Abstract
Cellular metabolism and reactive oxygen species (ROS) formation are interrelated processes in mitochondria and are implicated in a variety of human diseases including ischemic heart disease. During ischemia, mitochondrial respiration rates fall. Though seemingly paradoxical, reduced respiration has been observed to be cardioprotective due in part to reduced generation of ROS. Enhanced myocardial glucose uptake is considered beneficial for the myocardium under stress, as glucose is the primary substrate to support anaerobic metabolism. Thus, inhibition of mitochondrial respiration and uncoupling oxidative phosphorylation can protect the myocardium from irreversible ischemic damage. Growing evidence now positions the TXNIP/thioredoxin system at a nodal point linking pathways of antioxidant defense, cell survival, and energy metabolism. This emerging picture reveals TXNIP’s function as a regulator of glucose homeostasis and may prove central to regulation of mitochondrial function during ischemia. In this review, we summarize how TXNIP and its binding partner thioredoxin act as regulators of mitochondrial metabolism. While the precise mechanism remains incompletely defined, the TXNIP-thioredoxin interaction has the potential to affect signaling that regulates mitochondrial bioenergetics and respiratory function with potential cardioprotection against ischemic injury.
Ischemic heart disease and cardiac metabolism
The clinical profile of ischemic heart disease has changed profoundly during the last decade, with a growing prevalence of diabetes mellitus and the metabolic syndrome. Compared to individuals without diabetes, diabetic patients have a 2-fold higher risk of short-term mortality after acute myocardial infarction (Woodfield et al., 1996). The impact of metabolic disorders upon cardiac metabolism could contribute to the failure of conventional therapies to ischemic heart diseases. For example, insulin resistance can worsen myocardial ischemia; this is partially because insulin resistance increases myocardial fatty acid metabolism, leading to increased myocardial oxygen consumption (MVO2). Hyperglycemia is also associated with increased production of reactive oxygen species (ROS), which promotes worsened tissue damage. Thus, diabetes-associated cardiac damage is closely associated with mitochondrial metabolism and oxidative stress that can contribute to left ventricular dysfunction and heart failure following ischemic injury. Because of the impact of the diabetes epidemic on ischemic heart disease, a better understanding of cardiac metabolism is now crucial.
Mitochondrial respiration and anaerobic metabolism under ischemia
Fatty acids and pyruvate enter the mitochondrion and are then metabolized by the citric acid cycle, which produces NADH and FADH2. In the process of oxidative phosphorylation, high-energy electrons from NADH and FADH2 are passed to oxygen by means of the respiratory complex in the inner mitochondrial membrane to produce ATP by a chemiosmotic mechanism. ROS are byproducts of the electron flux through mitochondrial oxidative phosphorylation. Because of its role as an electron acceptor during mitochondrial respiration, oxygen is critical for the survival of multicellular organisms; however, many organs adapt to conditions of low oxygen (Kelly, 2008). Ischemia injury is characterized by release of tissue enzymes, oxidative modification of essential proteins and lipids, and inflammatory responses, which ultimately lead to tissue necrosis and apoptosis. The adaptation to ischemia is achieved by a dramatic reduction in cellular oxygen consumption with a shift in cellular fuel utilization from mitochondrial respiration to the generation of ATP outside of mitochondrion by burning glucose without the use of oxygen (anaerobic glycolysis) (Kelly, 2008). Accumulating data suggest that anaerobic glycolysis, with concomitant increased use of glucose, plays a significant role in promoting recoverability of the ischemic heart (Weissler et al., 1968). Inhibition of mitochondrial coupled respiration and oxidative phosphorylation can protect the myocardium from irreversible ischemic damage (Chen et al., 2007). The concept that cellular protection is mediated by a reduced capacity to produce energy during ischemia seems counterintuitive. However, the continuation of mitochondrial aerobic respiration in the absence of oxygen results in excessive or unneutralized ROS production, mitochondrial calcium overload, the onset of mitochondrial permeability transition, and activation of apoptotic cascades, all of which lead to mitochondrial-driven cardiomyocyte death (Chen et al., 2007). In this context, reduced mitochondrial respiration during and immediately following an ischemic episode attenuates the extent of myocardial injury (Chen and Lesnefsky, 2006). Blockade of electron transport (Chen et al., 2006) or the partial uncoupling of respiration during ischemia (McLeod et al., 2005; Nadtochiy et al., 2006) significantly lessens cardiac injury during ischemia and reperfusion. Stimuli that uncouple mitochondrial respiration prior to an ischemic insult have been suggested to underlie cardioprotection mediated by ischemic preconditioning (Nadtochiy et al., 2006). Hence, mechanisms that down-regulate mitochondrial function in anaerobic metabolism could be cardioprotective during and following ischemia. However, the ischemic cell still requires an energy source to regenerate its ATP stores. It would thus be necessary to increase glucose uptake into cells, as glucose is the primary substrate to support anaerobic metabolism in the heart. In diabetic conditions, this shift to anaerobic myocardial glucose metabolism during acute ischemia may be interrupted since the rate of glucose uptake can be decreased as a result of insulin resistance.
Thioredoxin-interacting protein (TXNIP) is a metabolic control protein that regulates cellular glucose uptake
TXNIP is a member of the alpha arrestin protein superfamily and serves as a tumor suppressor gene. A well known function of TXNIP is that TXNIP binds covalently to thioredoxin; this both inhibits thioredoxin’s ability to scavenge ROS and interferes with thioredoxin’s binding to other signaling molecules. TXNIP was identified as the gene most dramatically induced by glucose in an oligonucleotide microarray study (Shalev et al., 2002). This marked induction of TXNIP by glucose appears to be due to a carbohydrate response element in its promoter region (Shalev et al., 2002) and occurs in multiple cell types including cardiomyocytes (Figure 1). MondoA:Mlx complexes are glucose-dependent transcriptional regulators of TXNIP, activating expression after glucose-dependent nuclear accumulation and occupancy of the carbohydrate response elements of the TXNIP promoter (Stoltzman et al., 2008). TXNIP expression is elevated in the vasculature and the pancreas (Minn et al., 2005) of diabetic animals and in the skeletal muscle of human type 2 diabetes (Parikh et al., 2007). Genetic variation in TXNIP is associated with hypertriglyceridemia and blood pressure in human type 2 diabetes mellitus (van Greevenbroek et al., 2007).
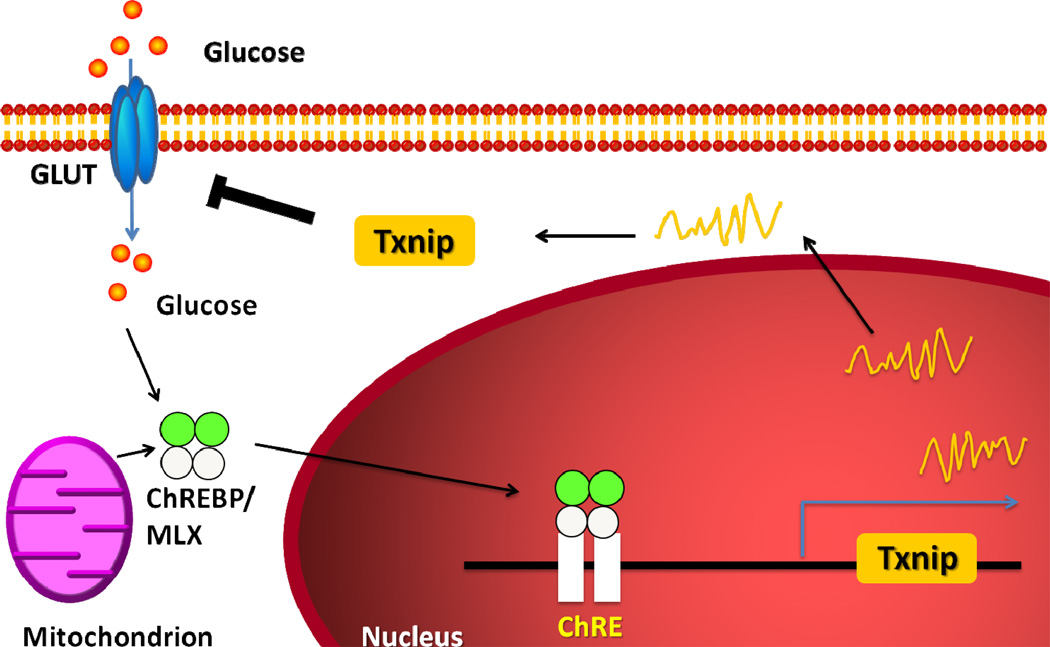
Figure 1. The feedback loop to regulate glucose transport by TXNIP.
TXNIP expression is increased by glucose via glucose-dependent transcriptional regulations by carbohydrate response element binding protein (ChREBP) and MondoA:Mlx. The induction of TXNIP under hyperglycemia inhibits further glucose uptake by feedback regulation.
More than simply a marker of glucose levels, TXNIP is a mediator of glucose homeostasis. Plasma-membrane-localized TXNIP mediates the acute suppression of glucose transporter (GLUT)1 function through endocytosis, whereas nuclear-localized TXNIP mediates suppression of GLUT1 mRNA and protein levels to reduce glucose uptake (Wu et al., 2013). TXNIP overexpression blunts adipocyte glucose uptake, while TXNIP gene-silencing augments both insulin-dependent and -independent glucose uptake in skeletal muscle and adipocytes (Chutkow et al., 2010; Parikh et al., 2007). Deletion of TXNIP robustly enhances myocardial glucose uptake (Yoshioka et al., 2007). These data reveal that TXNIP is a key regulator of glucose metabolism, and that the induction of TXNIP under hyperglycemia can inhibit further glucose uptake by feedback regulation (Figure 1).
While TXNIP is an endogenous inhibitor of antioxidant thioredoxin, TXNIP’s role as a glucose homeostatic regulator seems independent of its redox-mediated interaction with thioredoxin. A recent report identified both TXNIP and the related alpha arrestin ARRDC4 as inhibitors of glucose uptake (Patwari et al., 2009). Since ARRDC4 does not bind thioredoxin, regulation of glucose uptake may be intrinsic to the arrestin domains in TXNIP rather than its interaction with thioredoxin. TXNIP and five other alpha arrestins (ARRDC1–5) have two PPxY scaffolding motifs in their C-terminal tail. PPxY motifs are known to bind to WW domain proteins that regulate protein turnover, such as the Nedd4 family of E3 ubiquitin ligases. Thus, interaction between TXNIP and ubiquitin ligases may control endocytosis and degradation of substrate proteins such as glucose transporters.
Possible mechanisms by which TXNIP mediates mitochondrial respiration
The oxidative capacity of mitochondria is determined by the expression level of oxidative phosphorylation (OxPhos) subunits and by the number and size of mitochondria. Genes involved in mitochondrial OxPhos are regulated by mitochondrial biogenesis regulatory programs primarily through nuclear transcriptional regulation of mitochondrial proteins, which are mostly encoded by nuclear genes. Cellular hypoxic reprogramming in mitochondria involves a reduction of OxPhos accompanied by an increase in anaerobic glycolysis to maintain ATP production as an adaptive metabolic response, termed the Pasteur effect. One of major mechanisms by which cells respond to hypoxia is through hypoxia-inducible transcription factors (HIFs). In the setting of limited oxygen availability, HIFs drive an increase in the levels of pyruvate dehydrogenase kinase 4 (PDK4), a kinase that blocks the activity of the pyruvate dehydrogenase (PDH) complex. The decreased activity of PDH results in a reduction in mitochondrial respiration through the electron transport chain and a drop in cellular O2 consumption (Papandreou et al., 2006). This response reduces the generation of injurious ROS in mitochondria (Kelly, 2008), related to a shift in glucose metabolism from inside to outside of the mitochondrion, thereby leading to resistance to hypoxia (Aragones et al., 2008). TXNIP and thioredoxin may participate in these mitochondrial regulatory programs.
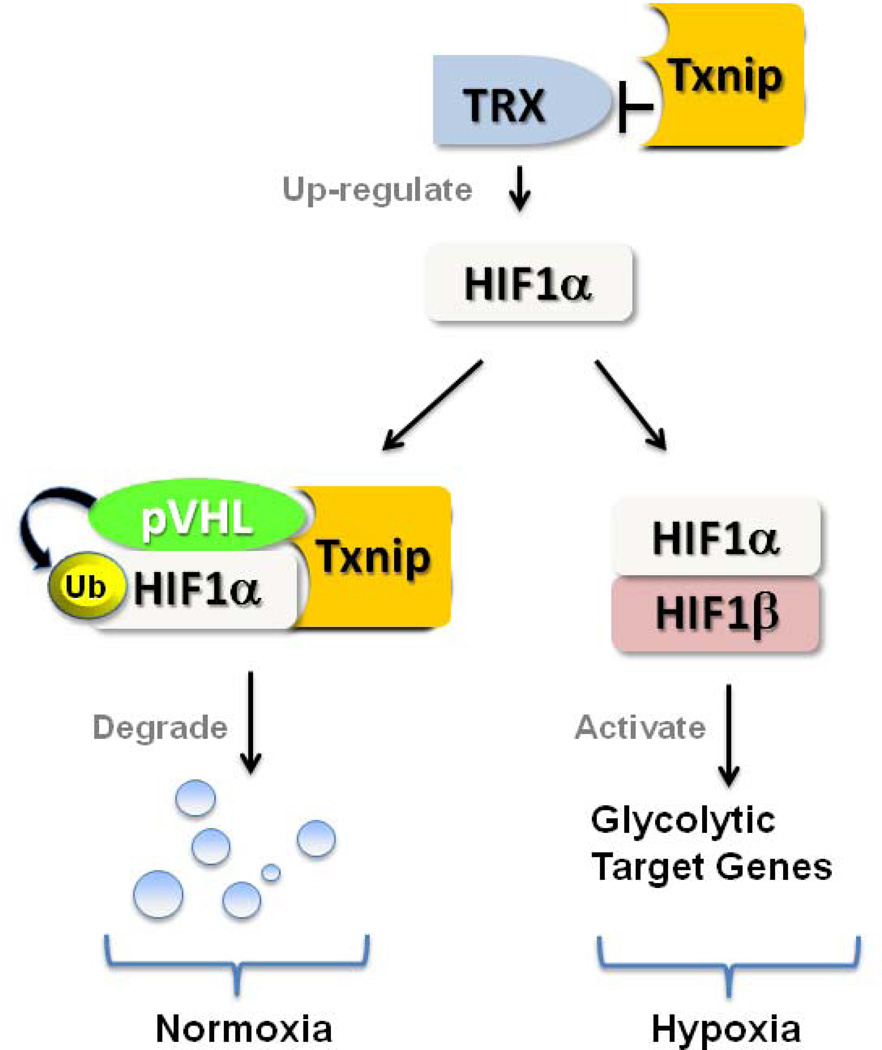
TXNIP has a putative hypoxia-regulated element binding sequence in its promoter region (Baker et al., 2008), and the expression level of TXNIP is highly sensitive to hypoxia. Evidence now demonstrates a functional interaction between TXNIP and the HIF-1 transcription factor (Figure 2). HIF-1 is a heterodimeric transcription factor consisting of HIF-1α and HIF-1β subunits. While HIF-1β levels are constitutive under aerobic conditions, HIF-1α protein is rapidly degraded following O2-dependent hydroxylation by prolyl-hydroxylases and von Hippel-Lindau protein (pVHL) under normoxia (Semenza, 2000). Under hypoxic conditions, HIF-1α degradation is inhibited and HIF-1α protein levels increase, leading to an increase in HIF-1 transactivating activity. HIF-1 signaling is a critical regulator of cellular hypoxic responses and glycolytic target genes. HIF-1α activation can repress mitochondrial function (Zhang et al., 2007) and oxygen consumption by inhibiting PDH activity (Zhang et al., 2007). TXNIP associates with the beta-domain of pVHL and enhances the interaction between pVHL and HIF1α to promote the ubiquitin-induced degradation of HIF1α. TXNIP mediates nuclear export of HIF-1α, resulting in HIF-1α degradation (Shin et al., 2007). Blocking of TXNIP inhibits this nuclear export of pVHL/HIF1α and relieves the destabilization of HIF1α (Shin et al., 2007). TXNIP deficiency leads to a metabolic switch, directing cells toward enhanced glycolytic metabolism even under normoxia in multiple organs (Chutkow et al., 2010; Yoshioka et al., 2012). Given these recent data demonstrating TXNIP’s interaction with the HIF-1 transcription factor, HIF-1α stability and its downstream signaling may be engaged in TXNIP regulation of mitochondrial function. On the other hand, HIF-1α expression itself is controlled by thioredoxin (Zhou et al., 2007). Thioredoxin dramatically increases both protein expression and transcriptional activity of HIF-1α under hypoxic conditions (Zhou et al., 2007), leading to increased expression of HIF-regulated genes (Welsh et al., 2003). Although it remains largely unknown whether thioredoxin directly contributes to the Pasteur effect, HIF-1α activation by thioredoxin may also play a role in increasing oxygen availability in response to low oxygen tension.
Figure 2. TXNIP interacts with von Hippel-Lindau protein (pVHL) to promote ubiquitin (Ub)-induced degradation of HIF-1α.
HIF-1α protein is rapidly degraded by pVHL with TXNIP under normoxia. Under hypoxic conditions, HIF-1α degradation is inhibited to activate glycolytic target genes that regulate mitochondrial oxidative phosphorylation. Blocking of TXNIP relieves the destabilization of HIF1α. HIF-1α expression is also up-regulated by thioredoxin.
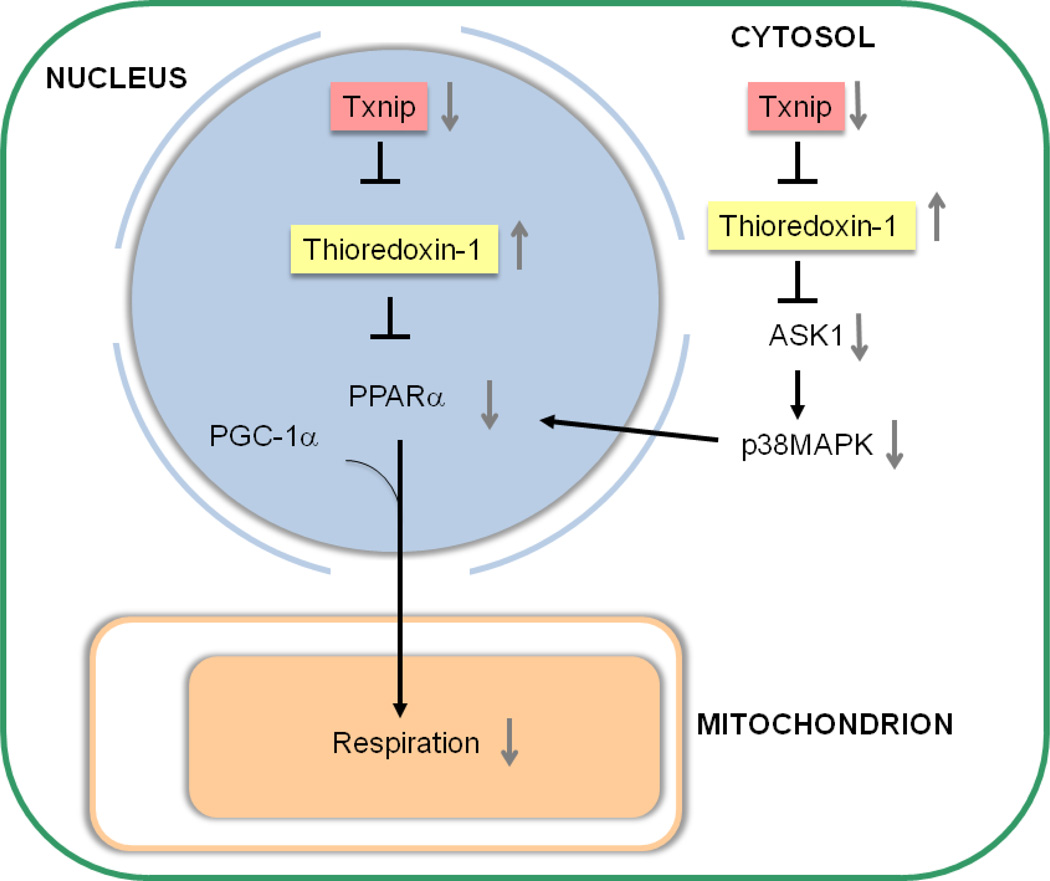
Another possible regulatory mechanism of mitochondrial function by TXNIP and thioredoxin is transcriptional activation of peroxisome proliferators-activated receptor (PPAR)α (Figure 3). Nuclear translocation of thioredoxin inhibits the transcriptional activity of PPARα (Liu et al., 2006). PPARα is a transcription factor belonging to the nuclear receptor superfamily and is required for activation of the mitochondrial biogenic response (Duncan et al., 2007). Thioredoxin’s inhibition of PPARα-mediated transcription is enhanced by TXNIP knockdown (Liu et al., 2006). Thioredoxin binds to the amino-terminal portion of apoptosis signal-regulating kinase 1 (ASK1), which can activate p38 MAP kinase. TXNIP binds to the catalytic cysteines of thioredoxin and displaces ASK1 from thioredoxin, leading to activation of p38 MAP kinase, which can then activate PPARα in cardiomyocytes (Barger et al., 2001). Thus, since TXNIP and thioredoxin have been shown to regulate PPARα, a critical control of mitochondrial respiration and biogenesis, TXNIP’s mitochondrial effects may operate via thioredoxin-mediated regulation of PPARα.
Figure 3. Hypothetical signaling pathways for mitochondrial regulation by TXNIP.
As both TXNIP and thioredoxin affect PPARα activity, TXNIP’s mitochondrial effects may operate via thioredoxin-mediated regulation of PPARα.
TXNIP can bind to the mitochondrial type of thioredoxin (thioredoxin-2) (Saxena et al., 2010). Mitochondrial thioredoxin-2 interacts with components of the mitochondrial respiratory chain and plays a role in the regulation of the mitochondrial membrane potential (Damdimopoulos et al., 2002). Thioredoxin-2 directly interacts with cytochrome-c to block its release (Tanaka et al., 2002; Wang et al., 2006), and protects cells from ROS-induced apoptosis (Chen et al., 2002; Tanaka et al., 2002). TXNIP binds to the catalytic cysteines of thioredoxin-2 and displaces ASK1 from thioredoxin-2, leading to activation of ASK1-mediated apoptosis (Zhang et al., 2004). While multiple potential pathways may be simultaneously involved, TXNIP and thioredoxins (thioredoxin-1 and -2) may be key participants in transcriptional regulatory circuits that control nuclear genes encoding mitochondrial proteins of oxidative phosphorylation as well as mitochondria-driven apoptosis.
Potential role of myocardial TXNIP in ischemia-reperfusion injury
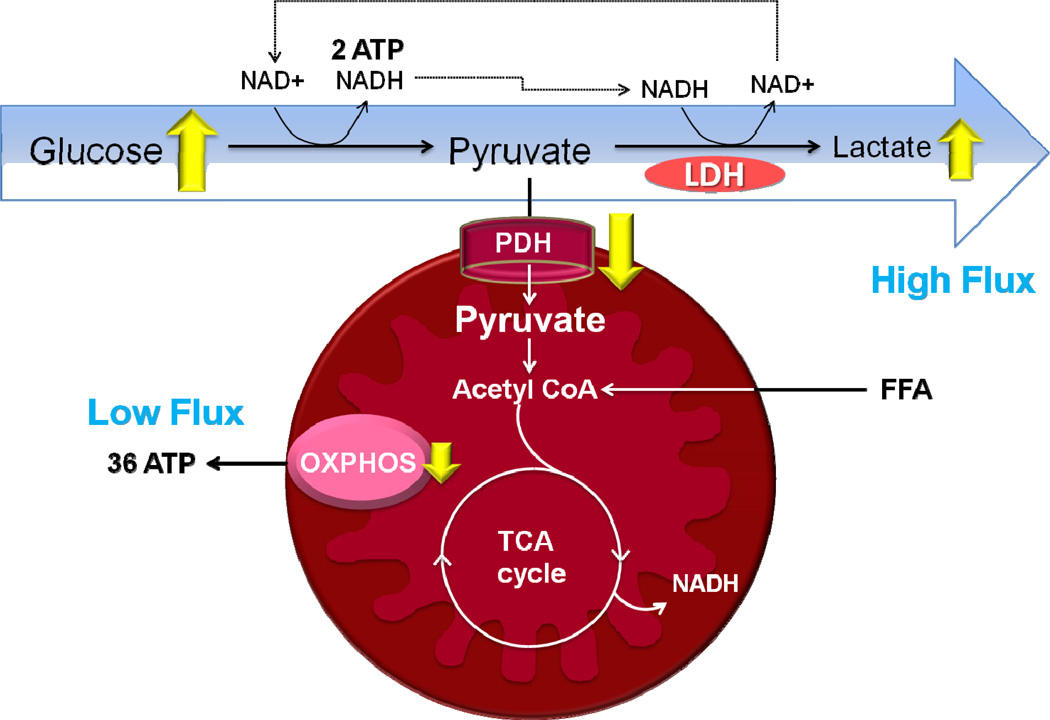
Recent results demonstrate that the metabolic alterations affected by TXNIP deletion enhance myocardial glucose uptake and suppress mitochondrial oxidative phosphorylation (Yoshioka et al., 2012; Yoshioka et al., 2007) (Figure 4). These reflect the myocardial type of the “Warburg effect” – a well-known phenomenon in tumors by which cells preferentially rely upon glycolytic metabolism, despite the availability of sufficient oxygen to support mitochondrial respiration. As discussed above, adaptation to hypoxia requires a dramatic reduction in cellular oxygen consumption. Mitochondrial thioredoxin-2 is inhibited by TXNIP that translocates into mitochondria under the influence of ROS (Saxena et al., 2010), but TXNIP-KO hearts exhibits unperturbed thioredoxin-2 activity after ischemic stress (Yoshioka et al., 2012). These responses induced by deletion of TXNIP can lead to survival adaptation with replenishing energy stores and reducing the generation of ROS in ischemic hearts (Figure 5). In fact, our previous data suggest that TXNIP-KO hearts had significantly improved postischemic left ventricular functional recovery in mice, despite reduced mitochondrial function (Yoshioka et al., 2012). Another study has also shown that targeted degradation of TXNIP mRNA by injection of a small nucleotide-based catalytic enzyme into ischemic myocardium leads to enhanced cardiomyocyte survival and reduced left ventricular remodeling (Xiang et al., 2005).
Figure 4. Mitochondrial metabolism in TXNIP-deletion cardiomyocytes.
TXNIP deficiency reprograms glucose metabolism in cardiomyocytes to a high flux glycolytic state in association with reduced activity of pyruvate dehydrogenase (PDH) and increased cytosolic lactate production maintaining energy homeostasis.
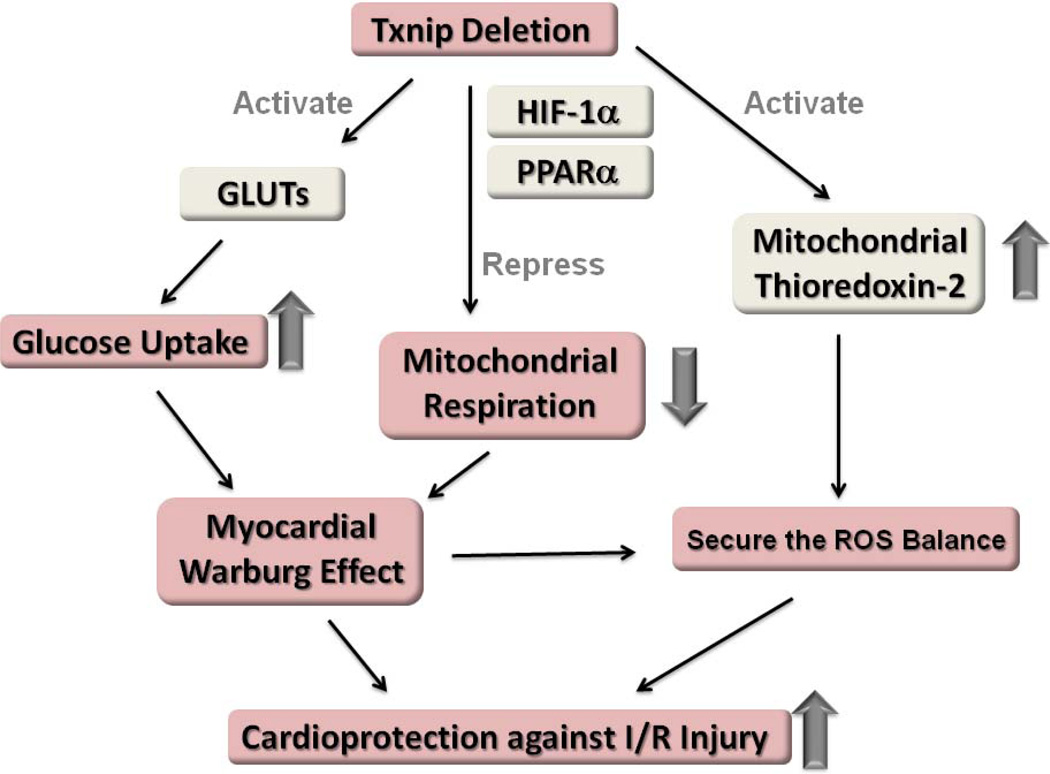
Figure 5. Possible mechanisms by which TXNIP deletion confers a protective advantage against ischemia-reperfusion (I/R) injury.
TXNIP deficiency leads to a significant metabolic switch to achieve cardioprotection by (1) enhancing myocardial glucose uptake, (2) reducing mitochondrial respiration, and (3) securing the ROS balance with the potent antioxidant activation by mitochondrial thioredoxin-2.
TXNIP expression is suppressed by a variety of cellular stimuli including oxidative stress, while overexpression of TXNIP sensitizes cardiomyocytes to ROS-induced apoptosis. Thus, under severe stress, cells may need to down-regulate TXNIP for their survival. However, a high concentration of glucose can counteract this defense mechanism, since TXNIP is one of the most dramatically up-regulated genes in the presence of elevated glucose (Shalev et al., 2002). Hyperglycemia increases cardiac expression of TXNIP and cleaved caspases-3, yet the increase in apoptosis is blunted by pharmacological TXNIP reduction in diabetic mouse hearts (Chen et al., 2009). There is strong evidence that diabetes sensitizes the heart to dysfunction after ischemic injury. Given the central role of TXNIP as a homeostatic switch that integrates stress sensing and controls glucose transport, down-regulation of TXNIP may afford an even greater protective advantage to the ischemic heart in the setting of diabetes. Although TXNIP can be an attractive therapeutic target for ischemic heart disease, pharmacological inhibitors of Txnip have not been developed. A report suggests that a calcium channel blocker can reduce cardiac TXNIP expression in the face of severe diabetes (Chen et al., 2009). Further studies are necessary to justify the development of pharmacological or genetic tools to inhibit TXNIP for cardioprotection.
In conclusion, TXNIP is emerging as a key mediator to integrate redox balance and energetic needs. While the precise mechanisms remain undefined, TXNIP and its binding partner thioredoxins have the potential to beneficially regulate mitochondrial bioenergetics and mitochondrial respiratory function, which confers a protective advantage against ischemic injury.
Acknowledgments
This work was supported by American Heart Association grants 0835484N and 13GRNT16870007 (to JY), and NIH grant HL103582 (to RTL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare that no conflict of interest exists.
References
- Aragones J, et al. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- Baker AF, et al. Identification of thioredoxin-interacting protein 1 as a hypoxia-inducible factor 1alpha-induced gene in pancreatic cancer. Pancreas. 2008;36:178–186. doi: 10.1097/MPA.0b013e31815929fe. [DOI] [PubMed] [Google Scholar]
- Barger PM, et al. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. The Journal of biological chemistry. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. Diabetes induces and calcium channel blockers prevent cardiac expression of proapoptotic thioredoxin-interacting protein. Am J Physiol Endocrinol Metab. 2009;296:E1133–E1139. doi: 10.1152/ajpendo.90944.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, et al. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007;292:C137–C147. doi: 10.1152/ajpcell.00270.2006. [DOI] [PubMed] [Google Scholar]
- Chen Q, et al. Blockade of electron transport before cardiac ischemia with the reversible inhibitor amobarbital protects rat heart mitochondria. J Pharmacol Exp Ther. 2006;316:200–207. doi: 10.1124/jpet.105.091702. [DOI] [PubMed] [Google Scholar]
- Chen Q, Lesnefsky EJ. "Hiding out" from chronic ischemia with help from the mitochondria? J Mol Cell Cardiol. 2006;41:956–958. doi: 10.1016/j.yjmcc.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J Biol Chem. 2002;277:33242–33248. doi: 10.1074/jbc.M202026200. [DOI] [PubMed] [Google Scholar]
- Chutkow WA, et al. Deletion of the alpha-arrestin protein Txnip in mice promotes adiposity and adipogenesis while preserving insulin sensitivity. Diabetes. 2010;59:1424–1434. doi: 10.2337/db09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damdimopoulos AE, et al. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- Duncan JG, et al. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DP. Hypoxic reprogramming. Nat Genet. 2008;40:132–134. doi: 10.1038/ng0208-132. [DOI] [PubMed] [Google Scholar]
- Liu GH, et al. Thioredoxin-mediated negative autoregulation of peroxisome proliferator-activated receptor alpha transcriptional activity. Mol Biol Cell. 2006;17:1822–1833. doi: 10.1091/mbc.E05-10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod CJ, et al. Uncoupling proteins 2 and 3 function in concert to augment tolerance to cardiac ischemia. J Biol Chem. 2005;280:33470–33476. doi: 10.1074/jbc.M505258200. [DOI] [PubMed] [Google Scholar]
- Minn AH, et al. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces beta-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- Nadtochiy SM, et al. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardioprotection. Biochem J. 2006;395:611–618. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Parikh H, et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007;4:e158. doi: 10.1371/journal.pmed.0040158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwari P, et al. Thioredoxin-independent regulation of metabolism by the alpha-arrestin proteins. J Biol Chem. 2009;284:24996–25003. doi: 10.1074/jbc.M109.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena G, et al. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. The Journal of biological chemistry. 2010;285:3997–4005. doi: 10.1074/jbc.M109.034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- Shalev A, et al. Oligonucleotide microarray analysis of intact human pancreatic islets: identification of glucose-responsive genes and a highly regulated TGFbeta signaling pathway. Endocrinology. 2002;143:3695–3698. doi: 10.1210/en.2002-220564. [DOI] [PubMed] [Google Scholar]
- Shin D, et al. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta. 2007 doi: 10.1016/j.bbamcr.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Stoltzman CA, et al. Glucose sensing by MondoA:Mlx complexes: A role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A. 2008;105:6912–6917. doi: 10.1073/pnas.0712199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Greevenbroek MM, et al. Genetic variation in thioredoxin interacting protein (TXNIP) is associated with hypertriglyceridaemia and blood pressure in diabetes mellitus. Diabet Med. 2007;24:498–504. doi: 10.1111/j.1464-5491.2007.02109.x. [DOI] [PubMed] [Google Scholar]
- Wang D, et al. Control of mitochondrial outer membrane permeabilization and Bcl-xL levels by thioredoxin 2 in DT40 cells. J Biol Chem. 2006;281:7384–7391. doi: 10.1074/jbc.M509876200. [DOI] [PubMed] [Google Scholar]
- Weissler AM, et al. Role of anaerobic metabolism in the preservation of functional capacity and structure of anoxic myocardium. J Clin Invest. 1968;47:403–416. doi: 10.1172/JCI105737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh SJ, et al. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2:235–243. [PubMed] [Google Scholar]
- Woodfield SL, et al. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996;28:1661–1669. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- Wu N, et al. AMPK-Dependent Degradation of TXNIP upon Energy Stress Leads to Enhanced Glucose Uptake via GLUT1. Molecular cell. 2013;49:1167–1175. doi: 10.1016/j.molcel.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang G, et al. Catalytic degradation of vitamin D up-regulated protein 1 mRNA enhances cardiomyocyte survival and prevents left ventricular remodeling after myocardial ischemia. J Biol Chem. 2005;280:39394–39402. doi: 10.1074/jbc.M502966200. [DOI] [PubMed] [Google Scholar]
- Yoshioka J, et al. Deletion of thioredoxin-interacting protein in mice impairs mitochondrial function but protects the myocardium from ischemia-reperfusion injury. J Clin Invest. 2012;122:267–279. doi: 10.1172/JCI44927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka J, et al. Targeted Deletion of Thioredoxin-Interacting Protein Regulates Cardiac Dysfunction in Response to Pressure Overload. Circ Res. 2007;101:1328–1338. doi: 10.1161/CIRCRESAHA.106.160515. [DOI] [PubMed] [Google Scholar]
- Zhang H, et al. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang R, et al. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–1491. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. Thioredoxin 1 and thioredoxin 2 have opposed regulatory functions on hypoxia-inducible factor-1alpha. J Biol Chem. 2007;282:7482–7490. doi: 10.1074/jbc.M608289200. [DOI] [PubMed] [Google Scholar]