Abstract
Objectives
We investigated whether selective ablation of the carotid body (CB) chemoreceptors improves cardiorespiratory control and survival during heart failure.
Background
Chronic heart failure (CHF) is a recognized health problem worldwide, and novel treatments are needed to better improve life quality and decrease mortality. Enhanced carotid chemoreflex drive from the CB is thought to contribute significantly to autonomic dysfunction, abnormal breathing patterns, and increased mortality in heart failure.
Methods
CHF was induced by coronary ligation in rats. Selective CB denervation (CBD) was performed to remove carotid chemoreflex drive in the CHF state (16 weeks post MI). Indices of autonomic and respiratory function were assessed in CB intact and CBD animals. CBD at 2 weeks post-MI was performed to evaluate whether early targeted CB ablation decreases the progression of left ventricular dysfunction, cardiac remodeling and arrhythmic episodes and improves survival.
Results
CHF rats developed increased CB chemoreflex drive and chronic central pre-sympathetic neuronal activation, increased indices of elevated sympathetic outflow, increased breathing variability and apnea incidence, and desensitization of the baroreflex. Selective CB ablation reduced the central pre-sympathetic neuronal activation by 40%, normalized indices of sympathetic outflow and baroreflex sensitivity, and reduced the incidence of apneas in CHF animals from 16.8 ± 1.8 events/h to 8.0 ± 1.4 events/h. Remarkably, when CB ablation was performed early, cardiac remodeling, deterioration of left ventricle ejection fraction, and cardiac arrhythmias were reduced. Most importantly, the rats that underwent early CB ablation exhibited an 85% survival rate compared to 45% survival in CHF rats without the intervention.
Conclusion
Carotid chemoreceptors play a seminal role in the pathogenesis of heart failure and their targeted ablation might be of therapeutic value to reduce cardiorespiratory dysfunction and improve survival during CHF.
Keywords: heart failure, autonomic function, breathing disorders, mortality, carotid body denervation
Introduction
Elevated sympathetic outflow and breathing disorders are two hallmarks of chronic heart failure (CHF), and both have been strongly related to decreased quality of life, poor prognosis, and increased mortality (1,2). Enhanced sympathetic drive and breathing instability during CHF have been associated with alterations in peripheral and central neural pathways that regulate autonomic function and breathing control (3,4). Despas et al. (2012) showed that CHF patients with high peripheral chemosensitivity displayed higher sympathetic outflow compared to CHF patients with normal chemosensitivity (5). In addition, impaired baroreflex function observed in CHF has been associated with an augmented peripheral chemosensitivity in patients with CHF (6). Previous studies from our laboratory have demonstrated an augmented afferent input from the carotid body (CB) chemoreceptors in pacing-induced CHF rabbits and myocardial infarcted CHF rats and have shown that reducing chemoreflex afferent traffic results in reductions in the sympathetic drive in CHF animals (7,8,9). Also, transient inhibition of the CB chemoreflex with brief hyperoxic stimulation in CHF patients results in a decreased sympathetic tone (5) and an improvement in the baroreflex function (6).
Patients with CHF exhibit a high incidence (up to 60%) of breathing disorders characterized by apnea/hypopneas and higher breathing variability (10,11) that have been related to the progression of the disease (12). Exaggerated CB-mediated ventilatory responses to apneas or hypopneas could contribute to respiratory instability. In support of this notion, we have previously shown that reductions in CB afferent activity during CHF decrease breathing variability and apnea/hypopnea incidence in rats (13).
Together these findings suggest that the CB chemoreflex sustains the cardiorespiratory dysfunction observed in CHF. Indeed, evidence showing higher mortality rates in CHF patients with high chemosensitivity compared to patients with normal chemosensitivity (3) suggests a crucial role for the CB chemoreflex in exacerbating the pathogenesis of CHF. Although a causal link between exaggerated CB chemoreflex drive and high mortality risk during CHF has not been proven, sympatho-excitation and the apnea-related hypoxemia exacerbated by the augmented chemoreflex could lead to an increased arrhythmogenesis and deterioration of cardiac function associated with a high mortality risk (2,14).
Our prior work has shown that the exaggerated chemoreflex in CHF emanates from elevated tonic afferent nerve traffic from the CB (7–9). Recently, it has been proposed that targeting the CB by denervation of the afferent inputs may be beneficial in cardiovascular diseases exacerbated by sympathetic hyperactivity (15). The impact of CB denervation (CBD) on autonomic function and survival during CHF has not been studied before and could represent a novel strategy to slow the progression of cardiac deterioration and lower mortality rates in CHF. In this study, we asked whether CBD improves autonomic balance and breathing regularity during CHF and whether CBD in the early stage of cardiac dysfunction reduces cardiac remodeling and arrhythmia incidence and increases survival of myocardial-infarcted CHF rats.
Methods
Induction of heart failure
Seventy one (2 month old) male Sprague-Dawley rats, weighing between 430 and 560 g, were studied. All animal procedures were reviewed and approved by the IACUC of the University of Nebraska Medical Center and were carried out under the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996). Chronic heart failure (CHF) was produced by coronary artery ligation (CAL) as previously described (13).
Selective carotid body denervation
At 16 weeks (Protocol 1) or 2 weeks (Protocol 2) post-CAL surgery, the rats underwent cryogenic destruction of the CBs (16). Graphic timelines of the protocols are provided in Supplemental Fig. 1. We found that this surgical approach allows the elimination of the CB chemosensory but not the carotid baroreceptor afferents (Supplemental Fig. 2 and 3). CBD did not affect water consumption or daily food intake (Supplemental Fig. 4). The sham CB denervation surgery performed in Sham and CHF rats showed no cardiorespiratory effects (See Supplemental Fig. 5–6, 8,10,11).
Echocardiography
Cardiac function was determined by echocardiography (Vevo 770; Visualsonics, Inc.) as previously described (13,17). M-mode tracings were recorded through the anterior and posterior LV walls, and anterior and posterior wall thicknesses (end-diastolic and end-systolic) and LV internal dimensions were measured. Rats with ejection fraction of less than 45% were considered to be in CHF (13,17).
Radiotelemetric monitoring of arterial blood pressure and heart rate
At 14 weeks, rats were chronically implanted with a radio-telemetry pressure transducer (TA11PA-C40, DSI, USA) with a catheter directed into the abdominal aorta. Blood pressure (BP) and heart rate (HR) were acquired in conscious resting state.
Autonomic balance
Heart rate variability (HRV) and the low frequency component of the systolic blood pressure variability (LF-SBPV) were assessed as indirect measures of autonomic balance (18) using power spectral analysis (19,20). Spontaneous baroreflex sensitivity was assessed by spectral calculation (21).
Arrhythmia score
Irregular heartbeats were visually inspected from HR time series (22). Arrhythmic episodes were defined as premature or delayed beats with changes greater than 3 standard deviations from the mean beat to beat interval duration and reported as events per hour.
Evaluation of respiratory variability and ventilatory chemoreflex function
Tidal volume (Vt), respiratory frequency (RR), and minute ventilation (VE: Vt x RR) were determined by whole body plethysmography as previously described (13). Respiratory stability was assessed from resting breathing recordings by Poincare plots and analysis of SD1 and SD2 of the inter-breath interval variability (13). Apnea and hypopnea incidence (cessation or ≥50% reduction in Vt over ≥3 consecutive breaths) was counted and reported as AHI (incidents/hr). Peripheral chemoreceptors were stimulated preferentially by allowing the rats to breathe hypoxic (10% O2/balance N2).
Western blotting
Neuronal activation in the rostral ventrolateral medulla (RVLM) was assessed by immunoblot of the fos-related antigen 1 (Fra-1, 1:100, Santa Cruz Biotechnology, USA) in RVLM micropunches as previously described (18). Fra-1 expression is induced during sustained neuronal activation and thus serves as a sensitive marker of chronically activated brainstem neurons (23). The relative amount of protein of interest was calculated as the ratio of intensity of the band relative to the intensity of the housekeeping gene β-actin.
Immunohistochemistry
Localization of Fra-1 immunoreactivity in RVLM catecholaminergic pre-sympathetic neurons was assessed in 4% PFA-fixed coronal sections (15 μm) of the brain. RVLM catecholaminergic pre-sympathetic neurons were identified by immunoreactivity to tyrosine hydroxylase (TH). Sections were visualized using a confocal laser microscope.
Mortality and cardiac remodeling studies
In a subset of animals, we assessed the effects of early CBD (eCBD, 2 weeks after CAL surgery) on survival rate and cardiac remodeling during CHF (Protocol 2, Supplemental Fig. 1). Echocardiography and survival rates were then followed through the remaining 14 weeks of the protocol. At the end of the study, the heart was removed and processed for histological quantification of tissue collagen levels from both the noninfarcted LV free wall and the interventricular septum (IVS).
Statistics
Data were expressed as means ± SEM. Differences among 3 or more groups were assessed with one or two-way ANOVA tests, followed by Newman-Keuls or Bonferroni posthoc comparisons. The Student’s t-test was employed to compare the differences between two groups. The log rank test was used to compare survival rates between CHF and CHF+eCBD rats. P < .05 was considered statistically significant.
Results
Respiratory disorders and CB ablation (Protocol 1)
CHF-rats displayed cardiac chamber dilation and a significant decrease in cardiac function after 16 weeks post-MI as described previously (13,18,24). Echocardiographic parameters were measured in sham and CHF rats (Supplemental Table 1). CHF rats exhibited reduced ejection fraction and fractional shortening (less that 40% of normal values) compared with non-infarcted sham rats (P < .05). Infarct size was 38 ± 2% of the total LV area in CHF rats (24).
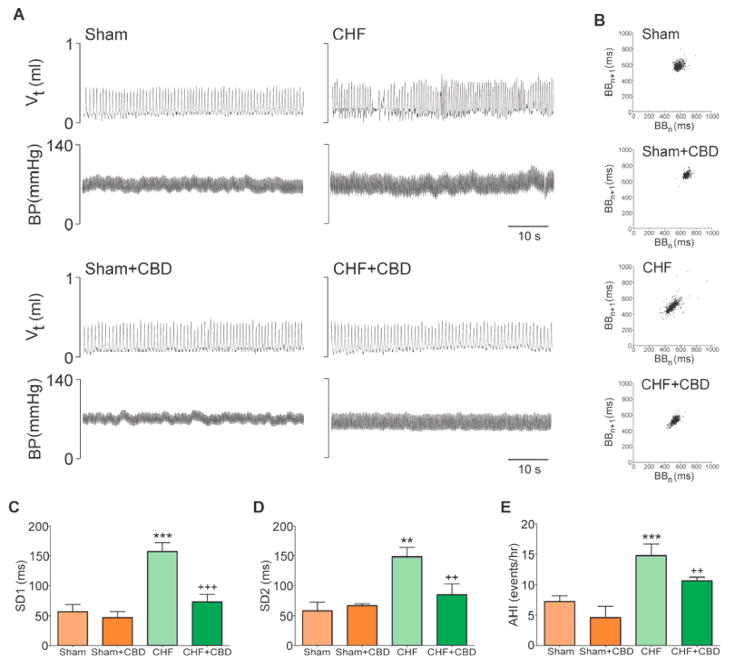
Sixteen weeks after infarct surgery, rats developed a significant increase in CB chemoreflex drive as evidenced by increased normoxic ventilation (Supplemental Table 2) and potentiation of the hypoxic ventilatory response (Fig. 1A–B and Supplemental Fig. 5). CHF rats displayed breathing disorders at rest (Fig. 2A–B). Breath-to-breath (SD1) and aggregate (SD2) breathing variability in CHF-rats increased 2-fold compared to the values obtained from sham rats (Fig. 2C–D). In addition, CHF rats displayed a greater number of apneas and hypopneas (Fig. 2E) compared to that in sham rats (16.8 ± 1.8 events/h vs. 8.0 ± 1.4 events/h, CHF vs. sham). The increased apnea index in CHF rats was not associated with post-sigh events, which remained unchanged (Supplemental Fig. 7).
Figure 1. Chemoreflex function and selective carotid body denervation in CHF.
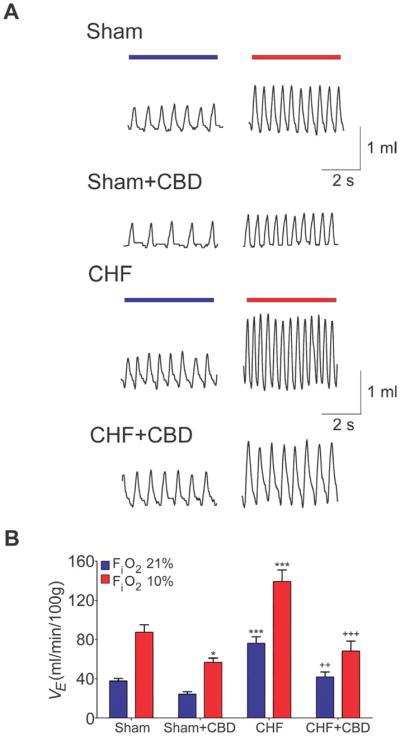
(A) Representative ventilatory recordings of the chemoreflex ventilatory response to acute hypoxia (FiO2 ~ 10%) in rats with and without CBD. (B) CBD abrogated the exaggerated hypoxic ventilatory response in CHF rats. VE: minute ventilation. *P<.05 vs. Sham, ***P<.001 vs. Sham, ++P<.01 vs. CHF, +++P<.001 vs. CHF. (Comparisons between groups at same FiO2.) Sham/Sham+CBD, n=6; CHF/CHF+CBD, n=8.
Figure 2. Selective CBD restored normal breathing control in CHF.
(A) Representative tracings of tidal volume (Vt) and arterial blood pressure (BP) taken at rest in a Sham and CHF rat before and after CBD. Breathing instability is shown in the Vt tracings from the CHF rat, and CBD restored normal breathing rhythm. (B) Poincare plots showing the breath-to-breath (BB) interval variability in the same rats illustrated in panel A. CBD reduced breathing variability in the CHF rat. (C) and (D) CBD reduced SD1 (short-term variability) and SD2 (long-term variability) of the breathing interval in CHF rats . **P<.01 vs. Sham, ***P<.001 vs. Sham, ++P<.01 vs. CHF; +++P<.001 vs. CHF (E) CBD suppressed the apnea-hypopnea incidence (AHI) in CHF rats. ***P<.001 vs. Sham, ++P<.01 vs. CHF. Sham/Sham+CBD, n=6; CHF/CHF+CBD, n=8.
The elevated resting ventilation and the enhanced ventilatory response to hypoxia in CHF rats were markedly reduced after CBD to values similar to sham rats that underwent CBD (Fig. 1B and Supplemental Fig. 5). Moreover, breathing variability was normalized in CHF-CBD rats (Fig. 2). After CBD, both SD1 and SD2 inter-breath variability were significantly reduced (53.4% and 57.2%, respectively) compared to the values obtained before CBD in CHF rats (Fig. 2C–D). CBD also significantly decreased AHI in CHF rats (Fig. 2E) to the level seen in sham rats (Fig. 2E).
Cardiac autonomic control following CBD in CHF rats
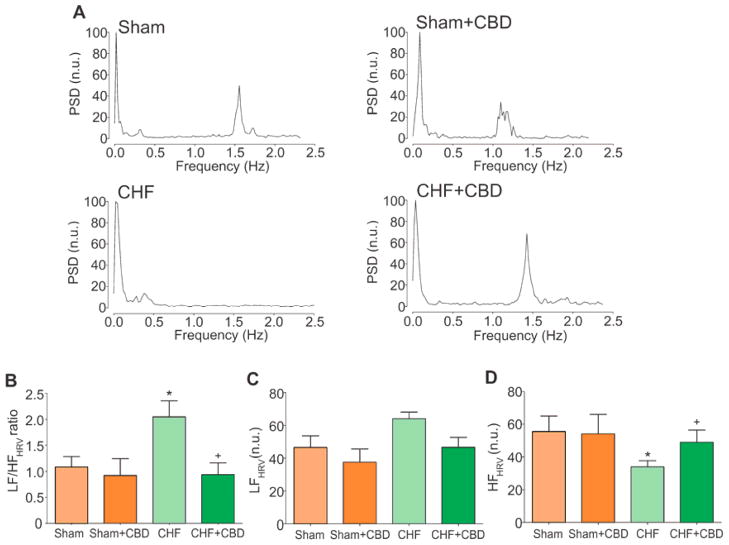
Autonomic imbalance was evident in CHF rats compared to Sham rats (Fig. 3). A shift in the HRV towards augmented sympathetic drive was found during CHF (Fig. 3B–D) as evidenced by a 2-fold increase in the low to high frequency ratio (LF/HF) of the HRV compared to sham rats (Fig. 3B). A major component to this shift was a significant reduction in the parasympathetic respiratory sinus arrhythmia during CHF as evidenced by a significant decrease in the HF component of the HRV (Fig. 3D).
Figure 3. Selective CBD normalized cardiac autonomic balance in CHF.
(A) Representative traces of the power spectral density (PSD) of heart rate variability (HRV) in Sham, Sham+CBD, CHF and CHF+CBD rats. CHF rats displayed a marked increase in the low frequency (LF) component of the PSD and a decrease in the high frequency (HF) component. (B) CBD markedly reduced low frequency/high frequency ratio (LF/HF) in CHF rats. (C) The LF band expressed in normalized units (n.u.) and (D) the HF band expressed in n.u. *P<.05 vs. Sham, +P< .05 vs. CHF. Sample size as in Fig. 3.
Ablation of the CB rescued normal HRV in CHF rats (Fig. 3 and Supplemental Fig. 8). The LF/HF of the HRV significantly decreased from 2.0 ± 0.3 to 0.9 ± 0.2 (before and after CBD, respectively, P < .05) in rats with CHF. Selective CBD did not affect resting BP or HR in either sham+CBD or CHF+CBD rats (Supplemental Table 2).
Carotid chemoreceptor ablation and brainstem pre-sympathetic neuronal activation during CHF
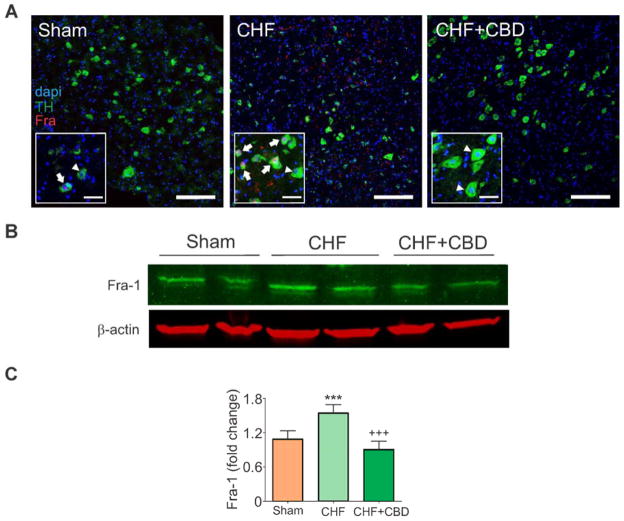
Catecholaminergic pre-sympathetic neurons (Fig. 4A) located in the RVLM represent a key locus in the central nervous system regulation of sympathetic outflow during CHF (25). Following CHF, RVLM catecholaminergic neurons (TH-positive) exhibited a 40% increase in Fra-1 immunoreactivity, a marker of chronic neural activation, compared to sham rats (Fig. 4A–C). By contrast, selective CBD significantly reduced Fra-1 expression in CHF rats (1.5 ± 0.1 vs. 0.9 ± 0.1 a.u., CHF vs. CHF+CBD) to levels comparable to the values obtained in sham rats (Fig. 4).
Figure 4. Selective CBD reduced pre-sympathetic neuron activation in CHF.
(A) The number of activated catecholaminergic neurons (arrow, yellow) in the RVLM were increased in CHF rats and reduced in CHF-CBD rats. Note that not all tyrosine hydroxylase (TH) positive cells (green) displayed Fra-1 immunostaining (arrowhead). Individual neurons are shown by nuclear stain, dapi (blue), catecholaminergic neurons by TH expression (green), and activated neurons by Fra-1 expression (red). Activated catecholaminergic neurons (co-expressing TH and Fra-1, yellow) were identified by merging color images. (B) Expression of Fra-1 protein in RVLM micropunches. All lanes were run on the same blot. (C) Quantitative analysis of Fra-1 expression. CBD significantly reduced Fra-1 expression in pre-sympathetic neurons from CHF rats as shown in panel A. βactin protein served as the gel loading control. ***P<.001 vs. Sham, +++P< .001 vs. CHF. n = 6 rats/group.
Sympatho-vasomotor tone and baroreflex gain following CBD
Sympatho-vasomotor tone as estimated by the LF-SBPV increased in CHF rats, which was normalized (from 18.4 ± 4.5 mmHg2 to 7.2 ± 2.0 mmHg2) after CBD (Supplemental Fig. 9A). In addition, the impaired baroreflex sensitivity displayed by CHF-rats was significantly improved after CBD (Supplemental Fig. 9B).
Progression of cardiac remodeling and arrhythmogenesis after early CBD (eCBD) in CHF (Protocol 2)
Necrotic myocytes in the infarct region were almost completely replaced with fibrotic scar tissue 16 weeks after CAL surgery in both CHF and CHF-eCBD rats (Fig. 5A). In addition, both groups exhibited nearly identical infarct size (Supplemental Fig. 11). Nevertheless, we hypothesized that restoration of autonomic control and breathing regularity by eCBD, 2 weeks after CAL, would influence remodeling in the non-infarcted regions.
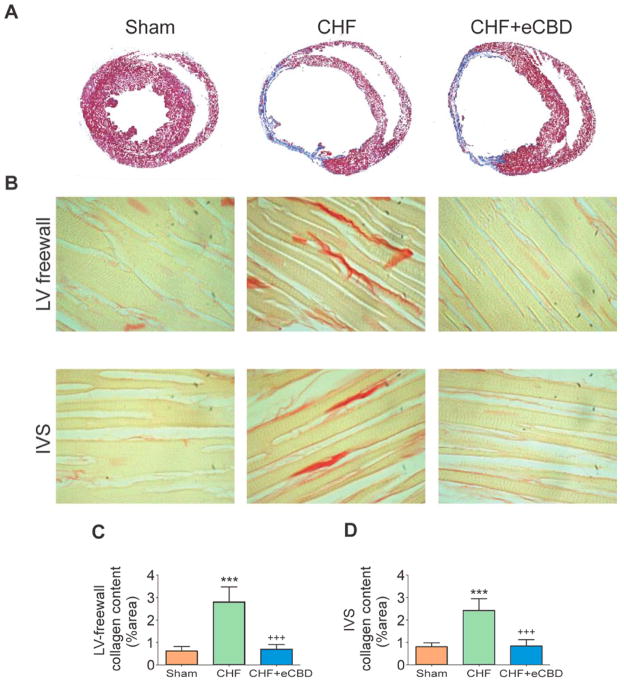
Figure 5. Early CBD (eCBD) reduced cardiac fibrosis in CHF.
(A) Rats with CHF and with CHF+eCBD displayed left ventricle (LV) dilation and tissue fibrosis in the infarcted region at 16 weeks post-infarct. (B) Marked collagen deposition was evident in the LV free wall and the interventricular septum (IVS) from CHF. Selective eCBD significantly reduced cardiac fibrosis in the LV-free wall (B–C) and in the IVS (B–D). ***P<.01 vs. Sham, +++P<.001 vs. CHF. n=4 rats/group.
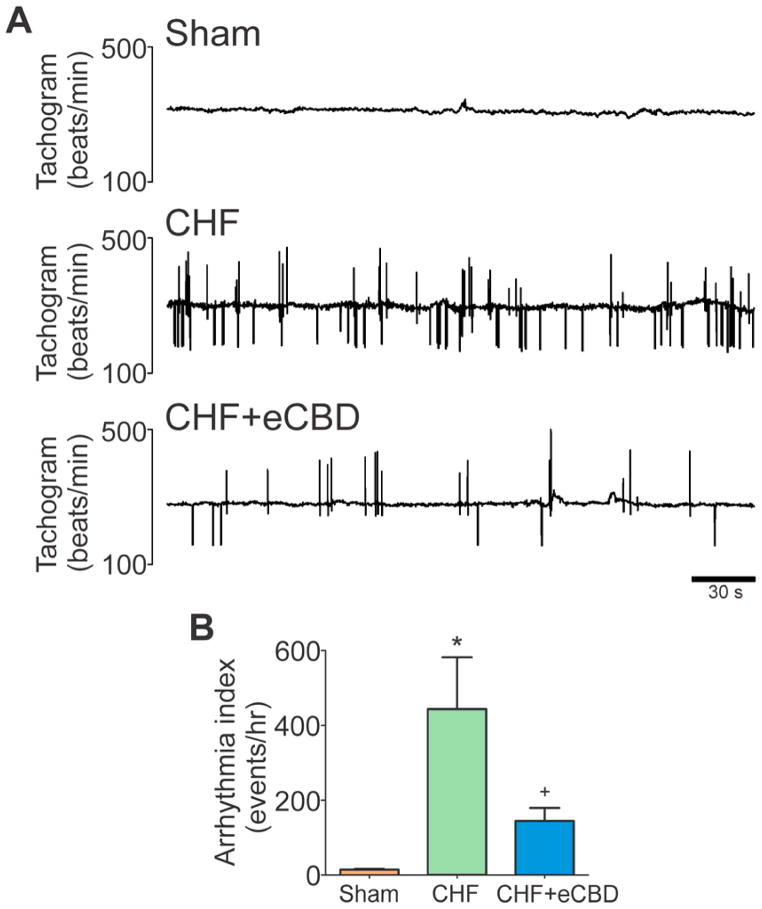
Non-infarcted cardiac tissue from CHF-rats displayed a significant 4.5-fold increase in the LV free wall and a 3-fold increase in the IVS collagen deposition compared to sham hearts (Fig. 5B). Notably, collagen content was reduced in non-infarcted cardiac tissue from CHF-eCBD rats (Fig. 5C–D), which was indistinguishable from the LV free wall and IVS collagen content obtained in sham rats. Analogously, the incidence of arrhythmias was markedly increased in CHF rats compared to Sham rats (Fig. 6), and CHF-eCBD decreased the arrhythmic episodes 2.5-fold compared to the CHF group (Fig. 6B).
Figure 6. Early CBD reduced arrhythmic episodes in CHF.
(A) Tracings show arrhythmic episodes in a CHF rat and a marked decreased in the arrhythmic events in a CHF+eCBD rat. (B) Arrhythmia incidence was increased in CHF and significantly reversed by eCBD at 16 weeks post CAL. *P<.05 vs. Sham, +P<.05 vs. CHF. n=4 rats/group.
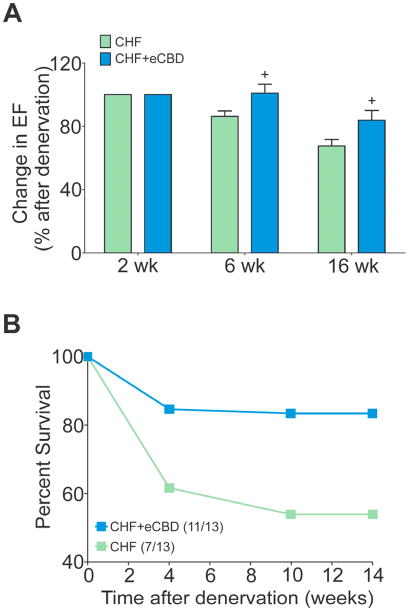
Both CHF and CHF+eCBD groups had comparable EF before CBD was performed at 2 week after CAL (Supplemental Table 3), but the progressive deterioration of EF in CHF-eCBD rats was blunted over the remaining 14 week period compared to CHF rats (Fig. 7A). A diminished expansion of LVsV accompanied the preservation of EF in CHF-eCBD rats (52.5 ± 16.6 vs. 98.0 ± 12.0% increase, CHF-eCBD vs. CHF, P < .05). The increase in LVdV also trended to be smaller in CHF-eCBD rats (37±12% vs. 61±10% increase, CHF-eCBD vs. CHF) but was not statistically significant (P =.14).
Figure 7. Early CBD delayed cardiac deterioration and improved survival in CHF.
(A) Selective eCBD blunted the deterioration in the EF during CHF (expressed as % of the EF values obtained at 2 weeks post-CAL surgery prior to CBD). +P<.01 vs. CHF. (B) Early CBD improved survival rate in CHF rats compared to those with intact CBs (P=.04).
Most remarkably, eCBD significantly reduced the mortality rate in CHF rats (P = .04) over the 14 weeks post-infarct compared to CHF rats with the CB intact (85% vs. 45% survival, CHF-eCBD vs. CHF) (Fig. 7B).
Discussion
The present study unveils a major role of the CB chemoreflex in the pathophysiology of heart failure. Here we show for the first time that selective CBD normalizes control of breathing, reduces pre-sympathetic neuronal activation in the RVLM in the brainstem, and restores normal autonomic and baroreflex function in CHF rats. Notably, CBD performed early after myocardial infarction results in significant reductions in aberrant ventricular remodeling and incidence of arrhythmias, and reduces the progressive deterioration of left ventricular function. The outcome being selective CBD improves survival in CHF rats.
Cardiac deterioration and mortality during the progression of heart failure
Autonomic dysfunction, breathing disorder, myocardial remodeling, and arrhythmogenesis constitute major predictors of morbidity and mortality in CHF (2,14,26–28). We have shown that CBD reduces sympathetic activation, respiratory instability, myocardial fibrosis, arrhythmias, and mortality during CHF. Moreover, our data strongly suggest that the effects of CBD are primarily associated with a normalization of altered central neural control of cardiovascular and ventilatory function arising from elevated input from the CB.
Selective CBD and cardiac remodeling during heart failure
While the present results suggest a seminal role of the CB in cardiac remodeling and progression of the disease, the molecular signaling pathways in the heart affected by CBD remain to be elucidated. Nevertheless, we can speculate that the effects of CBD on cardiac remodeling are likely due, at least in part, to a reduced sympathetic input and increased parasympathetic input to the heart. Indeed, it is well known that maladaptive heightened sympathetic/reduced parasympathetic outflow to the heart following myocardial infarction constitutes a major component in the progression into CHF (1,3,7,29). Moreover, it has been proposed that the mechanism underlying cardiac deterioration is associated with neurohumoral activation and increased catecholamines release by cardiac sympathetic nerve terminals during augmented sympathetic drive in CHF (30,31). Also, although CBD markedly improved cardiac systolic function and survival during CHF, it did not alter cardiac left ventricular chamber dilatation and hypertrophy (Supplemental Table 3). Clearly, other factors continue to play an important role in cardiac tissue remodeling after myocardial infarction that are not affected by CB denervation.
Selective CBD and sympathetic activation in heart failure
Activation of the RVLM is a critical component in the regulation of sympathetic outflow during CHF (17). We found that CBD decreases pre-sympathetic neuronal activation in the RVLM in CHF. Thus, it is reasonable to assume that CBD resulted in a general decrease in sympathetic outflow in the CHF rats, although the extent to specific vascular beds cannot be ascertained directly. However, the improvement in HRV and reduced arrhythmogenesis after CBD are consistent with a reduction in sympathetic outflow to the heart.
Besides the direct beneficial effects of CBD to reduce sympathetic activation of the heart during CHF, the reduction in sympathetic outflow may indirectly benefit the heart by impacting vascular peripheral resistance to decrease cardiac afterload and the kidneys to improve renal function and blood volume homeostasis. In support of improved afterload, we observed a trend (but not statistically significant) toward decreased BP after CBD (Supplemental Table 4). In addition, CBD decreased LF-SBPV suggesting that CBD may have decreased vasomotor tone in CHF rats. We did not assess renal function in this study; however, it is well known that sympatho-excitation impairs renal function in CHF (32). The impact of CBD on hemodynamics and renal function during the progression of CHF warrants further study.
Selective CBD and breathing in heart failure
The incidence of breathing disorders is high in patients with CHF (10–12), and the incidence of periodic breathing and Cheyne-Stokes respiration is associated with a deterioration of cardiac function (12). Remarkably, CBD reduced breathing variability and diminished the occurrence of apnea episodes in CHF rats, which supports a role for the CB in the generation of abnormal breathing patterns observed during CHF. The central neural mechanisms underlying induction of breathing instability by the CB in CHF deserve further study.
Clinical Implications
Despite the current advances in the treatment of heart diseases, mortality rates during CHF are still high (33). The progressive deterioration of cardiac function during CHF encompasses complex pathophysiological mechanisms that are resistant to treatment by a single therapeutic intervention. Breathing disorders and heightened sympathetic outflow are two major hallmarks of CHF and both are closely related to increased morbidity and mortality (1,2,5,6). Furthermore, cardiac remodeling and increases in cardiac arrhythmogenesis are both recognized to contribute to the progression of CHF. Our current findings indicate that targeted ablation of the CB is a potentially valuable therapeutic strategy that can effectively reverse autonomic and respiratory dysfunction and improve survival of CHF. Excitingly, a recent case report presented by Niewiński et al. (34) showed that surgical removal of the CB from a patient with systolic heart failure significantly decreased sympathetic tone. Thus, our results showing the more extensive beneficial effects of ablation of the CB should be relevant and potentially transferrable to humans.
Summary
The present study reports a major role of the CB chemoreflex in the cardiorespiratory alterations following CHF and shows that selective ablation of the CB effectively restores normal control of breathing and autonomic and baroreflex function during CHF. Furthermore, when performed earlier during the progression of CHF, selective CB ablation improves survival. Taken together, targeted CB denervation shows promise as a novel therapeutic strategy to improve autonomic control of the heart, decrease cardiac remodeling, reduce the incidence of arrhythmias, and increase life span in heart failure patients. Additionally, selective CB ablation may also have important implications for the treatment of breathing abnormalities during heart failure.
Supplementary Material
Acknowledgments
Supported by a Program Project Grant from the Heart, Lung and Blood Institute of NIH (PO1-HL62222).
This study was supported by a Program Project Grant from the Heart, Lung and Blood Institute of NIH (PO1-HL62222). The authors wish to thank Dr. Kurtis G. Cornish for his surgical assistance, Mary Ann Zink, Francisca Allendes, Johnnie F. Hackley, and Richard Robinson for their technical assistance.
Abbreviations list
- AHI
apnea and hypopnea index
- CAL
coronary artery ligation
- CB
carotid body
- CBD
carotid body denervation
- CHF
chronic heart failure
- eCBD
early carotid body denervation
- Fra-1
Fos related antigen 1
- HRV
heart rate variability
- LV
left ventricle
- RVLM
rostral ventrolateral medulla
Footnotes
Disclosures: The authors and have no conflict of interest to disclosure regarding funding or compensation from industry for this study. However, all authors collectively provided consultancy to Coridea NCI (now Cibiem, Inc.) regarding their interests in the efficacy of this procedure in animal models of CHF.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–62. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153:272–6. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 3.Ponikowski P, Chua TP, Anker SD, et al. Peripheral chemoreceptor hypersensitivity: an ominous sign in patients with chronic heart failure. Circulation. 2001;104:544–9. doi: 10.1161/hc3101.093699. [DOI] [PubMed] [Google Scholar]
- 4.Porter TR, Eckberg DL, Fritsch JM, et al. Autonomic pathophysiology in heart failure patients. Sympathetic-cholinergic interrelations. J Clin Invest. 1990;85:1362–71. doi: 10.1172/JCI114580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Despas F, Lambert E, Vaccaro A, et al. Peripheral chemoreflex activation contributes to sympathetic baroreflex impairment in chronic heart failure. J Hypertens. 2012;30:753–60. doi: 10.1097/HJH.0b013e328350136c. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Chua TP, Piepoli M, et al. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–94. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- 7.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 8.Li YL, Li YF, Liu D, et al. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res. 2005;97:260–7. doi: 10.1161/01.RES.0000175722.21555.55. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Li YL, Zimmerman MC, Davisson RL, Schultz HD. Role of CuZn superoxide dismutase on carotid body function in heart failure rabbits. Cardiovasc Res. 2009;81:678–85. doi: 10.1093/cvr/cvn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley TD, Floras JS. Sleep apnea and heart failure: Part I: obstructive sleep apnea. Circulation. 2003;107:1671–8. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 11.Bradley TD, Floras JS. Sleep apnea and heart failure: Part II: central sleep apnea. Circulation. 2003;107:1822–6. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 12.Leung RS, Floras JS, Lorenzi-Filho G, et al. Influence of cheyne-stokes respiration on cardiovascular oscillations in heart failure. Am J Respir Crit Care Med. 2003;167:1534–9. doi: 10.1164/rccm.200208-793OC. [DOI] [PubMed] [Google Scholar]
- 13.Del Rio R, Marcus NJ, Schultz HD. Inhibition of hydrogen sulfide restores normal breathing stability and improves autonomic control during experimental heart failure. J Appl Physiol. 2013;114:1141–50. doi: 10.1152/japplphysiol.01503.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannoni A, Emdin M, Poletti R, et al. Clinical significance of chemosensitivity in chronic heart failure: influence on neurohormonal derangement, Cheyne-Stokes respiration and arrhythmias. Clin Sci (Lond) 2008;114:489–97. doi: 10.1042/CS20070292. [DOI] [PubMed] [Google Scholar]
- 15.Paton JF, Sobotka PA, Fudim M, et al. The carotid body as a therapeutic target for the treatment of sympathetically mediated diseases. Hypertension. 2013;61:5–13. doi: 10.1161/HYPERTENSIONAHA.111.00064. [DOI] [PubMed] [Google Scholar]
- 16.Verna A, Roumy M, Leitner LM. Loss of chemoreceptive properties of the rabbit carotid body after destruction of the glomus cells. Brain Res. 1975;100:13–23. doi: 10.1016/0006-8993(75)90239-5. [DOI] [PubMed] [Google Scholar]
- 17.Haack KK, Engler CW, Papoutsi E, Pipinos II, Patel KP, Zucker IH. Parallel changes in neuronal AT1R and GRK5 expression following exercise training in heart failure. Hypertension. 2012;60:354–61. doi: 10.1161/HYPERTENSIONAHA.112.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagani M, Lombardi F, Guzetti S, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 19.Kuo TBJ, Yang CCH, Chan SHH. Selective activation of vasomotor component of SAP spectrum by nucleus reticularis ventrolateralis in rats. Am J Physiol. 1997;272:H485–92. doi: 10.1152/ajpheart.1997.272.1.H485. [DOI] [PubMed] [Google Scholar]
- 20.Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol. 2007;34:362–8. doi: 10.1111/j.1440-1681.2007.04588.x. [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- 22.Aboukhalil A, Nielsen L, Saeed M, Mark RG, Clifford GD. Reducing false alarm rates for critical arrhythmias using the arterial blood pressure waveform. J Biomed Inform. 2008;41:442–51. doi: 10.1016/j.jbi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol. 1998;275:H2140–6. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- 24.Wang HJ, Li YL, Zucker IH, Wang W. Exercise training prevents skeletal muscle afferent sensitization in rats with chronic heart failure. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1260–70. doi: 10.1152/ajpregu.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott SB, DePuy SD, Nguyen T, Coates MB, Stornetta RL, Guyenet PG. Selective optogenetic activation of rostral ventrolateral medullary catecholaminergic neurons produces cardiorespiratory stimulation in conscious mice. J Neurosci. 2013;33:3164–77. doi: 10.1523/JNEUROSCI.1046-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–82. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 27.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–84. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 28.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 29.Packer M. The neurohormonal hypothesis: a theory to explain the mechanism of disease progression in heart failure. J Am Coll Cardiol. 1992;20:248–54. doi: 10.1016/0735-1097(92)90167-l. [DOI] [PubMed] [Google Scholar]
- 30.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 31.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 32.Rea ME, Dunlap ME. Renal hemodynamics in heart failure: implications for treatment. Curr Opin Nephrol Hypertens. 2008;17:87–92. doi: 10.1097/MNH.0b013e3282f357da. [DOI] [PubMed] [Google Scholar]
- 33.Rich MW. Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. J Am Geriatr Soc. 1997;45:968–74. doi: 10.1111/j.1532-5415.1997.tb02968.x. [DOI] [PubMed] [Google Scholar]
- 34.Niewiński P, Janczak D, Rucinski A, et al. Carotid body removal for treatment of chronic systolic heart failure. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2013.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.