies OKT 11 and OKT 11A have pan-T reactivity and block sheep erythrocyte "receptors". Eur J Immunol. 1982 Jan;12(1):81–86. doi: 10.1002/eji.1830120115. [DOI] [PubMed] [Google Scholar]
- Wilkinson M., Morris A. The E receptor regulates interferon-gamma production: four-receptor model for human lymphocyte activation. Eur J Immunol. 1984 Aug;14(8):708–713. doi: 10.1002/eji.1830140807. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]