Abstract
Objective
We examined the association between 15 single nucleotide polymorphisms (SNPs) in HTR2A and characteristics of disordered eating, including weight/shape concerns, binge eating (with or without loss of control) and compensatory behaviors (purging and non-purging). Whether a lifetime history of major depressive disorder (MDD) moderated or mediated this association was also investigated.
Method
A sample of 1533 twin women of Caucasian descent that were part of the Missouri Adolescent Female Twin Study was used. Data were collected using self-report responses to a semi-structured interview. Logistic regression analyses were used to examine the association between weight/shape concerns, binge eating and compensatory behaviors and SNPs (where carriers of the minor allele were coded as 1).
Results
Two SNPs, rs6561333 and rs2296972, showed a protective influence against binge eating, with rs2296972 being significant at a trend level after application of the False Discovery Rate. The SNP was not associated with MDD nor did MDD moderate its putative relation with binge eating.
Discussion
Pending replication, our analyses provide preliminary evidence for intronic SNPs in HTR2A and their association with binge eating. Given the well-documented role of serotonergic dysfunction in eating psychopathology, this report warrants considerable further study.
Keywords: HTR2A, disordered eating, binge eating
Introduction
Weight and shape concerns, binge eating and compensatory behaviors are eating disorder symptoms that cut across multiple DSM-5 diagnoses of eating disorders (American Psychiatric Association, 2013). In addition to the growing prominence of these features in DSM-5, there is accumulating evidence for heritable influences on them (Thornton et al., 2011). In a longitudinal study of weight and shape concerns, Klump et al. report increasing heritability of the construct across development, with heritable factors contributing to 54% of the variance by age 13 (Klump et al., 2010). Further, both binge eating and compensatory behaviors (Munn-Chernoff et al., in press), such as self-induced vomiting have been found to be heritable, with significant overlap in the genetic factors influencing them (Sullivan et al., 1998; Wade et al., 2008). However, the genes that contribute to this heritable variation remain elusive (Helder and Collier, 2011).
Most gene-finding efforts have focused on diagnoses and subtypes of anorexia and bulimia nervosa in clinical populations, which may encompass a fair degree of etiological heterogeneity and may also exclude sub-threshold forms of these disorders, as well as specific eating disorder symptoms. Alterations in serotonin neurotransmission have been identified in patients with anorexia and bulimia nervosa (e.g. (Kaye, 2008; Norton and Owen, 2005; Scherag et al., 2010)). For instance, acute depletion of dietary tryptophan, the precursor to 5-HT (serotonin), has been found to correspond to increased food intake in those with bulimia nervosa (Weltzin et al., 1995). Furthermore, while those with bulimia have normal levels of 5-HIAA (5-hydroxyindoleatic acid, a serotonin metabolite), those in recovery show elevated levels in their cerebrospinal fluid (Bailer et al., 2012).
A gene that has garnered considerable interest encodes the 5-hydroxytryptamine (serotonin) receptor 2A (HTR2A) (Norton and Owen, 2005; Scherag et al., 2010). Generally, the serotonin receptor subtypes have been implicated in appetitive processes and satiety, as well as mood (Bailer et al., 2012; Herbeth et al., 2005). In particular, several studies have examined the association between the -1438G/A (rs6311) promoter polymorphism and anorexia nervosa (e.g. (Enoch et al., 1998; Hinney et al., 1997; Martaskova et al., 2009; Nacmias et al., 1999; Ricca et al., 2002; Sorbi et al., 1998)). Although an initial meta-analysis (Ziegler et al., 1999) and a multi-center study of trios reported an absence of an association between this genetic variant and anorexia nervosa, another meta-analysis (Gorwood et al., 2003) indicated an association accompanied by considerable across-sample heterogeneity. The meta-analysis concluded that variation in symptoms and clinical characteristics may obscure genetic association findings.
Research on HTR2A and bulimia nervosa are limited – women with bulimia nervosa tend to have normal 5-HT2A receptor binding (Goethals et al., 2004) although alterations have been noted in recovering patients. Furthermore, age-related changes in receptor binding tend to be absent in women recovering from bulimia nervosa and those with the binge-purge (but not restricting) subtype of anorexia nervosa (Bailer et al., 2012). While human association studies have explored the relationship between HTR2A variants and bulimia nervosa, albeit infrequently, samples have been modest and no clear evidence for association has emerged (Kiezebrink et al., 2010; Sorli et al., 2008).
We examined the association between 15 single nucleotide polymorphisms (SNPs) in HTR2A and disordered eating characteristics, including weight/shape concerns, binge eating (with or without loss of control) and compensatory behaviors (purging and non-purging) in a sample of 1533 European-American female twins. In addition, given the well-documented comorbidity between eating disorders and major depressive disorder (MDD) (Hudson et al., 2007), as well as associations between HTR2A variants and MDD, particularly antidepressant response (Kato and Serretti, 2010), we investigated whether MDD mediated or moderated any significant associations between HTR2A and these disordered eating characteristics.
Methods
Participants
Participants were drawn from the general population Missouri Adolescent Female Twin Study (MOAFTS). MOAFTS twins were born between 1975 and 1985 to parents residing in the state of Missouri. A baseline interview was conducted when the twins were adolescents (13–17 years old), but this assessment did not include measurements of eating disorders or their symptoms. All eligible twins were followed up with a detailed full-length interview in 2002–2005 (age range = 18–27 years). The full follow-up sample (N = 3787) is highly representative of the demographic characteristics of the state; 14% of twins self-identified as African-American – however, for the current analyses, only data on European-American subjects was utilized (there is no evidence for population stratification within the European-American participants). During this time, twins were also invited to provide a DNA sample (blood, buccal or saliva) for an ongoing multi-center genetic study. Saliva collection and genotyping is ongoing and currently, data on 1533 twins of European-American ancestry are available and were used in these analyses. Women who are currently genotyped did not significantly differ from the full sample on any disordered eating characteristic (p > 0.50). Additional details on sample recruitment and genetic data collection, quality control and tests for population stratification are available in related publications (Agrawal et al., 2010; Heath et al., 2002). All research was reviewed and approved by the institutional review board at the Washington University School of Medicine.
Assessment
The Semi-Structured Assessment of the Genetics of Alcoholism (SSAGA) was administered via a telephone interview to the twins (Bucholz et al., 1994). The eating disorders section was adapted from the Diagnostic Interview Schedule (version 4)( Robins et al., 1996). Although primarily aimed at obtaining DSM-IV diagnoses, some changes were made to the section – a detailed explanation of these modifications may be found in Duncan et al (Duncan et al., 2007). Most pertinent to the current study, unlike other assessments, participants were queried about compensatory behaviors regardless of their endorsement of binge eating. All items represent lifetime assessments, were dichotomously coded and based on single items that were presented to all participants without any study-related skips. Weight/shape concerns assessed whether the individual was ever greatly concerned about eating too much, looking too fat or gaining too much weight. Binge eating was assessed as ever eating a large amount of food in a short period of time, usually less than two hours. A follow-up question on whether the binge eating was accompanied by loss of control was used to further examine promising association signals. Finally, compensatory behaviors included items that assessed whether, in an effort to either lose weight, prevent weight gain or make-up for an eating binge, the respondent had: (a) made herself vomit, (b) taken laxatives, (c) taken water pills/diuretics, (d) dieted strictly, (e) fasted (not eat anything), or (e) exercised excessively. The first three methods are considered purging compensatory behaviors whereas the last three methods are considered non-purging compensatory behaviors.
Genotyping
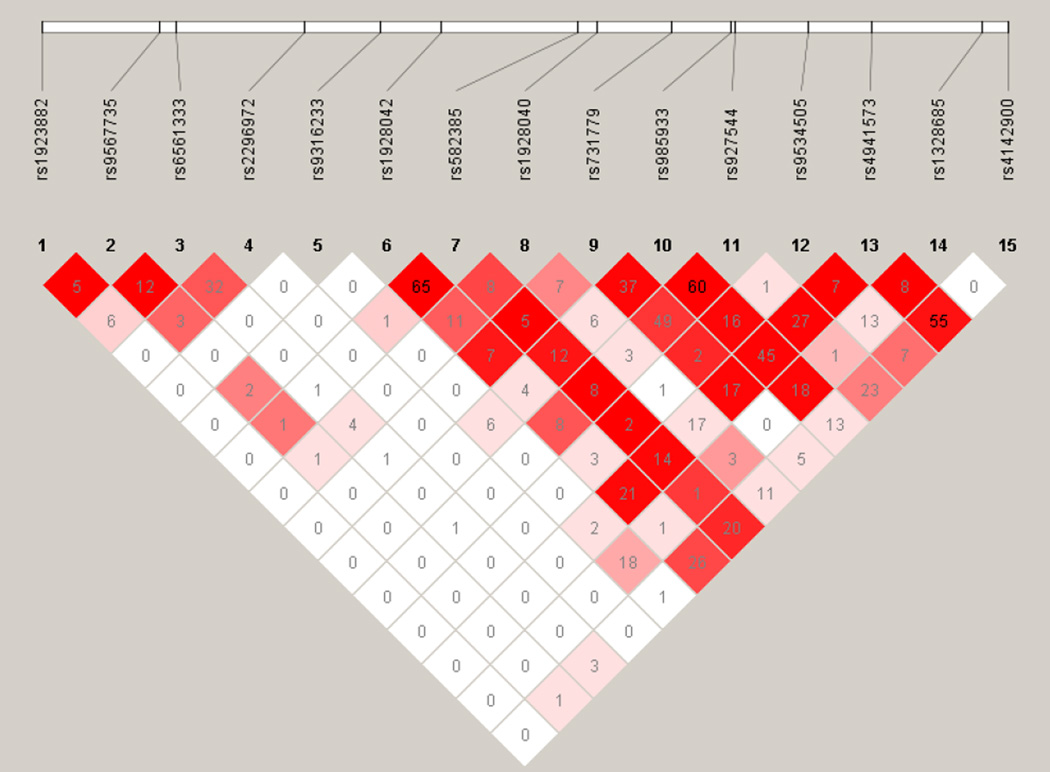
Typing of SNPs was conducted as part of a larger effort and was based on a panel developed by tagging genes selected primarily for studies of addiction (Hodgkinson et al., 2008). We selected to examine HTR2A alone due to its importance in the literature. The assay was developed for Illumina Golden Gate technology and genotyping was conducted on a subset of the sample at the Centers for Inherited Diseases Research (CIDR) and subsequently, at Washington University School of Medicine (WUSM). SNP selection involved gene tagging (including gene footprints), prioritization of exonic and published variants (please see Hodgkinson et al., 2008 for complete details; sequence information available upon request). None of the HTR2A SNPs showed significant deviations from Hardy-Weinberg Equilibrium (p > 0.05 for all but one SNP, rs1928040 with p = 0.01; however, this is well within the correction for multiple tests) and all had call rates greater than 99.5%. One SNP, rs6561333, was not genotyped in the second phase as the genotyping platform no longer supported the variant. Linkage disequilibrium (LD) between the SNPs in 831 unrelated women from the MOAFTS sample was calculated using Haploview (Barrett et al., 2005) (Figure 1).
Figure 1.
Linkage Disequilibrium (LD) across 15 SNPs in HTR2A in 831 unrelated women from the Missouri Adolescent Female Twin Study. The colors represent D’ (with darker colors representing greater LD), whereas the numeric inserts are r2 values.
Statistical Analyses
Each SNP was coded as a binary variable, where being homozygous for the major allele equaled 0 and being a carrier of the minor allele equaled 1. To examine the association between each SNP and the disordered eating characteristics, logistic regressions, adjusting for zygosity (monozygotic or not), age at interview and genotyping phase (WUSM or not), were conducted in STATA (Stata Corp, 2003) using a robust variance estimator that accounted for clustering of twin data. A conservative Bonferroni correction (p < 0.0033) and the more common q-value, an index of the False Discovery Rate (Storey and Tibshirani, 2003), were calculated. To examine the mediating effects of MDD, (i.e. whether the effect of HTR2A SNPs on disordered eating characteristics was due to their effect on MDD which, in turn, influenced disordered eating characteristics) first, the association between MDD and the SNP was tested. If the SNP was not associated with MDD, there was no possibility of mediation, even if MDD and disordered eating characteristics occurred comorbidly across the lifetime. To examine the potential moderating influence of MDD, an interaction between the SNP and MDD on disordered eating characteristics was examined.
Results
Sample characteristics
Of the 1533 European-American women with genotypic data, 25.8% reported weight/shape concerns, 6.7% endorsed binge eating (with 12 missing data) without or with loss of control (reported only by 33 subjects) and 19.4% endorsed compensatory behaviors. Purging behaviors (i.e., vomiting, taking laxatives or diuretics) were endorsed by 5.7% of the sample, whereas non-purging behaviors (i.e., strict dieting, fasting or excessive exercise) were endorsed by 16.8% of the sample.
Genetic association analyses
Results for the model where carriers of minor allele were contrasted with homozygotes for the major allele are shown in Table 1. Results for other models may be found in Supplemental Tables S1–S3. There was no evidence for association between the HTR2A SNPs and compensatory behaviors. For weight/shape concerns, even though rs1923882 was associated at p=0.04, it did not satisfy correction for multiple testing. For binge eating, carriers of the minor alleles of two SNPs, rs6561333 and rs2296972, were less likely to report binge eating than homozygotes of the major allele (rs6561333: p = 0.01, q-value = 0.06; rs2296972: p = 0.006, q-value = 0.06), with both SNPs showing a protective effect against binge eating (rs6561333: Odds Ratio (O.R.) = 0.56, 95% Confidence Interval (C.I.) = 0.34–0.91; rs22976972: O.R. = 0.57, 95% C.I. = 0.38–0.86). These two SNPs were only in moderate LD (r2 = 0.32, D’ = 0.80; see Figure 1 for complete LD information). After a False Discovery Rate and a conservative Bonferroni correction, rs2296972 was only significant at a trend level.
Table 1.
For 15 SNPs in HTR2A (chromosome 13q14-q21), the percentage of women who reported weight/shape concerns, binge eating and compensatory behaviors who also carry one or more copies of the minor allele (+) and those homozygous for the major allele (−) in 1534 European-American female twins aged 18–29 years.
| SNP | Base-pair | Minor Allele Frequency# |
Weight/Shape Concerns |
Binge Eating | Compensatory Behaviors |
|||
|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | |||
| rs1923882 | 46309662 | 0.23 (A/G) | 24.4 | 26.7 | 7.3 | 6.4 | 16.9* | 21.2* |
| rs9567735 | 46317205 | 0.15 (G/A) | 26.7 | 25.5 | 7.3 | 6.6 | 21.8 | 18.6 |
| rs6561333% | 46318313 | 0.45 (A/G) | 24.8 | 25.8 | 5.6* | 9.9* | 19.8 | 19.5 |
| rs2296972 | 46326472 | 0.28 (A/C) | 24.1 | 27.4 | 5.0** | 8.5** | 19.9 | 19.1 |
| rs9316233 | 46331356 | 0.18 (C/G) | 24.6 | 26.4 | 7.3 | 6.5 | 19.1 | 19.5 |
| rs1928042 | 46335217 | 0.24 (C/A) | 26.1 | 25.5 | 6.8 | 6.8 | 18.8 | 19.9 |
| rs582385 | 46343995 | 0.18 (G/A) | 26.9 | 25.3 | 7.1 | 6.6 | 18.8 | 19.7 |
| rs1928040 | 46345237 | 0.35 (A/G) | 26.5 | 24.7 | 6.5 | 7.1 | 19.8 | 18.9 |
| rs731779 | 46350039 | 0.21 (C/A) | 24.0 | 26.8 | 6.1 | 7.2 | 20.3 | 19.0 |
| rs985933 | 46353864 | 0.39 (A/G) | 24.8 | 27.3 | 6.5 | 7.2 | 20.5 | 17.6 |
| rs927544 | 46354052 | 0.30 (G/A) | 25.4 | 26.3 | 6.3 | 7.3 | 20.6 | 18.3 |
| rs9534505 | 46358745 | 0.09 (A/G) | 23.3 | 26.3 | 6.3 | 6.9 | 18.8 | 19.4 |
| rs4941573 | 46362858 | 0.42 (G/A) | 25.30 | 26.8 | 6.9 | 6.6 | 19.4 | 19.7 |
| rs1328685 | 46369891 | 0.10 (G/A) | 24.1 | 26.2 | 7.8 | 6.5 | 19.4 | 19.5 |
| rs4142900 | 46371551 | 0.48 (A/C) | 25.0 | 28.0 | 6.3 | 8.0 | 19.0 | 20.7 |
Alleles presented as minor/major. Positions based on NCBI build 36.3; “+” carriers of minor allele; “−“ homozygous for major allele.
p-value less than 0.05
p-value less than 0.005;
only typed in the first round of genotyping; no longer supported by assay design, N=1039
Binge eating with or without loss of control
We examined whether rs6561333 and rs22976972 were associated with binge eating that only occurred with loss of control. For both SNPs, there was no evidence that the association was attributable to those reporting loss of control (post-hoc comparison of odds-ratios p-values > 0.05), although the modest number of subjects endorsing loss of control (N = 33) warrant caution in this post-hoc finding.
Effect of major depressive disorder
Although MDD (O.R. = 3.46, 95% C.I. 2.62–4.56) was associated with binge eating, rs2296972 was not associated with MDD (O.R. = 0.92, 95% C.I. 0.71–1.19) and consequently, there was no evidence for mediation. Evidence for moderation (genotype × MDD interaction) was also absent (interaction O.R. = 1.56, 95% C.I. 0.66–3.70), indicating the independent protective main effects of this variant on binge eating.
Discussion
In a sample of 1533 European-American female twins, we found that two SNPs in HTR2A, rs6561333 and rs2296972, were associated with a lower likelihood of binge eating. As these SNPs are intronic and, to our knowledge, do not correlate with functional variants in HTR2A or neighboring genes, the putative role of these variants will require replication and further biological validation.
A majority of human gene association studies of HTR2A have focused on a single promoter polymorphism (-1438G/A, rs6311) whose precise role in gene regulation remains somewhat unclear. We failed to find an association between rs4941573, which is a proxy (r2 = 1, D’ = 1) for the frequently studied -1438G/A promoter polymorphism (rs6311) and disordered eating characteristics. This is consistent with findings from a large multi-center family study (Gorwood et al., 2002). Nonetheless, it is also possible that the lack of association is attributable to our focus on disordered eating characteristics versus a diagnosis of anorexia or bulimia nervosa. Few studies have also examined this variant in the context of bulimia nervosa. For instance, the low functioning allele of -1438G/A (G) has been found to be associated with poorer treatment response (Steiger et al., 2008). Another study implicates the G allele in increased impulsivity and reduced sensitivity to post-synaptic serotonin in women with bulimia nervosa (Bruce et al., 2005). One study, however, examined multiple SNPs in the gene and reported a similar protective association between rs3742278 and the binge-purge anorexia nervosa subtype (Kiezebrink et al., 2010) – this SNP is in low r2 (0.07–0.17) but high D’ (1.0) with the SNPs associated with binge eating in our sample.
Some limitations of this study are worth noting. First, ours is a study of women in the Midwestern United States and may not generalize to other populations. Second, we were unable to examine clinical diagnoses of anorexia or bulimia nervosa, or severity as well as continuous indices of disordered eating due to small sample sizes. Third, we elected to exclude the African-American participants from these analyses due to concerns regarding admixture. This group is worth considerable future study as the genetic etiology of these disordered eating characteristics may differ between ethnic/racial groups. Finally, these results should be viewed as preliminary pending independent replication. Power to detect genetic main effects of less than 1.15 was less than 80%, even with minor allele frequencies of 0.4 and greater. Thus, the likelihood of false positives and negatives is a concern.
In conclusion, our examination of the genetic underpinnings of weight/shape concerns, binge eating and compensatory behaviors implicates variants in HTR2A for its role in binge eating. As we progress towards revised diagnostic definitions of eating psychopathology, our study underscores the importance of examining specific eating disorder symptoms, such as binge eating, in genetic analyses (Birgegard et al., 2012; Walsh, 2009).
Supplementary Material
Acknowledgment
This project is supported by AA11998 (AA, ACH, KKB, MTL), AA07728, AA09022 & K05AA17688 (ACH). MMC is supported by T32- AA07580.
Reference List
- Agrawal A, Lynskey MT, Todorov AA, et al. A Candidate Gene Association Study of Alcohol Consumption in Young Women*. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2010.01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Bailer UF, Frank GK, Price JC, et al. Interaction between serotonin transporter and dopamine D2/D3 receptor radioligand measures is associated with harm avoidant symptoms in anorexia and bulimia nervosa. Psychiatry Res. 2012 doi: 10.1016/j.pscychresns.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Birgegard A, Norring C, Clinton D. DSM-IV versus DSM-5: implementation of proposed DSM-5 criteria in a large naturalistic database. Int J Eat Disord. 2012;45(3):353–361. doi: 10.1002/eat.20968. [DOI] [PubMed] [Google Scholar]
- Bruce KR, Steiger H, Joober R, et al. Association of the promoter polymorphism-1438G/A of the 5-HT2A receptor gene with behavioral impulsiveness and serotonin function in women with bulimia nervosa. Am J Med Genet B Neuropsychiatr Genet. 2005;137B(1):40–44. doi: 10.1002/ajmg.b.30205. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret RJ, Cloninger RC, et al. A New, Semi-Structured Psychiatric Interview For Use In Genetic Linkage Studies. J Stud Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Bucholz KK, Neuman RJ, et al. Clustering of eating disorder symptoms in a general population female twin sample: a latent class analysis. Psychol Med. 2007;37(8):1097–1107. doi: 10.1017/S0033291707000505. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Kaye WH, Rotondo A, et al. 5-HT2A promoter polymorphism-1438G/A, anorexia nervosa, and obsessive-compulsive disorder. Lancet. 1998;351(9118):1785–1786. doi: 10.1016/S0140-6736(05)78746-8. [DOI] [PubMed] [Google Scholar]
- Goethals I, Vervaet M, Audenaert K, et al. Comparison of cortical 5-HT2A receptor binding in bulimia nervosa patients and healthy volunteers. Am J Psychiatry. 2004;161(10):1916–1918. doi: 10.1176/ajp.161.10.1916. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Ades J, Bellodi L, et al. The 5-HT(2A)-1438G/A polymorphism in anorexia nervosa: a combined analysis of 316 trios from six European centres. Mol Psychiatry. 2002;7(1):90–94. doi: 10.1038/sj.mp.4000938. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Kipman A, Foulon C. The human genetics of anorexia nervosa. Eur J Pharmacol. 2003;480(1–3):163–170. doi: 10.1016/j.ejphar.2003.08.103. [DOI] [PubMed] [Google Scholar]
- Heath AC, Howells W, Bucholz KK, et al. Ascertainment of a mid-western US female adolescent twin cohort for alcohol studies: assessment of sample representativeness using birth record data. Twin Res. 2002;5(2):107–112. doi: 10.1375/1369052022974. [DOI] [PubMed] [Google Scholar]
- Helder SG, Collier DA. The genetics of eating disorders. Curr Top Behav Neurosci. 2011;6:157–175. 157–175. doi: 10.1007/7854_2010_79. [DOI] [PubMed] [Google Scholar]
- Herbeth B, Aubry E, Fumeron F, et al. Polymorphism of the 5-HT2A receptor gene and food intakes in children and adolescents: the Stanislas Family Study. Am J Clin Nutr. 2005;82(2):467–470. doi: 10.1093/ajcn.82.2.467. [DOI] [PubMed] [Google Scholar]
- Hinney A, Ziegler A, Nothen MM, Remschmidt H, Hebebrand J. 5-HT2A receptor gene polymorphisms, anorexia nervosa, and obesity. Lancet. 1997;350(9087):1324–1325. doi: 10.1016/S0140-6736(05)62485-3. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Yuan Q, Xu K, et al. Addictions biology: haplotype-based analysis for 130 candidate genes on a single array. Alcohol Alcohol. 2008;43(5):505–515. doi: 10.1093/alcalc/agn032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biol Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Serretti A. Review and meta-analysis of antidepressant pharmacogenetic findings in major depressive disorder. Mol Psychiatry. 2010;15(5):473–500. doi: 10.1038/mp.2008.116. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94(1):121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiezebrink K, Mann ET, Bujac SR, et al. Evidence of complex involvement of serotonergic genes with restrictive and binge purge subtypes of anorexia nervosa. World J Biol Psychiatry. 2010;11(6):824–833. doi: 10.3109/15622975.2010.484550. [DOI] [PubMed] [Google Scholar]
- Klump KL, Burt SA, Spanos A, et al. Age differences in genetic and environmental influences on weight and shape concerns. Int J Eat Disord. 2010;43(8):679–688. doi: 10.1002/eat.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martaskova D, Slachtova L, Kemlink D, Zahorakova D, Papezova H. Polymorphisms in serotonin-related genes in anorexia nervosa. The first study in Czech population and metaanalyses with previously performed studies. Folia Biol (Praha) 2009;55(5):192–197. [PubMed] [Google Scholar]
- Munn-Chernoff MA, Duncan AE, Grant JD, et al. A twin study of alcohol dependence, binge eating and compensatory behaviors. J Stud Alc Drugs. 2014 doi: 10.15288/jsad.2013.74.664. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacmias B, Ricca V, Tedde A, et al. 5-HT2A receptor gene polymorphisms in anorexia nervosa and bulimia nervosa. Neurosci Lett. 1999;277(2):134–136. doi: 10.1016/s0304-3940(99)00859-9. [DOI] [PubMed] [Google Scholar]
- Norton N, Owen MJ. HTR2A: association and expression studies in neuropsychiatric genetics. Ann Med. 2005;37(2):121–129. doi: 10.1080/07853890510037347. [DOI] [PubMed] [Google Scholar]
- Ricca V, Nacmias B, Cellini E, et al. 5-HT2A receptor gene polymorphism and eating disorders. Neurosci Lett. 2002;323(2):105–108. doi: 10.1016/s0304-3940(02)00088-5. [DOI] [PubMed] [Google Scholar]
- Robins LN, Cottler LB, Bucholz K, Compton W. DIS-IV. Saint Louis; 1996. [Google Scholar]
- Scherag S, Hebebrand J, Hinney A. Eating disorders: the current status of molecular genetic research. Eur Child Adolesc Psychiatry. 2010;19(3):211–226. doi: 10.1007/s00787-009-0085-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbi S, Nacmias B, Tedde A, et al. 5-HT2A promoter polymorphism in anorexia nervosa. Lancet. 1998;351(9118):1785. doi: 10.1016/S0140-6736(05)78745-6. [DOI] [PubMed] [Google Scholar]
- Sorli JV, Frances F, Gonzalez JI, et al. Impact of the-1438G>a polymorphism in the serotonin 2A receptor gene on anthropometric profile and obesity risk: a case-control study in a Spanish Mediterranean population. Appetite. 2008;50(1–):260–265. doi: 10.1016/j.appet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Stata Corp. STATA. College Station, TX: 2003. [Google Scholar]
- Steiger H, Joober R, Gauvin L, et al. Serotonin-system polymorphisms (5-HTTLPR and -1438G/A) and responses of patients with bulimic syndromes to multimodal treatments. J Clin Psychiatry. 2008;69(10):1565–1571. doi: 10.4088/jcp.v69n1006. [DOI] [PubMed] [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Bulik CM, Kendler KS. Genetic epidemiology of binging and vomiting. Br J Psychiatry. 1998;173:75–79. doi: 10.1192/bjp.173.1.75. [DOI] [PubMed] [Google Scholar]
- Thornton LM, Mazzeo SE, Bulik CM. The heritability of eating disorders: methods and current findings. Curr Top Behav Neurosci. 2011;6:141–56. 141–156. doi: 10.1007/7854_2010_91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TD, Treloar S, Martin NG. Shared and unique risk factors between lifetime purging and objective binge eating: a twin study. Psychol Med. 2008;38(10):1455–1464. doi: 10.1017/S0033291708002791. [DOI] [PubMed] [Google Scholar]
- Walsh BT. Eating disorders in DSM-V: review of existing literature (Part 1) Int J Eat Disord. 2009;42(7):579–580. doi: 10.1002/eat.20756. [DOI] [PubMed] [Google Scholar]
- Weltzin TE, Fernstrom MH, Fernstrom JD, Neuberger SK, Kaye WH. Acute tryptophan depletion and increased food intake and irritability in bulimia nervosa. Am J Psychiatry. 1995;152(11):1668–1671. doi: 10.1176/ajp.152.11.1668. [DOI] [PubMed] [Google Scholar]
- Ziegler A, Hebebrand J, Gorg T, et al. Further lack of association between the 5-HT2A gene promoter polymorphism and susceptibility to eating disorders and a meta-analysis pertaining to anorexia nervosa. Mol Psychiatry. 1999;4(5):410–412. doi: 10.1038/sj.mp.4000561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.