Abstract
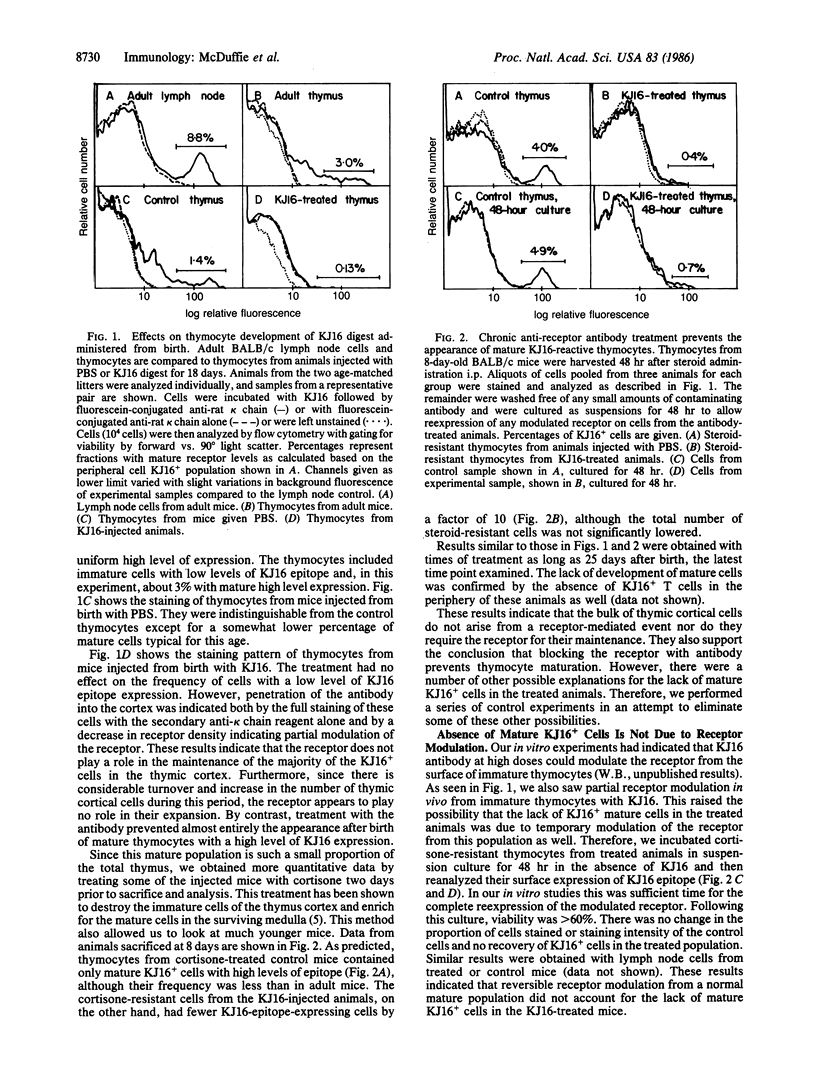
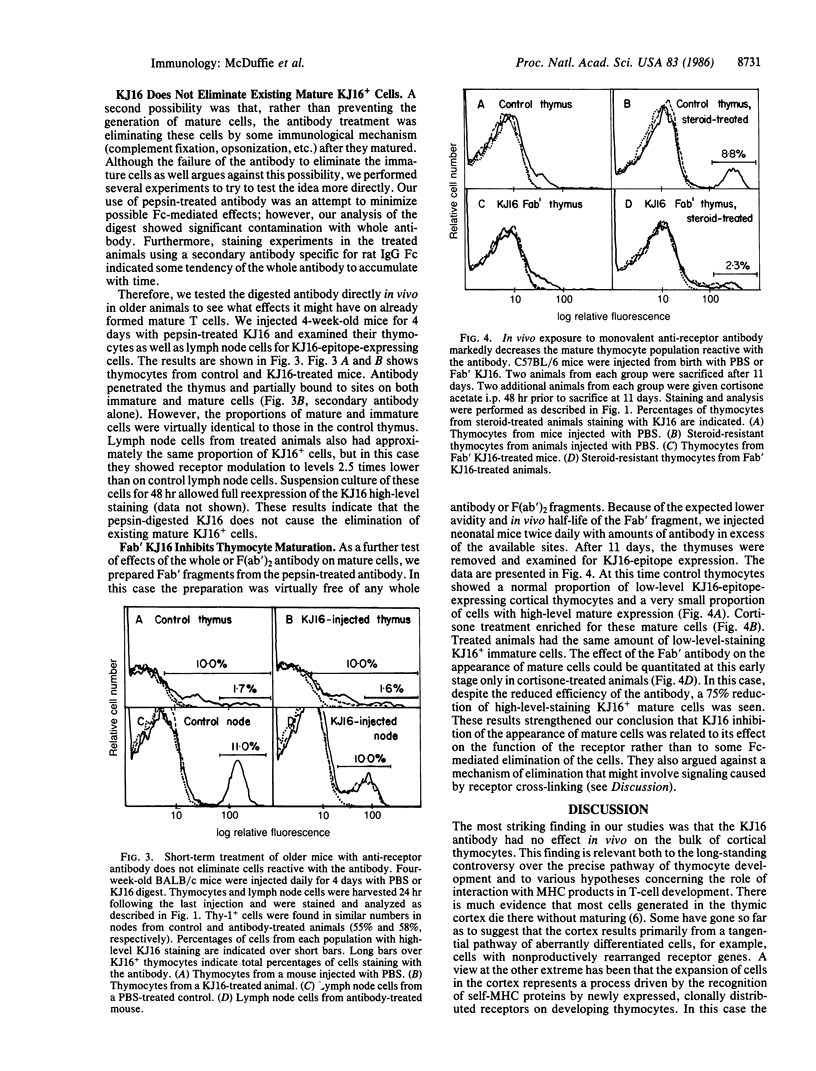
The T-cell receptor, which recognizes antigen plus a product of the major histocompatibility complex, has been postulated to drive T-cell maturation in the thymus by engaging major histocompatibility complex proteins expressed on thymic stromal cells. We tested this idea by injecting neonatal animals with an anti-receptor antibody, KJ16, that binds to about 20% of T cells and is capable of blocking receptor function. In the presence of this antibody, mature T cells bearing the KJ16 epitope failed to develop. On the other hand, although the antibody could be shown to bind to receptors on cortical thymocytes, it did not prevent the rapid expansion or survival of the bulk of the KJ16+ cells in this population. These results are consistent with the hypothesis that most cortical thymocytes arise by a receptor-independent mechanism and that only a small proportion of these cells mature by a process dependent on receptor-major histocompatibility complex interactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M. J. In a radiation chimaera, host H-2 antigens determine immune responsiveness of donor cytotoxic cells. Nature. 1977 Sep 29;269(5627):417–418. doi: 10.1038/269417a0. [DOI] [PubMed] [Google Scholar]
- Born W., Yagüe J., Palmer E., Kappler J., Marrack P. Rearrangement of T-cell receptor beta-chain genes during T-cell development. Proc Natl Acad Sci U S A. 1985 May;82(9):2925–2929. doi: 10.1073/pnas.82.9.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor H., Weissman I. Development and function of subpopulations of thymocytes and T lymphocytes. Prog Allergy. 1976;20:1–64. [PubMed] [Google Scholar]
- Farr A. G., Anderson S. K., Marrack P., Kappler J. Expression of antigen-specific, major histocompatibility complex-restricted receptors by cortical and medullary thymocytes in situ. Cell. 1985 Dec;43(2 Pt 1):543–550. doi: 10.1016/0092-8674(85)90183-7. [DOI] [PubMed] [Google Scholar]
- Haskins K., Hannum C., White J., Roehm N., Kubo R., Kappler J., Marrack P. The antigen-specific, major histocompatibility complex-restricted receptor on T cells. VI. An antibody to a receptor allotype. J Exp Med. 1984 Aug 1;160(2):452–471. doi: 10.1084/jem.160.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J. W., Marrack P. C. Helper T cells recognise antigen and macrophage surface components simultaneously. Nature. 1976 Aug 26;262(5571):797–799. doi: 10.1038/262797a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., Kubo R., Haskins K., White J., Marrack P. The mouse T cell receptor: comparison of MHC-restricted receptors on two T cell hybridomas. Cell. 1983 Oct;34(3):727–737. doi: 10.1016/0092-8674(83)90529-9. [DOI] [PubMed] [Google Scholar]
- Kruisbeek A. M., Fultz M. J., Sharrow S. O., Singer A., Mond J. J. Early development of the T cell repertoire. In vivo treatment of neonatal mice with anti-Ia antibodies interferes with differentiation of I-restricted T cells but not K/D-restricted T cells. J Exp Med. 1983 Jun 1;157(6):1932–1946. doi: 10.1084/jem.157.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruisbeek A. M., Mond J. J., Fowlkes B. J., Carmen J. A., Bridges S., Longo D. L. Absence of the Lyt-2-,L3T4+ lineage of T cells in mice treated neonatally with anti-I-A correlates with absence of intrathymic I-A-bearing antigen-presenting cell function. J Exp Med. 1985 May 1;161(5):1029–1047. doi: 10.1084/jem.161.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettgen H. C., Terhorst C., Cantley L. C., Rosoff P. M. Stimulation of the T3-T cell receptor complex induces a membrane-potential-sensitive calcium influx. Cell. 1985 Mar;40(3):583–590. doi: 10.1016/0092-8674(85)90206-5. [DOI] [PubMed] [Google Scholar]
- Raulet D. H., Garman R. D., Saito H., Tonegawa S. Developmental regulation of T-cell receptor gene expression. Nature. 1985 Mar 7;314(6006):103–107. doi: 10.1038/314103a0. [DOI] [PubMed] [Google Scholar]
- Roehm N., Herron L., Cambier J., DiGuisto D., Haskins K., Kappler J., Marrack P. The major histocompatibility complex-restricted antigen receptor on T cells: distribution on thymus and peripheral T cells. Cell. 1984 Sep;38(2):577–584. doi: 10.1016/0092-8674(84)90512-9. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Lugo J. P. Differentiation and cell division in the mammalian thymus. Dev Biol. 1985 Nov;112(1):1–17. doi: 10.1016/0012-1606(85)90114-9. [DOI] [PubMed] [Google Scholar]
- Sim G. K., Augustin A. A. V beta gene polymorphism and a major polyclonal T cell receptor idiotype. Cell. 1985 Aug;42(1):89–92. doi: 10.1016/s0092-8674(85)80104-5. [DOI] [PubMed] [Google Scholar]
- Snodgrass H. R., Kisielow P., Kiefer M., Steinmetz M., von Boehmer H. Ontogeny of the T-cell antigen receptor within the thymus. Nature. 1985 Feb 14;313(6003):592–595. doi: 10.1038/313592a0. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Lesley J., Trotter J., Hyman R. Thymocyte subpopulation enriched for progenitors with an unrearranged T-cell receptor beta-chain gene. Nature. 1985 Jun 20;315(6021):666–669. doi: 10.1038/315666a0. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Landgraf B., Bentin J., Valentine M., Lotz M., Vaughan J. H., Carson D. A. Activation of resting T lymphocytes by anti-CD3 (T3) antibodies in the absence of monocytes. J Immunol. 1985 Sep;135(3):1719–1723. [PubMed] [Google Scholar]
- Zinkernagel R. M., Callahan G. N., Althage A., Cooper S., Klein P. A., Klein J. On the thymus in the differentiation of "H-2 self-recognition" by T cells: evidence for dual recognition? J Exp Med. 1978 Mar 1;147(3):882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974 Oct 11;251(5475):547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]



