Abstract
“Cognitive unbinding” refers to the impaired synthesis of specialized cognitive activities in the brain and has been proposed as a mechanistic paradigm of unconsciousness. This article draws on recent neuroscientific data to revisit the tenets and predictions of cognitive unbinding, using general anesthesia as a representative state of unconsciousness. Current evidence from neuroimaging and neurophysiology supports the proposition that cognitive unbinding is a parsimonious explanation for the direct mechanism (or “proximate cause”) of anesthetic-induced unconsciousness across multiple drug classes. The relevance of cognitive unbinding to sleep, disorders of consciousness, and psychological processes is also explored. It is concluded that cognitive unbinding is a viable neuroscientific framework for unconscious processes across the fields of anesthesiology, sleep neurobiology, neurology and psychoanalysis.
Keywords: consciousness, unconsciousness, cognitive binding, cognitive unbinding, information integration, anesthesia, vegetative state, sleep, psychoanalysis
Unconsciousness is a fundamental neural state that can be achieved through the pathways of physiology (e.g., sleep), pharmacology (e.g., general anesthesia), and pathology (e.g., seizure). Within the pharmacological pathway of general anesthesia, there are multiple classes of drugs with diverse molecular and neurophysiological mechanisms that can suppress consciousness of the environment in a characteristic way (Alkire et al., 2008). As such, understanding a common neural principle that explains the actions of multiple general anesthetics could provide a foundation for a more general theory of unconsciousness.
The mechanism of anesthetic-induced unconsciousness has persisted as a scientific problem since the first public demonstration of surgical anesthesia in 1846 (Bigelow, 1846) and the first attempt at a unifying theory in 1847 (von Bibra and Harless, 1847). Studies of anesthetic mechanism have been conducted using a variety of approaches at the biophysical, molecular, neuroanatomical, systems neuroscience and information processing scales. In the past 165 years, numerous theories have been proposed either to explain a common mechanism across all anesthetic drugs or to account for the effects of individual anesthetics (Perouansky, 2012). Approximately 10 years ago, I proposed the “cognitive unbinding paradigm” as a framework for anesthetic-induced unconsciousness (Mashour, 2004) and related states (Mashour, 2006, 2008). “Cognitive unbinding” refers to impaired synthesis of functionally specialized cognitive modules in the brain, which is posited to interrupt conscious representation. Given the relative paucity of data at that time regarding brain connectivity and communication during general anesthesia, the initial description of the paradigm was speculative in nature. In the past decade, however, extensive investigation of general anesthesia using neuroimaging and neurophysiology has been conducted. In this article, I will (1) reframe terminology related to “mechanisms” of unconsciousness as “root” and “proximate” causes, (2) review the basic tenets and predictions of the cognitive unbinding paradigm, (3) distinguish theories related to cognitive binding and information integration, (4) evaluate current scientific evidence that supports cognitive unbinding as a general theory of anesthetic-induced unconsciousness, (5) argue that cognitive unbinding is a viable framework for other unconscious states and processes, (6) describe the scientific and clinical impact of the theory, then (7–8) discuss limitations, future directions, and conclusions.
1. Reconciling unity and diversity
The goal of the cognitive unbinding paradigm is to create a framework that shows how a diversity of physiological, pharmacological, and pathological events converge upon a final common pathway of unconsciousness. One obstacle to achieving this goal is the problematic term “mechanism.” Using an example from general anesthesia, one investigator could claim that the mechanism of unconsciousness induced by propofol (a commonly used intravenous anesthetic) is agonism of the gamma-aminobutyric acid (GABA) type A receptor. Indeed, a mutation of the β 3 subunit of that receptor can attenuate (but not ablate) the hypnotic properties of propofol (Jurd et al., 2003). The claim is therefore evidence-based, but it is not entirely clear how this information explains the loss of consciousness. This is especially true given the regional differences in GABA function, with higher levels of GABA in the pontine reticular formation correlating with wakefulness rather than unconsciousness (Vanini and Baghdoyan, 2013; Vanini et al., 2008). In contrast, another investigator could claim that the mechanism of propofol-induced unconsciousness is inhibition of top-down processing from the prefrontal cortex. Although this neurophysiological phenomenon has been demonstrated (Boly et al., 2012) and is, in principle, closely related to the neurobiology of consciousness, the explanation would also be unsatisfying as a “mechanism” because it appears phenomenological and insensitive to the underlying molecular events. Furthermore, given multiple scales of research, it is unclear how to ascribe weight or value to various mechanistic levels. Which, using the current example, is more mechanistically relevant: GABAA agonism or disruption of corticocortical connectivity?
In order to avoid this confusion, I would argue that this neuroscientific problem is better addressed by referring to root and proximate causes. A root cause is something that initiates a causal chain of events, whereas a proximate cause is that which more directly results in the observed effect. Continuing with the example of propofol, the root cause of unconsciousness could be the binding to the β 3 subunit of the GABAA receptor, while the proximate cause could be impaired corticocortical connectivity, with mediating steps at the systems neuroscience level. In the context of anesthesiology, cognitive unbinding would be proposed as the proximate cause for the functional outcome of anesthetic-induced unconsciousness. This mechanistic framework of root and proximate causation suggests activity at different scales, from molecular targets to network dynamics. A complete explanation of consciousness and unconsciousness will require an understanding of the details by which root causes are translated across scales to proximate causes.
By distinguishing these types of causes, one can assert a single proximate cause while embracing a diversity of root causes (e.g., differing molecular targets). This reconciles unity and diversity, creating an approach that can achieve coherence without sacrificing nuance. Thus, the cognitive unbinding paradigm does not discount the details of causal pathways leading to anesthetic effects, but rather argues that there is a common proximate cause on which these individual pathways converge. Such an approach also allows cognitive unbinding to be explored as a proximate cause for other states of unconsciousness, despite the fact that they may be induced physiologically (e.g., sleep) or pathologically (e.g., brain injury) rather than pharmacologically.
2. Basic tenets of the cognitive unbinding paradigm
The essential tenet of the cognitive unbinding paradigm is that disrupting the synthesis of neural information is sufficient to cause unconsciousness. This implies that cognitive processing can persist in an anesthetized or other unconscious state while the binding of this activity into a meaningful conscious representation is inhibited. This basic principle of cognitive unbinding was originally derived from theoretical and empirical arguments regarding the importance of cognitive binding in the generation of consciousness (Singer, 2001). It is important to note that the “consciousness” of interest in this context is external consciousness (i.e., consciousness of an environmental event such as surgery). Internal consciousness (e.g., dreaming) will not be addressed, since it is of considerably less relevance to the anesthetized state. Although dreaming is sometimes reported after general anesthesia, there is a strong possibility that such reports are related to experiences during emergence from unconsciousness rather than to the anesthetized state itself.
Put simply, the brain undergoes a “division of labor” in processing environmental stimuli by breaking them down into sensory modalities (e.g., seeing, hearing) and submodalities (e.g., seeing color, hearing pitch), but our experience of the world is nonetheless unified. How does the brain construct a single experience out of diverse information processing streams? The question persists. When cognitive unbinding was originally described, there were two proposed strategies for binding considered to be of particular relevance to general anesthesia: binding by synchrony and binding by convergence (Mashour, 2004). Synchrony and convergence provide, respectively, temporal and spatial answers to the question of how the brain synthesizes neural processing. Binding by synchrony is thought to coordinate neural events in time, while amplifying brain responses to a stimulus (temporal dimension) (Engel and Singer, 2001); binding by convergence implies a higher order neural area or network upon which primary sensory processing converges for synthesis (spatial dimension). The two can clearly be inter-related, as convergent binding likely requires temporal binding as well.
These basic principles gave rise to predictions related to anesthetic-induced unconsciousness that, if demonstrated, would support the cognitive unbinding paradigm:
Neural activity can persist despite anesthetic-induced unconsciousness
Primary sensory networks/processing can persist despite anesthetic-induced unconsciousness
Inter- or cross-modal processing is disrupted in association with anesthetic-induced unconsciousness
Temporal coordination of neural activity is disrupted in association with anesthetic-induced unconsciousness
Areas, networks, or processes in the brain involved in information synthesis are disrupted in association with anesthetic-induced unconsciousness
As a general framework for the proximate cause of anesthetic-induced unconsciousness, we would also predict that anesthetics with diverse molecular or neurophysiological profiles would satisfy key aspects of these predictions.
3. Distinguishing theories related to binding and integration
The cognitive unbinding paradigm of anesthetic-induced unconsciousness is theoretically related to the integrated information theory of consciousness (Tononi 2004, 2008, 2012). Both theories propose that consciousness is lost during anesthesia (and sleep) due to impaired communication across brain networks and the consequent isolation of cognitive processing modules. Before addressing a key difference, it is first important to note that the potential involvement of “synchrony” in cognitive binding is not a distinguishing feature between the theories. There has been a tacit assumption that cognitive binding is somehow equivalent to synchrony, but this assumption is not justified. Although some have focused on temporal dimensions of the process (Engel and Singer, 2001), synchrony is not intrinsic to the concept of cognitive binding. Accordingly, initial descriptions of the cognitive unbinding paradigm stated explicitly that other mechanisms of binding are not only possible, but likely (Mashour, 2004, 2006). Thus, it is incorrect to associate binding exclusively with temporal covariation (e.g., measured by functional connectivity, coherence or phase synchrony) and to associate information integration with communication and causal influence (e.g., measured by directional or effective connectivity).
The cognitive unbinding paradigm predicts that it is the isolation rather than the extinction of neural activity or sensory processing that is sufficient for anesthetic- or sleep-induced unconsciousness. The integrated information theory is proposed as a comprehensive explanation for both the levels of consciousness (e.g., alert vs. anesthetized) and contents of consciousness (e.g., seeing a red circle vs. a blue triangle). Although integrated information theory is much richer in detail and far wider in scope, it rests on more tenuous epistemological grounds. The cognitive unbinding paradigm makes an assumption that the integration of information is necessary for normal consciousness, a relatively uncontroversial proposition. If cognitive binding is necessary, then the unbinding of neural information would therefore be sufficient for anesthetic- or sleep-induced unconsciousness. The integrated information theory makes a far more radical proposition, namely, that consciousness is integrated information. As such, the theory moves beyond necessity and sufficiency to an identity relationship between consciousness and integrated information. The accompanying evidentiary burden is therefore severe; currently, only a correlation between surrogate measures of integration and levels of consciousness can be supported. Thus, investigators basing theories of physiological, pharmacological, or pathological states of unconsciousness on integrated information theory should be cognizant of the fact that they are also subscribing to an identity relationship between consciousness and integrated information that is untested and potentially untestable. In summary, the cognitive unbinding paradigm is not intrinsically linked to neural synchrony, is consistent with some predictions of integrated information theory, but depends only on the necessity of neural synthesis for consciousness rather than identity. Since it posits specific network disconnections rather than general network disruption, it is more readily supported by current neuroscientific evidence and does not need to explain the so-called “hard problem” of consciousness, i.e., how neural activity could result in subjectivity (Chalmers, 1995). Indeed, the cognitive unbinding paradigm does not attempt to account for which activities beyond the synthesis of neural information are required for phenomenal consciousness. This circumspection can be construed as a limitation (in terms of theoretical scope) or strength (in terms of theoretical defensibility), but is motivated by the fact that there is no phenomenological or scientific evidence to suggest that integrated information is sufficient for or identical to consciousness.
4. Evidence for cognitive unbinding during general anesthesia
The cognitive unbinding paradigm predicts persistent sensory processing but impaired network communication in association with anesthetic-induced unconsciousness. Evidence supporting these predictions can be found in a number of neuroimaging and neurophysiology studies conducted since the theory was first proposed.
4.1 Preserved primary sensory networks vs. Impaired higher-order networks (Evidence for predictions A & B)
Two studies of functional connectivity suggest that the functional architecture of sensory networks in the human brain is relatively unperturbed after unconsciousness induced by the inhaled anesthetic sevoflurane (Martuzzi et al., 2010) or the intravenous anesthetic propofol (Boveroux et al., 2010) (Figure 1). In contrast, both studies identified a reduction of functional connectivity in higher-order cognitive areas. Such higher-order areas included lateral frontal-parietal networks, which are thought to be important for the conscious experience of environmental stimuli (Baars et al., 2003). This finding is consistent with neurophysiological studies in animals (Imas et al., 2005) and humans (Boly et al., 2012; Ku et al., 2011) demonstrating that posterior-to-anterior processing (often associated with primary sensory processing) persists during general anesthesia, while top-down processing (often associated with conscious experience) is impaired. Indeed, long-latency sensory potentials (posited to reflect the integration of information) are depressed during anesthesia in a dose-dependent manner, while early potentials (posited to reflect primary sensory processing) are preserved (Hudetz et al., 2009). Collectively, these data support the cognitive unbinding paradigm by suggesting that primary sensory information may still be processed during general anesthesia, pointing to a higher-order synthesis as the process impaired by general anesthetics. Such findings during anesthetic-induced unconsciousness are consistent with the cognitive neuroscience of consciousness, in which conscious processing is thought to be mediated by longer-latency potentials in higher-order networks rather than early potentials in primary sensory cortices (Dehaene and Changeux, 2011; del Cul et al., 2007). Furthermore, it is not clear if integrated information theory would predict the specific breakdown of multisensory integration (as opposed to a breakdown of integration, per se) as the proximate cause of anesthetic-induced unconsciousness, whereas the theory of cognitive unbinding does.
Figure 1. Primary sensory networks are preserved after propofol-induced unconsciousness.
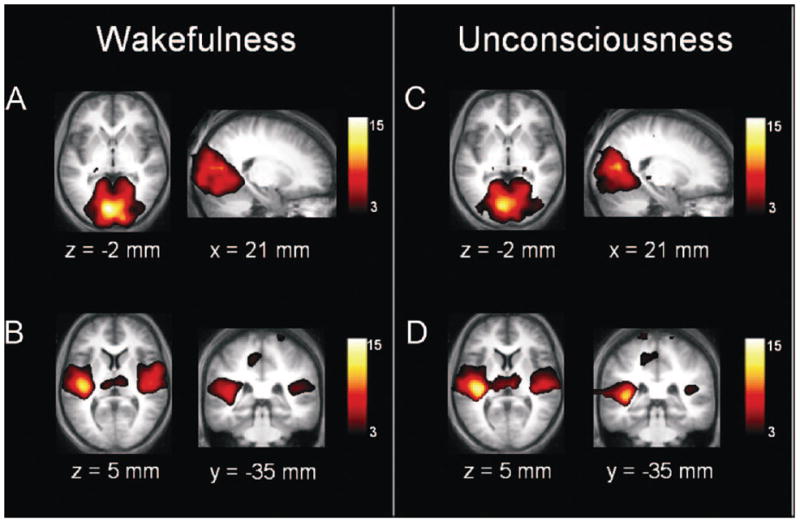
Data from functional magnetic resonance imaging was gathered in healthy volunteers exposed to propofol. Panels A and C show primary visual cortex, whereas panels B and D show primary auditory cortex, during consciousness and unconsciousness. Reproduced from Boveroux et al, 2010, with permission.
4.2 Impaired cross-modal communication and temporal coordination (Evidence for predictions C & D)
Although cognitive modules such as primary sensory cortex may be relatively maintained despite unconsciousness, cortical-cortical and cortical-subcortical connectivity are disrupted. Neuroimaging studies have demonstrated decreased functional integration and decreased cortical-subcortical connectivity after propofol-induced unconsciousness (Mhuircheartaigh et al., 2010; Schroter et al., 2012; Schrouff et al., 2011; White and Alkire, 2003). Of note, functional disconnections of the cortex from the thalamus preferentially affect “nonspecific” thalamic nuclei, which are thought to integrate cortical computations, as opposed to the sensory-related thalamic nuclei (Liu et al., 2013). A study using high-density electroencephalography and transcranial magnetic stimulation showed that induction of anesthesia with midazolam is associated with decreased effective connectivity across the cortex (Ferrarelli et al., 2010). Decreases in effective connectivity in this context imply the inhibition of corticocortical communication, i.e., a disruption of causal influences among different cortical regions.
A study of electrocorticography and single-unit recordings in humans undergoing induction of anesthesia with propofol is particularly illuminating with respect to how cortical communication may be interrupted during general anesthesia (Figure 2) (Lewis et al., 2012). Propofol-induced unconsciousness was correlated with a dramatic increase in the power of slow oscillations (0.1–1 Hz). Mean neuronal spike activity was markedly suppressed after loss of consciousness, but activity subsequently returned to baseline in some cases—and above baseline in others—despite the fact that the patients were still unconscious (direct evidence for prediction A). This is consistent with cognitive unbinding in that neuronal activity itself was not suppressed but rather temporally fragmented, with periods of high activity interrupted by quiescence. Neural spike activity became coupled to the phase of the slow oscillation at loss of consciousness, but the phase coupling of these slow oscillations across different areas of the cortex dropped significantly as the distance increased. Thus, spike activity was coupled to the slow oscillation but fragmented into “on” and “off” states, while the slow oscillations became uncoupled with increasing cortical distance. In this way, neuronal activity in one cortical area (spiking “on”) could be occurring while neuronal activity in another cortical area is suppressed (spiking “off”) due to the variable phase relationship of the slow oscillation. This represents a process of “temporal unbinding” (evidence for prediction D) that would (1) impair the effective communication required for consciousness and (2) dramatically decrease the probability of synthesizing diverse cognitive processes across the brain. It is important to note that these findings were derived from only three human patients who had diagnoses of medically-refractory epilepsy and only one anesthetic agent (propofol) was studied.
Figure 2. Schematic of temporal unbinding after propofol-induced unconsciousness.
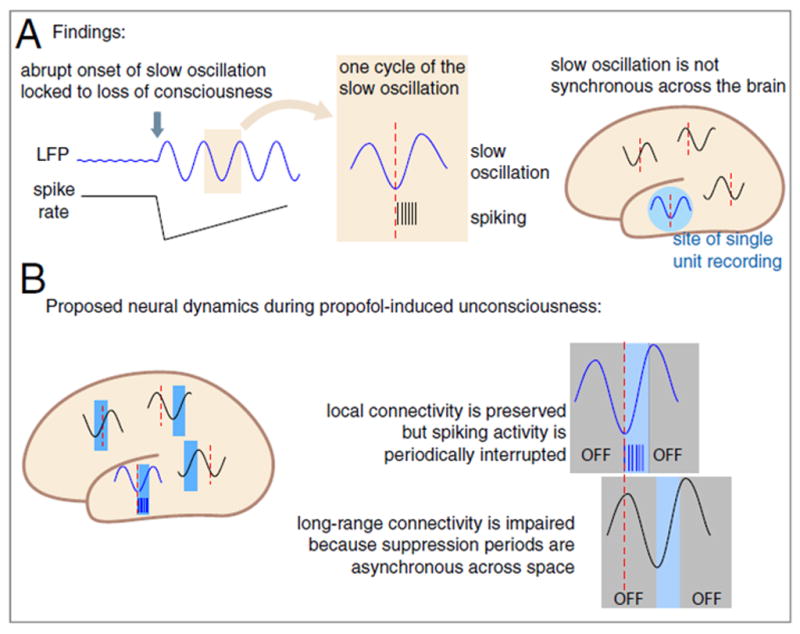
Data gathered from electrocorticography in human epilepsy patients shows temporal unbinding after administration of propofol, based on the relationship of single-unit activity and local field potentials. Reproduced from Lewis et al, 2012, with permission.
4.3 Disruption of neural areas and processes important for information synthesis (Evidence for prediction E)
The directionality of corticocortical communication after anesthetic-induced unconsciousness has also been investigated. Electroencephalography in humans has shown a preferential inhibition of “top-down” or feedback frontal-to-parietal connectivity during general anesthesia, as evidenced by the analytic techniques of evolution map approach (propofol) (Lee et al., 2009), symbolic transfer entropy (propofol and sevoflurane) (Ku et al., 2011), normalized symbolic transfer entropy (ketamine, propofol, and sevoflurane) (Lee et al., 2013a), and dynamic causal modeling (propofol) (Boly et al., 2012). It is notable that feedback connectivity from frontal to parietal regions was also identified for the intravenous anesthetic ketamine. Ketamine leads to distinct spectral features of the electroencephalogram, including an increase in high-frequency waveforms. The selective loss of feedback connectivity after ketamine administration has been cited as the first demonstration of disrupted corticocortical communication during general anesthesia in the setting of an activated cortex (Sleigh et al., 2013).
Graph-theoretical analysis of human electroencephalographic recordings and directed phase lag index have shown that propofol-induced unconsciousness is associated with a suppression of posterior parietal hub dominance, which may account for the appearance of selective feedback suppression (Lee et al, 2013b). That is, the posterior parietal region no longer serves as a highly-connected “sink” toward which information flows, therefore attenuating feedback from the prefrontal cortex. The consistent inhibition of the posterior parietal cortex by multiple and diverse anesthetics (Kuhlman et al., 2013; Lee et al., 2011) is of direct relevance to cognitive unbinding, since this brain region is thought to be a key point of convergence for visual feature binding (Baumgartner et al., 2013) as well as the integration of other diverse inputs.
The disruption of top-down processing and posterior parietal network connectivity is highly relevant to current theories of cognitive binding. A recent model proposes two key processes for the binding of an object’s features (O’Reilly et al., 2013; Wyatte et al., 2012a, 2012b). The first is neural inhibition, in which neurons most actively responding to a salient stimulus “out-compete” and inhibit neighboring neurons representing another object. The second is top-down feedback, in which the lower-level neurons that have “won” the representational competition are rewarded with further excitatory bias. This feedback/top-down processing (from prefrontal cortex or higher-order sensory processing areas) allows for more robust representation compared with the relatively weak and ambiguous feedforward/bottom-up processing. In a simulation of multiple-object feature binding, unbalanced feedforward processing resulted in markedly higher error rates of binding, while inhibition of feedback processing dramatically decreased the ratio of relevant to irrelevant neuronal responses (i.e., it reduced signal-to-noise ratios) (Figure 3). Given this framework, the finding that all major anesthetic classes preserve feedforward processing while inhibiting feedback processing in human brain networks strongly supports cognitive unbinding as the proximate cause for disruption of consciousness (Figure 4). At one extreme, a high percentage of binding errors and a significant suppression of relevant responses (i.e., signal/noise ratio) would extinguish consciousness, as is observed during general anesthesia. This is especially true given the likely reduction of neuronal contrast/competition, which would be accomplished by anesthetics causing either more consistent depression (e.g., propofol) or more consistent and disorganized activation (e.g., ketamine). Thus, a complete state of general anesthesia impairs both the bottom-up and top-down processes currently postulated to mediate cognitive binding. However, an intermediate disruption of these binding processes could result in illusory conjunctions or other sensory abnormalities in a conscious individual, which may provide a mechanism for post-anesthetic delirium.
Figure 3. Theoretical relevance of impaired feedback and preserved feedforward processing to cognitive binding.
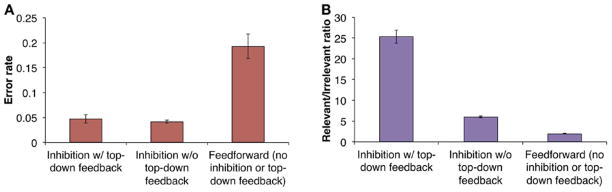
These modeling data demonstrate the effects of neural inhibition, feedback processing and feedforward processing on error rate and signal-to-noise ratio during cognitive binding of multiple objects. Importantly, feedforward processing alone results in the highest error rate and lowest signal-to-noise (relevant/irrelevant) ratio. Reproduced from Wyatte et al, 2012, with permission (open access journal).
Figure 4. Empirical demonstration of impaired feedback and preserved feedforward connectivity after anesthetic-induced unconsciousness.
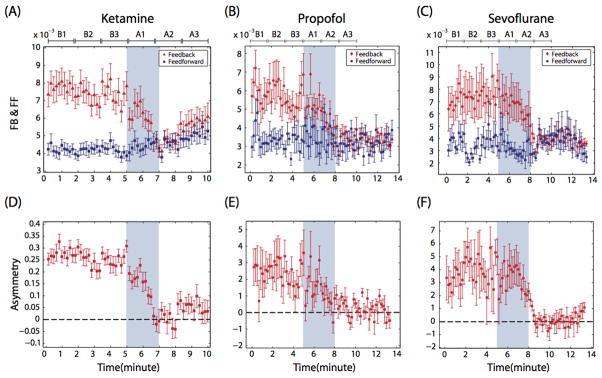
This figure demonstrates feedback and feedforward connectivity in frontal-parietal networks during and after anesthetic-induced unconsciousness in surgical patients (A–C). Lower panels (D–F) show asymmetry of directional connectivity, with positive values representing feedback dominance and negative values representing feedforward dominance. Connectivity was measured using electroencephalography and symbolic transfer entropy, which is rooted in information theory. Blue shaded area represents induction of anesthesia; the period before induction is baseline consciousness and the period after is anesthetic-induced unconsciousness. Each state is separated into three substates of Baseline (B1, B2, B3) and Anesthetized (A1, A2, A3) conditions; the timescale is different because patients receiving ketamine were studied using a different protocol than patients receiving propofol and sevoflurane. FB=Feedback, FF= Feedforward. Reproduced from Lee et al, 2013a, with permission.
4.4 Summary of evidence
In summary, current neuroscientific evidence supports the cognitive unbinding paradigm: average neuronal spike activity persists or is even increased during general anesthesia, primary sensory networks are relatively preserved at anesthetic doses associated with unconsciousness, temporal coordination is impaired, top-down/feedback processing is disrupted consistently and major network nodes such as the posterior parietal cortex—a site at which neural information converges—lose hub status as sites of integration.
It is important to note, however, that not all of the original predictions regarding cognitive unbinding have been supported by the literature. At the time of the original description (Mashour, 2004, 2006), “40 Hz” or gamma oscillations were of significant interest in the field of neuroscience for their potential role in feature binding and consciousness (Joliot et al., 1994; Rodriguez et al., 1999). Furthermore, a study of multiple anesthetics revealed an uncoupling of gamma oscillations in association with anesthetic-induced unconsciousness (John et al., 2001). As such, gamma was predicted to play a role in cognitive unbinding, but this prediction has not been supported by all subsequent studies. With respect to consciousness, Merker has recently argued that gamma oscillations reflect network activation rather than cognitive processing per se (Merker, 2013). With respect to general anesthesia, gamma synchrony can persist and even be augmented in the anesthetized state (Imas et al., 2005; Murphy et al., 2011), although this may not be the transient and coordinated gamma previously associated with conscious processing (Rodriguez et al., 1999). Studies in humans suggest that higher gamma is suppressed during anesthesia (Breshears et al., 2010), while “40 Hz” activity (shorthand for low gamma) is preserved. Thus, the role of gamma synchrony in cognitive binding or unbinding has not been robustly or consistently supported.
5. Cognitive unbinding and other unconscious states or processes
Since the concept of cognitive unbinding can provide a framework for states of unconsciousness induced by diverse anesthetic drugs, it may also be applicable to other unconscious processes. This is especially true because general anesthesia can be accomplished either via an inhibitory or excitatory pathway. Non-pharmacological counterparts to these two pathways exist. Sleep and stroke are, respectively, examples of physiological and pathological etiologies of the inhibitory pathway to unconsciousness. Seizures, on the other hand, represent an excitatory pathway to unconsciousness. As would be predicted by the cognitive binding paradigm and integrated information theory, cortical effective connectivity (a surrogate for brain communication) decreases (Massimini et al., 2005) and modularity of brain networks increases (Boly et al., 2012) with non-rapid eye movement sleep. These findings are commensurate with general anesthesia (Ferrarelli et al., 2010; Lee et al, 2013b) and suggest that the cognitive modules normally bound together during consciousness are isolated and non-communicative during slow-wave sleep. Unresponsive wakefulness syndrome, which could be caused by stroke or trauma, is associated with decreases in effective connectivity (Laureys et al., 1999) and a return of such connectivity with recovery of consciousness (Rosanova et al., 2012). Indeed, intact “cognitive islands” have been identified in patients with this disorder of consciousness (Schiff et al., 2002). Again, to draw a parallel with anesthetic-induced unconsciousness, unresponsive wakefulness syndrome has been associated with preserved feedforward connectivity and impaired top-down connectivity (Boly et al., 2011) although the methodology leading to this finding has been questioned (King et al., 2011). Finally, in terms of cortical activation and cognitive unbinding, it has recently been demonstrated that absence seizures—during which consciousness is lost—are associated with retained primary sensory processing, suggesting that the cause of unconsciousness is impaired binding or higher-order processing of such cognitive activity (Chipaux et al., 2013). This unbinding could be caused by altered temporal dynamics during a seizure or a depression of important sites of integration, such as the frontoparietal association cortices (Blumenfeld, 2012).
It is critical to note that cognitive unbinding is not intended to explain every form of unconsciousness
In an extreme example, a brain-dead individual is unconscious because there is no longer any neuronal function, not because there is cognitive unbinding. With respect to certain pathologic states or deep planes of general anesthesia, primary sensory or cognitive processing is likely non-existent, rendering cognitive unbinding irrelevant because there is no meaningful information to be bound. Instead, the cognitive unbinding paradigm provides something analogous to proposed neural correlates of consciousness. Just as the neural correlates of consciousness are intended to represent the minimal and sufficient neural requirements for a conscious state, so too is cognitive unbinding intended to be the minimal and sufficient requirement for an unconscious state. In other words, even with environmental information being processed in primary sensory cortices and perhaps even higher sensory areas, the unbinding of cognitive modules in the brain would still be sufficient as the most proximate cause that renders an individual unconscious. However, this does not imply that cognitive unbinding is operational for every “depth” of unconsciousness, just as the neural correlates for the richness of normal human consciousness are likely far more extensive than those required for a minimally conscious state.
Thus far, the discussion of cognitive unbinding has been restricted primarily to levels of consciousness (i.e., awake vs. asleep vs. anesthetized). However, there is also an interesting application of this theory to the targeted exclusion of certain contents of consciousness. While general anesthesia leads to the global exclusion from consciousness of contents that would normally be represented but intolerable in the case of surgery, certain psychoanalytic defense mechanisms such as repression lead to the focal exclusion from consciousness of contents that might result in an intolerable affect. As I have previously described (Mashour, 2008), there is a remarkable correlate of cognitive unbinding found in the work of Wilfred Bion, a psychoanalyst in the tradition of British object relations. Bion, in articles published in the late 1950s and early 1960s, described a theory of consciousness in which “beta particles” (analogous to the raw data of sensory experience) needed to be synthesized by an “alpha function” to generate the “alpha particles” that formed the building blocks of consciousness (Bion, 1962). Importantly, Bion posited that certain psychoanalytic processes were mediated by what he called “attacking links” (Bion,1959), in which fragments of information could be behaviorally functional yet kept out of consciousness. In other words, repressed content was not extinguished at the level of the beta particle (i.e., the raw information), but rather isolated through an attack on the links generated by the alpha function, which would normally have served to bind such emotion or cognition to conscious perception. Bion’s concept of “attacks on linking” is strikingly similar to a process of cognitive unbinding, in which a focal representation is suppressed not because information is absent, but rather because it is not integrated. Although the resonance of cognitive binding/unbinding is most obvious in the work of Bion, cognitive unbinding provides a mechanism by which a dynamic unconscious could be possible. As such, cognitive unbinding is a scientific framework that meaningfully explains unconsciousness across the fields of anesthesiology, sleep neurobiology, coma science and psychoanalysis.
6. Scientific and clinical impact
In considering the value of the cognitive unbinding paradigm, there are arguments for both scientific and clinical relevance. First, focusing the paradigm of unconsciousness on the proximate cause vs. the root cause is advantageous. Investigation into cognitive unbinding as a proximate cause keeps us closer to the phenomenon of interest, namely, consciousness. This yields a more organized framework that can better guide exploration of root causes (be they pharmacological, physiological, pathological, or psychological) in a top-down manner. Cognitive unbinding also creates a common nomenclature for unconscious processes that transcends discipline.
Just as importantly, linking cognitive unbinding to the common functional outcome that can be achieved with drugs such as propofol, sevoflurane, and ketamine has clinical relevance. The assumption that these three drugs have individual cause-effect pathways with no final common mediator limits the development of a monitor that can reflect the unconscious state. By starting with the empirical effects of various anesthetic drugs on (for example) the electroencephalogram—rather than starting with the neurobiology of consciousness and analyzing correlates after exposure to various drugs—we will continue to generate anesthesia monitors rather than consciousness monitors. The anesthesia monitor is limited to the time period in which a particular anesthetic is delivered and it may not be valid for a future anesthetic associated with an alternative root cause. In contrast, a consciousness monitor should behave in a predictable way for all drugs that cause the characteristic phenotype of general anesthesia. A proximate-cause paradigm such as cognitive unbinding keeps the focus on the neurobiological target of consciousness rather than different drug actions, per se, and thus should be able to form the basis for monitoring a variety of anesthetics, sleep, vegetative states, and potentially states of altered consciousness such as delirium.
7. Limitations and Future Directions
The cognitive unbinding paradigm is limited in scope. First, in terms of anesthesiology, it does not attempt to account for therapeutic endpoints of general anesthesia beyond unconsciousness. However, cognitive unbinding may be relevant to anesthetic-induced amnesia, which, like unconsciousness, is mediated in the brain. Neurophysiological studies have suggested that loss of inter-regional synchrony (i.e., temporal unbinding) may be a common mechanism of impaired memory encoding due to a variety of drugs (Pryor et al., 2010). Similarly, a functional neuroimaging study explicitly tested and supported the hypothesis that cognitive unbinding accounted for propofol’s effects on impaired auditory verbal memory (Liu et al., 2012). In contrast, the proximate cause of anesthetic-induced immobility (mediated primarily in the spinal cord, as shown by Antognini and Schwartz, 1993; Rampil, 1994) is likely quite different than the proximate cause of anesthetic-induced unconsciousness and amnesia (mediated in the brain). Thus, almost by definition, the unbinding paradigm is limited to cognitive phenomena induced by general anesthetics. Second, the focus on higher cognition raises the question of whether this paradigm can be applied to other species and organisms that are clearly susceptible to the effects of anesthesia. Although there are findings consistent with unbinding in mammalian studies of general anesthesia using rodents, it is unclear how cognitive unbinding would apply, for example, to the nematode C. Elegans. Since impaired movement is also the endpoint of anesthesia in this experimental model, cognitive unbinding may not be relevant. However, a study of anesthetic effects in Drosophila explicitly suggested that there was evidence of perceptual “unbinding,” as well as an uncoupling of perceptual and motor processing (van Swinderen, 2006). It remains to be seen whether unbinding is a principle that can be applied to other anesthetic or “unconscious” endpoints and other species. In terms of other unconscious states, cognitive unbinding may not be operational as a process once primary cognitive activity itself is extinguished (as in coma).
The cognitive unbinding paradigm continues to provide direction for coherent research activity. Neuroimaging methods and experimental protocols to test feature binding could be conducted during exposure to general anesthetics as well as during sleep and other unconscious states. This would be direct confirmation of the unbinding hypothesis and would complement ongoing investigations into anesthetic effects on sites of information convergence such as the posterior parietal cortex or the “global neuronal workspace,” which includes the frontal-parietal networks (Changeux, 2012; Dehaene and Changeux, 2011). Current data suggest that impaired top-down processing may be a final common mediator, i.e., a potential proximate cause, of anesthetic-induced unconsciousness by diverse drugs such as propofol, sevoflurane and ketamine (Lee et al., 2013a). This is of relevance to the cognitive unbinding paradigm because top-down or feedback information processing is thought to be important for synthesizing feature processing (Figure 3). Further work is required to trace the pathway from this potentially common proximate cause (impaired top-down processing) through the neurophysiological findings (decreased gamma/increased alpha power for propofol and sevoflurane; increased gamma/decreased alpha power for ketamine) to molecular events (GABAA agonism for propofol; GABAA agonism and various other targets for sevoflurane; N-methyl-D-aspartate glutamatergic receptors for nitrous and ketamine, the latter of which may also act through HCN1 channels) (Chen et al., 2009; Jevtovic-Todorovic et al., 1998; Lee et al., 2013a). Such pathways will need to be tested empirically with protocols that specifically assess feature binding or other processes related to the synthesis of neural information. The use of genetically-modified mice or emerging optogenetic techniques in conjunction with neurophysiology and graph-theoretical network analysis will be valuable in linking root and proximate causes.
Clinically, the cognitive unbinding paradigm provides direction for monitoring in ways that other frameworks of unconsciousness and general anesthesia, especially those focused on the subcortical or molecular level, do not. Since unbinding is thought to be mediated, in part, through impaired corticocortical communication, the paradigm implies that monitors could be developed that reflect the effects of all major classes of general anesthetics. There is already emerging evidence supporting this claim (Lee et al., 2013a). However, since the cognitive unbinding approach to anesthetic mechanism explicitly focuses on principles of consciousness, related monitoring strategies should be valuable beyond the period of anesthetic exposure. As noted, the focus should be on the development of a consciousness monitor rather than merely an anesthesia monitor. As such, other states of consciousness beyond general anesthesia could potentially be monitored using the principles of cognitive unbinding. For example, delirium is a state of altered consciousness that occurs commonly in the postoperative period. It is conceivable that disorders of attention relate to impaired top-down processing from prefrontal cortex and that altered perceptions or hallucinations could be due to an incomplete recovery of perceptual binding processes after general anesthesia, leading to illusory conjunctions.
8. Conclusion
Cognitive unbinding is a viable paradigm for the proximate cause of anesthetic-induced unconsciousness and provides an answer to the clinical question of how diverse anesthetics can be used to achieve the same functional goal. Neuroimaging and neurophysiological studies over the past decade have provided consistent support to the key tenets of the paradigm, while shifting the focus of temporal unbinding away from low gamma activity and shifting the focus of convergence unbinding toward the posterior parietal cortex and frontal-parietal network. Emerging evidence suggests that the principles of the cognitive unbinding paradigm can form the basis of a more sophisticated monitor that measures processes related to consciousness rather than individual drug effects. The scientific basis and clinical implications of the cognitive unbinding paradigm also extend to sleep neurobiology, coma science, and psychoanalysis. Further and substantial work is clearly required, but the framework of cognitive unbinding provides direction for both mechanistic and clinical research across numerous disciplines, with the potential to advance understanding and improve patient care.
Highlights.
Cognitive unbinding is the disrupted synthesis of specialized neural activity
Current evidence suggests that diverse anesthetics cause cognitive unbinding
Other unconscious states are also consistent with cognitive unbinding
Cognitive unbinding may be a general theory of unconsciousness
Acknowledgments
Funding: Dr. Mashour is supported by grant RO1 GM098578 from the National Institutes of Health, Bethesda, MD, and the James S. McDonnell Foundation, St. Louis, MO.
The author would like to thank the following investigators for their critical review of the manuscript: Dr. Max Kelz (University of Pennsylvania, Philadelphia); Dr. Michael Avidan (Washington University, St. Louis); Drs. UnCheol Lee, Dinesh Pal, Stefanie Blain-Moraes, Joon-Young Moon (University of Michigan, Ann Arbor); Chelsea Cummiford, HeonSoo Lee (University of Michigan, Ann Arbor).
Footnotes
Disclosure: Dr. Mashour holds a patent (pending) on measuring directional brain connectivity as an index of levels of consciousness.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antognini JF, Schwartz K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. doi: 10.1097/00000542-199312000-00015. [DOI] [PubMed] [Google Scholar]
- Baars BJ, Ramsoy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Baumgartner F, Hanke M, Geringswald F, Zinke W, Speck O, Pollmann S. Evidence for feature binding in the superior parietal lobule. NeuroImage. 2013;68:173–180. doi: 10.1016/j.neuroimage.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Bigelow H. Insensibility during surgical operations produced by inhalation. Boston Med Surg J. 1846;35:309–317. [PMC free article] [PubMed] [Google Scholar]
- Bion WR. Attacks on linking. Int J Psychoanal. 1959;40:308. [Google Scholar]
- Bion WR. The psycho-analytic study of thinking. A theory of thinking. Int J Psychoanal. 1962;43:306–310. [PubMed] [Google Scholar]
- Blumenfeld H. Impaired consciousness in epilepsy. Lancet Neurol. 2012;11:814–826. doi: 10.1016/S1474-4422(12)70188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Garrido MI, Gosseries O, Bruno MA, Boveroux P, Schnakers C, Massimini M, Litvak V, Laureys S, Friston K. Preserved feedforward but impaired top-down processes in the vegetative state. Science. 2011;332:858–862. doi: 10.1126/science.1202043. [DOI] [PubMed] [Google Scholar]
- Boly M, Moran R, Murphy M, Boveroux P, Bruno MA, Noirhomme Q, Ledoux D, Bonhomme V, Brichant JF, Tononi G, Laureys S, Friston K. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, Pelegrini-Issac M, Maquet P, Benali H. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc Natl Acad Sci U S A. 2012;109:5856–5861. doi: 10.1073/pnas.1111133109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveroux P, Vanhaudenhuyse A, Bruno MA, Noirhomme Q, Lauwick S, Luxen A, Degueldre C, Plenevaux A, Schnakers C, Phillips C, Brichant JF, Bonhomme V, Maquet P, Greicius MD, Laureys S, Boly M. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- Breshears JD, Roland JL, Sharma M, Gaona CM, Freudenburg ZV, Tempelhoff R, Avidan MS, Leuthardt EC. Stable and dynamic cortical electrophysiology of induction and emergence with propofol anesthesia. Proc Natl Acad Sci U S A. 2010;107:21170–21175. doi: 10.1073/pnas.1011949107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers D. Facing up to the problem of consciousness. J Conscious Stud. 1995;2:200–219. [Google Scholar]
- Changeux JP. Conscious processing: implications for general anesthesia. Curr Opin Anaesthesiol. 2012;25:397–404. doi: 10.1097/ACO.0b013e32835561de. [DOI] [PubMed] [Google Scholar]
- Chen X, Shu S, Bayliss DA. HCN1 Channel Subunits Are a Molecular Substrate for Hypnotic Actions of Ketamine. J Neurosci. 2009;29:600–609. doi: 10.1523/JNEUROSCI.3481-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipaux M, Vercueil L, Kaminska A, Mahon S, Charpier S. Persistence of cortical sensory processing during absence seizures in human and an animal model: evidence from EEG and intracellular recordings. PLoS ONE. 2013;8:e58180. doi: 10.1371/journal.pone.0058180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Changeux JP. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Del Cul A, Baillet S, Dehaene S. Brain dynamics underlying the nonlinear threshold for access to consciousness. PLoS Biol. 2007;5:e260. doi: 10.1371/journal.pbio.0050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Sarasso S, Casali A, Riedner BA, Angelini G, Tononi G, Pearce RA. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz AG, Vizuete JA, Imas OA. Desflurane selectively suppresses long-latency cortical neuronal response to flash in the rat. Anesthesiology. 2009;111:231–239. doi: 10.1097/ALN.0b013e3181ab671e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Imas OA, Ropella KM, Ward BD, Wood JD, Hudetz AG. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 2005;102:937–947. doi: 10.1097/00000542-200505000-00012. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Todorovic SM, Mennerick S, Powell S, Dikranian K, Benshoff N, Zorumski CF, Olney JW. Nitrous oxide (laughing gas) is an NMDA antagonist, neuroprotectant and neurotoxin. Nat Med. 1998;4:460–463. doi: 10.1038/nm0498-460. [DOI] [PubMed] [Google Scholar]
- John ER, Prichep LS, Kox W, Valdes-Sosa P, Bosch-Bayard J, Aubert E, Tom M, di Michele F, Gugino LD. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10:165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- Joliot M, Ribary U, Llinas R. Human oscillatory brain activity near 40 Hz coexists with cognitive temporal binding. Proc Natl Acad Sci U S A. 1994;91:11748–11751. doi: 10.1073/pnas.91.24.11748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkowiak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. Faseb J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- King JR, Bekinschtein T, Dehaene S. Comment on “Preserved feedforward but impaired top-down processes in the vegetative state”. Science. 2011;334:1203. doi: 10.1126/science.1210012. author reply 1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku SW, Lee U, Noh GJ, Jun IG, Mashour GA. Preferential inhibition of frontal-to-parietal feedback connectivity is a neurophysiologic correlate of general anesthesia in surgical patients. PLoS ONE. 2011;6:e25155. doi: 10.1371/journal.pone.0025155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann L, Foster BL, Liley DT. Modulation of functional EEG networks by the NMDA antagonist nitrous oxide. PLoS ONE. 2013;8:e56434. doi: 10.1371/journal.pone.0056434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Goldman S, Phillips C, Van Bogaert P, Aerts J, Luxen A, Franck G, Maquet P. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. NeuroImage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Lee U, Kim S, Noh GJ, Choi BM, Hwang E, Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009;18:1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lee U, Ku S, Noh G, Baek S, Choi B, Mashour GA. Disruption of Frontal-Parietal Communication by Ketamine, Propofol, and Sevoflurane. Anesthesiology. 2013a;118:1264–1275. doi: 10.1097/ALN.0b013e31829103f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Mashour GA, Noh GJ, Kim S, Lee U. Reconfiguration of Network Hub Structure after Propofol-Induced Unconsciousness. Anesthesiology. 2013b doi: 10.1097/ALN.0b013e3182a8ec8c. In-Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee U, Muller M, Noh GJ, Choi B, Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–881. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- Lewis LD, Weiner VS, Mukamel EA, Donoghue JA, Eskandar EN, Madsen JR, Anderson WS, Hochberg LR, Cash SS, Brown EN, Purdon PL. Rapid fragmentation of neuronal networks at the onset of propofol-induced unconsciousness. Proc Natl Acad Sci U S A. 2012;109:E3377–3386. doi: 10.1073/pnas.1210907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lauer K, Ward BD, Rao SM, Li S, Hudetz AG. Propofol disrupts fuctional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012;33:2487–2498. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Lauer KK, Ward BD, Li SJ, Hudetz AG. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology. 2013;118:59–69. doi: 10.1097/ALN.0b013e318277a801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. NeuroImage. 2010;49:823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA. Consciousness unbound: toward a paradigm of general anesthesia. Anesthesiology. 2004;100:428–433. doi: 10.1097/00000542-200402000-00035. [DOI] [PubMed] [Google Scholar]
- Mashour GA. Integrating the science of consciousness and anesthesia. Anesth Analg. 2006;103:975–982. doi: 10.1213/01.ane.0000232442.69757.4a. [DOI] [PubMed] [Google Scholar]
- Mashour GA. Toward a general theory of unconscious processes in psychoanalysis and anesthesiology. J Am Psychoanal Assoc. 2008;56:203–222. doi: 10.1177/0003065108315692. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Merker B. Cortical gamma oscillations: the functional key is activation, not cognition. Neurosci Biobehav Rev. 2013;37:401–417. doi: 10.1016/j.neubiorev.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Bruno MA, Riedner BA, Boveroux P, Noirhomme Q, Landsness EC, Brichant JF, Phillips C, Massimini M, Laureys S, Tononi G, Boly M. Propofol anesthesia and sleep: a high-density EEG study. Sleep. 2011;34:283–291A. doi: 10.1093/sleep/34.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Wyatte D, Herd S, Mingus B, Jilk DJ. Recurrent processing during object recognition. Front Psychol. 2013;4:124. doi: 10.3389/fpsyg.2013.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perouansky M. The quest for a unified model of anesthetic action: a century in Claude Bernard’s shadow. Anesthesiology. 2012;117:465–474. doi: 10.1097/ALN.0b013e318264492e. [DOI] [PubMed] [Google Scholar]
- Pryor KO, Reinsel RA, Mehta M, Li Y, Wixted JT, Veselis RA. Visual P2-N2 complex and arousal at the time of encoding predict the time domain characteristics of amnesia for multiple intravenous anesthetic drugs in humans. Anesthesiology. 2010;113:313–326. doi: 10.1097/ALN.0b013e3181dfd401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampil IJ. Anesthetic potency is not altered after hypothermic spinal cord transection in rats. Anesthesiology. 1994;80:606–610. doi: 10.1097/00000542-199403000-00017. [DOI] [PubMed] [Google Scholar]
- Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Gosseries O, Casarotto S, Boly M, Casali AG, Bruno MA, Mariotti M, Boveroux P, Tononi G, Laureys S, Massimini M. Recovery of cortical effective connectivity and recovery of consciousness in vegetative patients. Brain. 2012;135:1308–1320. doi: 10.1093/brain/awr340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Ribary U, Moreno DR, Beattie B, Kronberg E, Blasberg R, Giacino J, McCagg C, Fins JJ, Llinas R, Plum F. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125:1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- Schroter MS, Spoormaker VI, Schorer A, Wohlschlager A, Czisch M, Kochs EF, Zimmer C, Hemmer B, Schneider G, Jordan D, Ilg R. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J Neurosci. 2012;32:12832–12840. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J, Perlbarg V, Boly M, Marrelec G, Boveroux P, Vanhaudenhuyse A, Bruno MA, Laureys S, Phillips C, Pelegrini-Issac M, Maquet P, Benali H. Brain functional integration decreases during propofol-induced loss of consciousness. NeuroImage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Singer W. Consciousness and the binding problem. Ann N Y Acad Sci. 2001;929:123–146. doi: 10.1111/j.1749-6632.2001.tb05712.x. [DOI] [PubMed] [Google Scholar]
- Sleigh JW. The study of consciousness comes of age. Anesthesiology. 2013;118:1245–1246. doi: 10.1097/ALN.0b013e318291031f. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC neuroscience. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- Tononi G. Integrated information theory of consciousness: an updated account. Archives italiennes de biologie. 2012;150:56–90. doi: 10.4449/aib.v149i5.1388. [DOI] [PubMed] [Google Scholar]
- van Swinderen B. A succession of anesthetic endpoints in the Drosophila brain. J Neurobiol. 2006;66:1195–1211. doi: 10.1002/neu.20300. [DOI] [PubMed] [Google Scholar]
- Vanini G, Baghdoyan HA. Extrasynaptic GABAA receptors in rat pontine reticular formation increase wakefulness. Sleep. 2013;36:337–343. doi: 10.5665/sleep.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bibra E, Harless E. Die wirkung des schwefelathers in chemischer und physiologischer beziehung. Erlangen, Bavaria, Heyder 1847 [Google Scholar]
- White NS, Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. NeuroImage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Wyatte D, Curran T, O’Reilly R. The limits of feedforward vision: recurrent processing promotes robust object recognition when objects are degraded. J Cogn Neurosci. 2012;24:2248–2261. doi: 10.1162/jocn_a_00282. [DOI] [PubMed] [Google Scholar]
- Wyatte D, Herd S, Mingus B, O’Reilly R. The role of competitive inhibition and top-down feedback in binding during object recognition. Front Psychol. 2012;3:182. doi: 10.3389/fpsyg.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]


