Abstract
Background & Aims
Metaplasias often have characteristics of developmentally related tissues. Pancreatic metaplastic ducts are usually associated with pancreatitis and pancreatic ductal adenocarcinoma. The tuft cell is a chemosensory cell that responds to signals in the extracellular environment via effector molecules. Commonly found in the biliary tract, tuft cells are absent from normal murine pancreas. Using the aberrant appearance of tuft cells as an indicator, we tested if pancreatic metaplasia represents transdifferentiation to a biliary phenotype and what effect this has on pancreatic tumorigenesis.
Methods
We analyzed pancreatic tissue and tumors that developed in mice that express an activated form of Kras (KrasLSL−G12D/+;Ptf1aCre/+ mice). Normal bile duct, pancreatic duct, and tumor-associated metaplasias from the mice were analyzed for tuft cell and biliary progenitor markers, including SOX17, a transcription factor that regulates biliary development. We also analyzed pancreatic tissues from mice expressing transgenic SOX17 alone (ROSAtTa/+;Ptf1 CreERTM/+;tetO-SOX17) or along with activated Kras (ROSAtT a/+;Ptf1a CreERTM/+;tetO-SOX17;KrasLSL−G12D;+).
Results
Tuft cells were frequently found in areas of pancreatic metaplasia, decreased throughout tumor progression, and were absent from invasive tumors. Analysis of the pancreatobiliary ductal systems of mice revealed tuft cells in the biliary tract, but not the normal pancreatic duct. Analysis for biliary markers revealed expression of SOX17 in pancreatic metaplasia and tumors. Pancreas-specific overexpression of SOX17 led to ductal metaplasia along with inflammation and collagen deposition. Mice that overexpressed SOX17 along with KrasG12D had a greater degree of transformed tissue compared with mice expressing only KrasG12D. Immunofluorescence analysis of human pancreatic tissue arrays revealed the presence of tuft cells in metaplasia and early-stage tumors, along with SOX17 expression, consistent with a biliary phenotype.
Conclusions
Expression of KrasG12D and SOX17 in mice induces development of metaplasias with a biliary phenotype, containing tuft cells. Tuft cells express a number of tumorigenic factors that can alter the microenvironment. Expression of SOX17 induces pancreatitis and promotes KrasG12D-induced tumorigenesis in mice.
Keywords: pancreatic cancer, pathogenesis, mouse model, signal transduction
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) is currently the fourth leading cause of cancer death, with an overall five-year survival rate of 6% and abysmal median survival of just 4–6 months1. As symptoms appear late in disease progression and metastasis has typically occurred by the time of diagnosis, earlier detection is likely to be invaluable. Elucidation of early detection markers requires a greater understanding of early disease pathology, such as pancreatic intraepithelial neoplasia (PanINs), proposed precursors to PDA, and acinar-to-ductal metaplasia (ADM), a process that results in the formation of highly reactive metaplastic ducts. The consistent association of ADM with PDA suggests that lesions arise as a consequence of disruption of nearby normal tissue. Recent studies implicate them as a source of PanIN2, 3. Consistent with this, metaplastic ducts are a hallmark of chronic pancreatitis (CP), which may be part of the reason why CP is a significant risk factor for PDA.
Epithelial metaplasia is a hallmark of inflammatory and neoplastic disease in several organs. In many tissues, normal epithelium is replaced by epithelium usually confined to a developmentally-related organ; this phenomenon occurs in adenocarcinoma of the esophagus in areas of Barrett’s metaplasia, where the epithelium takes on gastric and intestinal characteristics marked by CDX2 expression or cystitis glandularis where bladder metaplasia becomes phenotypically colon-like4. In the pancreas, ADM is generally described as pancreatic acinar cells being replaced by pancreatic duct cells, with no prior description of their mimicry of epithelia of related tissues.
One cell type absent from the exocrine pancreas is the tuft cell. Tuft cells are a type of solitary chemosensory cell (SCC) and are found in multiple organs, but are prevalent in the developmentally-related common bile duct and murine pancreatobiliary duct (the segment of duct following the intersection of the main pancreatic duct and the bile duct, prior to fusion with the duodenum), and associated peribiliary glands (PBGs). SCCs are part of the diffuse chemosensory system (DCS) and are analogous to taste cells, though they do not aggregate in buds. SCCs are thought to link chemosensation of intraluminal content to local control of absorptive and secretory processes, as well as central nervous system (CNS) activity5.
In our analysis of pancreatic metaplasia, we have discovered that the aberrant genesis of pancreatic tuft cells is common in KrasG12D-induced pancreatic disease and is accompanied by epithelial expression of SOX17, a master control factor of biliary development and differentiation6. Lineage tracing showed pancreatic tuft cells transdifferentiate from adult acinar cells and express a full array of markers associated with mature tuft cells found in other tissues. ADM and mPanINs consistently contained a PDX1+/SOX17+ cell population reminiscent of the common pancreatobiliary progenitor6. Forced expression of SOX17 in adult pancreas was sufficient to induce acinar cell transdifferentiation into a tuft cell-containing metaplasia, accompanied by a chronic pancreatitis-like phenotype, including fibrosis and an adaptive immune response. Transgenic expression of SOX17 in concert with oncogenic K-ras expression enhanced the degree of transformation of normal pancreas, suggesting that SOX17-induced metaplasia was fully susceptible to Kras-induced transformation. We conclude that pancreatic ADM found in PDA represents transdifferentiation to a biliary phenotype and contributes to disease progression through the assumption of a pancreatobiliary progenitor cell phenotype.
MATERIALS AND METHODS
Mouse strains
LSL-KrasG12D/+, Ptf1aCre/+, Ptf1aCre−ERTM/+, tetO-SOX17, MT-Tgfa and ROSAtTa/+ strains have been described previously and were genotyped accordingly7–12. ROSAYFP mice were obtained from Jackson Laboratories. Experiments were conducted in accordance with the Office of Laboratory Animal Welfare and approved by the Institutional Animal Care and Use Committees at Stony Brook University and the Mayo Clinic.
Mouse Tissue Microarrays
Custom 5mm TMAs were assembled by a hand corer and pre-cast recipient molds. Pancreatic ductal adenocarcinoma from 10 LSL-KrasG12D;P53R172H/+;PDX1Cre/+ mice were included. Adjacent PanIN-containing tissues were included for 5 tumors, while distant metastases from several organ sites were included for 5 other tumors.
Human samples
Distribution and use of all human samples was approved by the Institutional Review Boards of Vanderbilt University Medical Center and the Mayo Clinic.
Induction of experimental pancreatitis
Cerulein-induced pancreatitis was achieved by treating mice twice daily with 250μg/kg cerulein (Sigma-Aldrich, St. Louis, MO), for seven days, and allowing mice to recover for one day. Metaplasia was induced in the MT-Tgfa strain by administration of 25mM ZnSO4 in drinking water for either 3, 6, or 9.5 months.
Overexpression of SOX17
Adult acinar cell-specific overexpression of SOX17 was accomplished by treating 6–12 week old mice with 5 daily doses of 5mg/kg tamoxifen administered through oral gavage to ROSAtTa/+;Ptf1aCreERTM/+;tetO-SOX17 mice with 6 weeks recovery. The identical protocol was used with ROSAtTa/+;Ptf1aCreERTM/+;tetO-SOX17;KrasLSL−G12D;+mice.
Tumor quantitation was performed by scanning 4 random H&E stained slides from each pancreata with an Aperio slide scanner (Vista, CA). Aperio ImageScope software was used to delineate and quantitate areas flanked by fibrosis and containing metaplasia or neoplasia compared to total tissue area.
Immunostaining
Tissues were harvested and fixed overnight in 4% paraformaldehyde. Immunohistochemistry was performed as previously described13. Slides were counterstained with hematoxylin and photographed on an Olympus BX41 light microscope (Olympus, Tokyo, Japan). Immunofluorescence was performed as previously described with some modifications (supplementary materials and methods)14.
Electron Microscopy
Tissue was prepared for EM by perfusion of mice with 2% paraformaldehyde/2.5% EM grade glutaraldehyde in 0.1M PBS, pH 7.4. Samples were viewed with a FEI Tecnail2 BioTwinG2 transmission electron microscope at 80 kV. Digital images were acquired with an AMT XR-60 CCD Digital Camera System.
Tuft cell quantitation
DCLK1 immunohistochemistry (IHC) was performed on paraffin-embedded tissue from 11 LSL-KrasG12D;Ptf1aCre/+ mice ranging in age from 4 months to 1 year using a Ventana Discovery XT autostainer. A minimum of twenty images at 40x were acquired per slide and lesions staged. Tuft cells were quantitated as DCLK1+ cells per number of nuclei per lesion. For quantitation in MT-Tgfa mice, DCLK1 IHC was performed on paraffin-embedded tissue from 9 mice treated with ZnS04 from 3–10 months. Ten images were taken at 40x per slide and tuft cells were quantitated as DCLK1+ cells per number of nuclei per metaplastic lesion.
Lineage Tracing
Recombination was induced in 8 week old KrasG12D/+;ROSAYFP;Ptf1aCre−ERTM/+ mice with one daily intraperitoneal (i.p.) injection of 3mg of tamoxifen (Sigma-Aldrich) for 5 d. Tumorigenesis was accelerated by a daily i.p. injection of 250 μg/kg of cerulein for 5 d. Mice were sacrificed 9 weeks later and tissue was prepared for immunofluorescence.
Cell Culture
Human PDA cell lines were purchased from the American Type Culture Collection and maintained at 37°C in 5% CO2 in ATCC-recommended medium, supplemented with 10% fetal bovine serum and 0.5μg/mL gentamicin.
Western Blotting
Pre-confluent cells were harvested in ice cold RIPA buffer supplemented with PhosStop phosphatase inhibitor and cOmplete EDTA-free protease inhibitor (Roche, Indianapolis, IN). 75μg of protein was run on a 7% SDS-gel and blotted to PVDF membrane for antibody incubation.
RESULTS
Tuft cells are a consistent component of epithelial metaplasia in a mouse model of pancreatic tumorigenesis
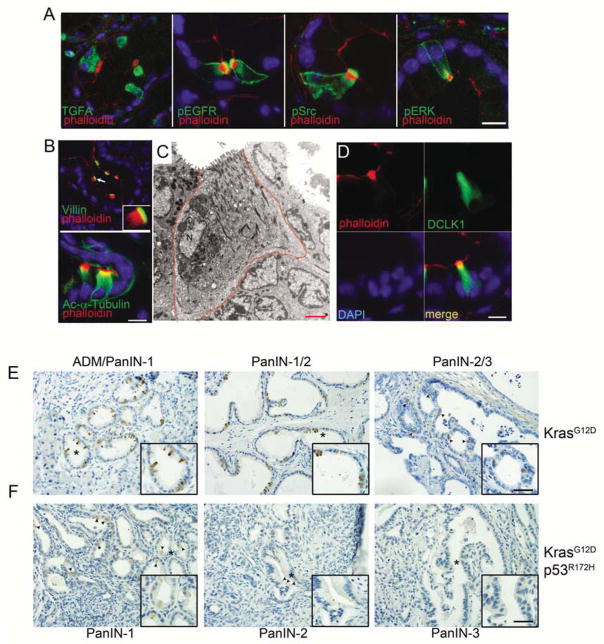
The LSL-KrasG12D/+;Ptf1aCre/+ murine model of pancreatic tumorigenesis presents with mainly ductal metaplasia and early murine PanIns (mPanINs), up to ~1 year of age, when later stage mPanINs and, occasionally, PDA, is found. The EGFR pathway has been associated with PDA progression and, recently, we found that activity was required for induction of tumorigenesis3, 15. Upon examining EGFR pathway activity by immunofluorescence (IF) in this model, we observed significant cellular heterogeneity within metaplastic structures. While EGFR activity was elevated throughout the metaplastic epithelium, we found highly elevated positivity for pY1068 EGFR, pY416 Src, pT202/pY204 ERK and the EGFR ligand TGFα, within isolated cells of metaplastic ducts (Figure 1A). This staining pattern was never observed in ducts of wild type control pancreata, but could readily be identified in the nearby pancreatobiliary tract (data not shown). Using phalloidin to costain for F-actin, we noted that these phospho-EGFR positive cells had a unique arrangement of microfilaments, marked by a perpendicular orientation to the apical membrane, typical of a tuft cell. IF for villin and acetylated-α-tubulin confirmed the presence of both prominent microvilli and the tubulovesicular system, respectively (Figure 1B). Using the unique microfilament arrangement as a guide, electron microscopy of a four-month-old LSL-KrasG12D/+;Ptf1aCre/+pancreas confirmed that tuft cells were commonly integrated into metaplastic ducts (Figure 1C).
Figure 1. Tuft cells in pancreatic metaplasia.
Co-immunofluorescence staining in 4–6 month old LSL-KrasG12D/+;Ptf1acre/+ mice including (A) TGFA, phospho-EGFR (pY1068), phospho-Src (pY416), or phospho-ERK (pT202/pY204) (green) with phalloidin (red). (B) Tuft cell structural components villin and acetylated alpha tubulin (green) with phalloidin (red). Scale bars = 10μm. (C) Electron microscopy of a metaplastic tuft cell in a 4 month old LSL-KrasG12D;Ptf1acre/+ mouse. Scale bar = 2μm. (D) Co-immunofluorescence for DCLK1 (green) and phalloidin (red). Nuclei are stained with DAPI. (E) Immunohistochemistry for DCLK1 in either LSL-KrasG12D;Ptf1acre/+ or (F) LSL-KrasG12D;P53R172H/+ mice in PanIN1–3 and invasive PDA. Scale bars =50μm for panels and 33μm for insets.
Doublecortin-like kinase 1 (DCLK1), a tubulin polymerization serine/threonine kinase, has been proposed to be both a marker of quiescent stem cells in the pancreas, as well as a marker of tuft cells in the stomach and intestine, where they are thought to represent a terminally differentiation cell population16–18. To address whether metaplastic tuft cells phenocopy mature tuft cells in other organs, DCLK1 expression was assessed relative to the expression level of other tuft cell markers. Co-IF with phalloidin revealed that 100% of tuft cells expressed DCLK1 (n=300), whereas 72% of DCLK1 positive cells were identified unambiguously as tuft cells (n=418, Figure 1D). The remaining 28% of DCLK1 positive cells that were not obviously tuft cells were possibly obscured due to section planarity or, alternatively, represent a legitimate non-tuft cell population, such as an adult stem cell population identified previously. Tuft cells of the normal biliary and intestinal tract have been shown to express an array of markers, including G-α-gustducin, TRPM5, β-endorphin, COX1, COX2, HPGDS and Gfi1b (Table S1). We found that metaplastic pancreatic tuft cells expressed each of these proteins at highly elevated levels, confirming their identity as bona fide tuft cells, indistinguishable from those found in normal tissue16, 19–21 (Figure S1).
To determine the association of tuft cells with disease progression, we quantitiated the number of DCLK1+ tuft cells at different points in tumor progression using pancreata from LSL-KrasG12D;Ptf1aCre/+ mice of various ages. Tuft cells were most abundant in ADM (an average 15.0% of the epithelium per lesion) and became less frequent throughout disease progression, constituting 11.2% of the epithelium in mPanIN-1, 8.7% in mPanIN-2 and 2.9% in mPanIN-3, whereas invasive disease had no tuft cells (Figure 1E). Analysis of pancreata from the LSL-KrasG12D;p53R172H/+;Pdx1-Cre model of PDAC was consistent with these results, though the overall number of cells per lesion were generally reduced to 4.8% in PanIN1s, 2.1% in PanIN2s and <1% in PanIN3s, with several being entirely negative (Figure 1F). To determine if tuft cells accompanied non-tumor models of pancreatic metaplasia, we examined DCLK1 expression in TGFα-driven and cerulein-induced metaplasia. Tuft cells were very commonly associated with metaplasia in MT-Tgfa mice, present in 73.5% of metaplastic lesions, constituting 14.4% of the epithelium (Figure S2A). Tuft cell presence was confirmed by electron microscopy (Figure S2B). Interestingly, tuft cells were rarely found in cerulein-induced, transient metaplasia (Figure S2C).
Metaplastic tuft cells suggest adoption of a biliary phenotype
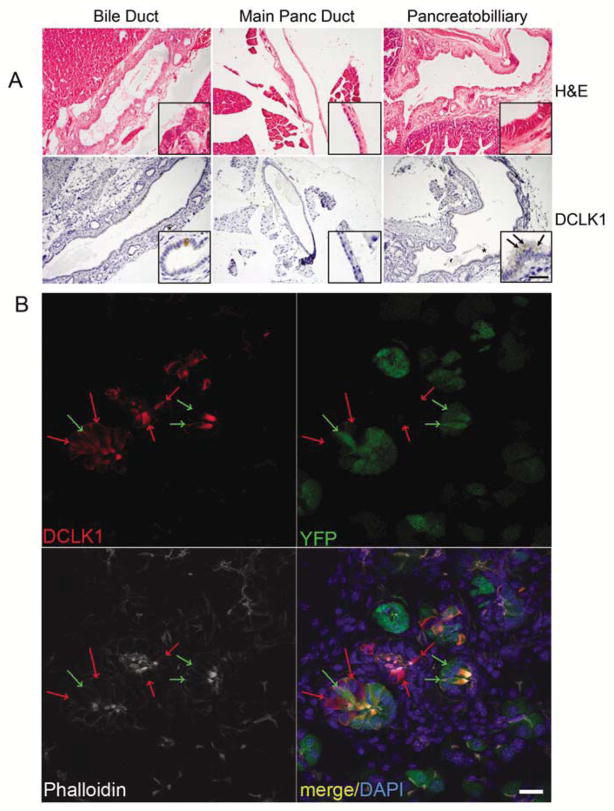
Tuft cells have been previously described within the normal murine biliary tract and intestine, however, in our experiments, we never observed these cells in random sections of wild type pancreata16, 22. To definitively determine if the normal main pancreatic duct harbored a tuft cell population, we dissected the main pancreatic duct away from the common duct of the biliary tract and the pancreatobiliary duct and examined them histologically. The murine biliary tract was composed of a central duct, decorated along its length by ancilliary peribiliary glands (PBGs), which were lined with columnar epithelium, including numerous tuft cells, identified by DCLK1 IHC23. In contrast, the murine main pancreatic duct was composed of low cuboidal epithelium, lacked PBGs and was entirely devoid of tuft cells. The pancreatobiliary duct was morphologically similar to bile duct, including PBGs and numerous tuft cells (Figure 2A).
Figure 2. Pancreatic tuft cells are disease-specific and transdifferentiate from Ptf1a+ epithelium.
(A) Histological analysis of the murine bile duct, pancreatic duct, and pancreatobiliary duct by hemotoxylin and eosin analysis and DCLK1 immunohistochemistry. Scale bar = 100μm for all panels, 25μm for insets. (B) Lineage tracing and immunofluorescent analysis in LSL-KrasG12D;ROSAYFP;Ptf1aCre−ERTM/+ pancreata by DCLK1 (red), YFP (green) and phalloidin (white). Nuclei are stained with DAPI. YFP negative tuft cells are indicated by red arrows. YFP positive tuft cells are indicated by green arrows. Scale bar =20μm.
Pancreatic tuft cells transdifferentiate from acinar cells
PBGs associated with the common and pancreatobiliary ducts (Figure 2A) have been hypothesized to be a source of progenitor cells for the liver, biliary tract, and pancreas, in part due to their expression of several stem and progenitor markers23. Strobel et al. have labeled related structures associated with the main pancreatic duct “pancreatic duct glands” (PDGs), describing them as a potential source of pancreatic disease24. In this study, it is hypothesized that ADM may directly emanate from the expansion of PDGs, with a popular alternative being that acinar cells transdifferentiate to form metaplasia. To distinguish these possibilities in our system, we conducted lineage tracing in the LSL-KrasG12D/+;ROSAYFP;Ptf1aCre−ERTM/+ murine model. This model initiates expression of both KrasG12D and yellow fluorescent protein (YFP) exclusively in Ptf1a+ adult acinar cells upon tamoxifen induction of CRE activity8. YFP fluorescence was found in 68.7% of tuft cells identified by phalloidin staining (n=300) and 76.3% of DCLK1+ cells (n=300) (Figure 2B), indicating that they are commonly derived from transdifferentiation of acinar cells. YFP-negative tuft cells could represent partial derivation from normal PDGs, incomplete recombination of the ROSA locus or ROSA promoter activity silencing, as has been observed in other adult tissues25. We conclude that while Kras-induced metaplasia is not primarily derived from PDGs, it does take on a PDG-like phenotype, including their possible regenerative function.
Pancreatic metaplasia takes on a pancreato-biliary progenitor phenotype
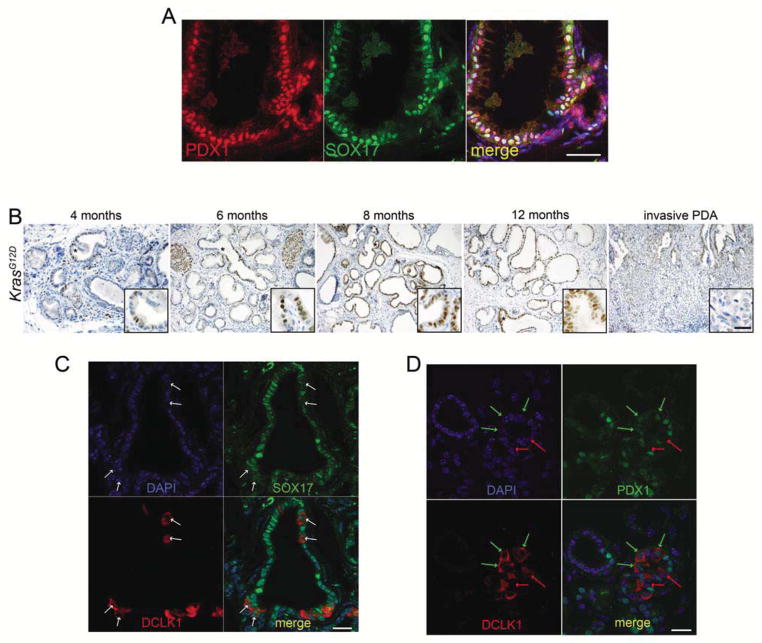
Metaplasia has been described as a developmental reversion, taking on the phenotype of cells normally confined to other developmentally-related organs4. Recent studies have shown that the murine biliary tract is developmentally more closely related to the pancreas, sharing a common progenitor cell population6. The observation that ADM resembles tuft cell-containing PBGs led us to hypothesize that ADM adopts other characteristics of the biliary tract. To test this, we examined the expression of SOX17, a transcription factor critical for bile duct development and recently described as being expressed in human IPMNs26.
The pancreas and biliary tract share a common progenitor cell population characterized by co-expression of PDX1 and SOX17. In the adult, however, epithelial SOX17 expression is restricted to the biliary tract, though endothelial SOX17 can be found lining blood vessels6 (Figure S3). Co-IF for SOX17 and PDX1 revealed two cell subtypes within metaplastic ducts: a PDX1+SOX17− population and a PDX1+/SOX17+ population (Figure 3A), the latter being reminiscent of the common pancreatobiliary progenitor cell6. To determine if this expression pattern persisted, LSL-KrasG12D/+;Ptf1aCre/+ mice of various ages were assessed for SOX17 expression by IHC. The frequency and intensity of SOX17 expression increased throughout disease progression over time in PanINs of all stages. Like tuft cells, SOX17 was absent from invasive disease (Figure 3B). Interestingly, liver metastases from both the KrasG12D/+;Ptf1aCre/+ and LSL-KrasG12D;P53R172H/+;Pdx1-Cre models often expressed SOX17, suggesting reversion to a more differentiated phenotype (data not shown). SOX17 was not expressed in transient metaplastic ducts induced by chronic cerulein treatment (data not shown).
Figure 3. SOX17 is expressed during pancreatic tumorigenesis.
(A) Co-immunofluorescence for SOX17 (green) and PDX1 (red) in a 6 month old LSL-KrasG12D/+;Ptf1acre/+ pancreas. Nuclei are stained with DAPI. Scale bar = 50μm. (B) Immunohistochemistry for SOX17 in 4–12 month old LSL-KrasG12D/+;Ptf1acre/+ mice. Scale bar = 50μm for all panels, 25μm for insets. (C) Co-IF for SOX17 (green) and DCLK1 (red). Arrows indicate several DCLK1 positive cells that have low SOX17 expression compared to nearby cells. Nuclei are stained with DAPI (blue). Scale bar =20 μm. (D) Co-IF for PDX1 (green) and DCLK1 (red). Green arrows indicate co-positive cells. Red arrows indicate DCLK1-only positive cells. Nuclei are stained with DAPI (blue). Scale bar =20 μm
The apparent inverse relationship between increasingly-common SOX17 expression and the gradual loss of tuft cells in the mPanINs of KrasG12D/+;Ptf1aCre/+ mice led us to examine their possible causal relationship more directly. We performed co-IF for SOX17 and DCLK1 on 4 month old KrasG12D/+;Ptf1aCre/+ pancreata. Interestingly, DCLK1+ tuft cells consistently expressed relatively low levels of SOX17 compared to most other cells within the metaplastic epithelium (Figure 3C), indicating that elevated levels of SOX17 are not required, and may have to be suppressed, for the derivation or maintenance of metaplastic tuft cells. PDX1 expression was found in some DCLK1+ tuft cells, but not all, suggesting no relationship with tuft cell maintenance (Figure 3D).
SOX17+ PBGs are mucinous, tuft cell-containing glands that express stem cell factors such as OCT4 and LGR5, and endoderm-specific markers SOX9, PDX1, EPCAM, CXCR4, and FOXA223. Many of these markers have been previously described to play a role in pancreatic ADM and tumorigenesis2, 27–31. To examine the extent of PBG mimicry in acinar cell-derived metaplasia, we induced KrasG12D expression by treating 8 week old LSL-KrasG12D;ROSAYFP;Ptf1aCre−ERTM/+ mice with tamoxifen, to induce recombination, and cerulein, to accelerate tumorigenesis, and examined their pancreata 9 weeks later. We found that this acinar cell-derived pancreatic metaplasia widely expressed SOX17, PDX1, SOX9, and EPCAM, LGR5, consistent with a PBG-like phenotype (Figure S3).
SOX17 expression drives a pancreatitis-like disease state and accelerates Kras-driven tumorigenesis
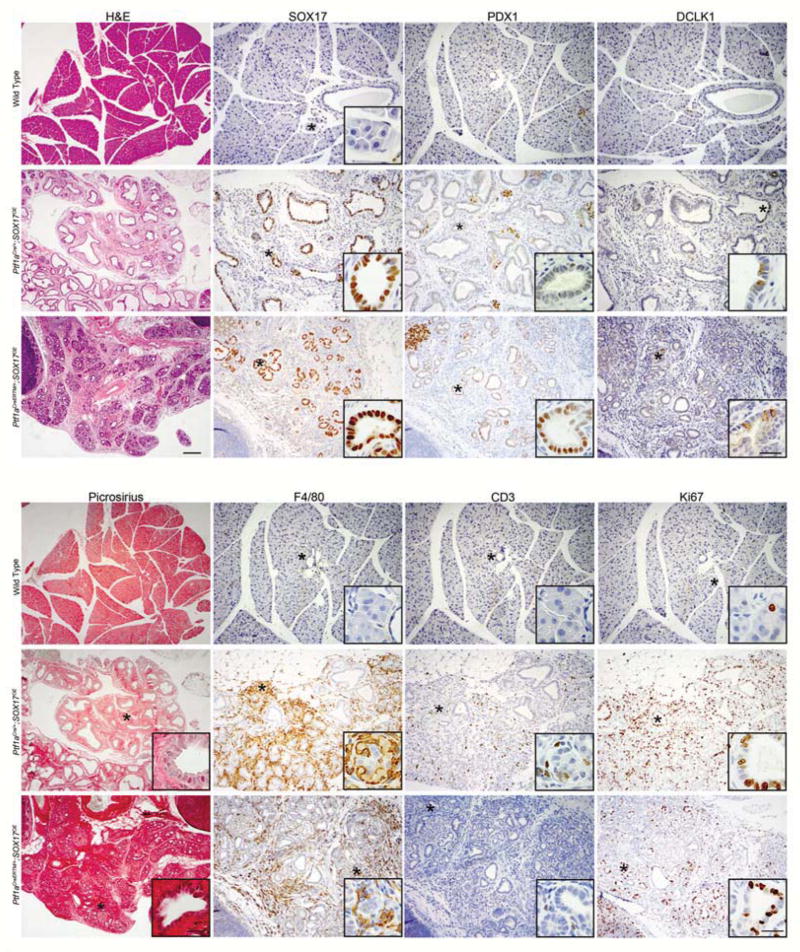
SOX17 is a known determinant of biliary development and differentiation. Its presence in pancreatic metaplasia suggests that it may play a causal role in acinar cell reprogramming during disease. It has been shown previously that SOX17 overexpression in PDX1+ cells during early pancreatic development causes severe ductal expansion. Using a similar model that induces a more universal pancreatic expression of SOX17, ROSAtTa/+;Ptf1aCre/+;tetO-SOX17 (Ptf1aCre/+;SOX17OE) and an adult acinar cell-specific inducible model of SOX17 overexpression, ROSAtTa/+;Ptf1aCreERTM/+;tetO-SOX17 (Ptf1aCreERTM/+;SOX17OE), we set out to determine the full spectrum of downstream consequences of SOX17 expression in the pancreas. In the Ptf1aCre/+;SOX17OE model, mice express SOX17 in Ptf1a+ positive progenitor cell populations and exhibit an expansion of PDX1 positive, tuft cell-containing ductal structures at the expense of the acinar and islet compartments by 6 weeks of age (Figure 4), consistent with previous findings6. We found that ductal structures were constituted by Ki67 positive epithelia and contained a subpopulation of DCLK1+ tuft cells, consistent with Kras-induced ADM and the assumption of a biliary phenotype, respectively. Surprisingly, these pancreata showed a robust innate and adaptive inflammatory reaction, as determined by IHC for macrophages (F4/80+) and T-cells (CD3+). Picrosirius red staining demonstrated a vigorous fibrotic response (Figure 4).
Figure 4. Transgenic expression of SOX17 induces a biliary phenotype and a pancreatitis-like disease state.
Immunohistochemical comparison of the pancreata from a wild type mouse to that of a Ptf1aCre/+;SOX17OE mouse, where SOX17 has been overexpressed from development, and a Ptf1aCreERTM/+;SOX17OE mouse in which SOX17 expression was induced in the adult. The pancreata of both experimental models exhibit a biliary-like phenotype highly expressing SOX17 and DCLK1+ tuft cells. Consistent with biliary differentiation, PDX1 expression is low in the Ptf1aCre/+;SOX17OE model. Consistent with metaplasia, the Ptf1aCreERTM/+;SOX17OE model highly expresses PDX1. Exhibiting characteristics of chronic pancreatitis, both models demonstrate both an innate (F4/80) and adaptive (CD3) immune response accompanying a fibrotic response (picrosirius) and proliferative (Ki67) ducts. Scale bars for H&E and picrosirius =200μm, all IHC =100μm, and all insets =25μm.
To determine whether SOX17 was capable of reprogramming adult acinar cells, we induced SOX17 expression in the pancreata of adult mice using the Ptf1aCreERTM/+;SOX17OE model. Mice, 5–10 weeks of age, were treated with tamoxifen to induce acinar cell-specific CRE activity, leading to tTA induction of SOX17 in these cells. Similar to what we observed with SOX17 expression initiated during organogenesis, expression of SOX17 for six weeks in adult acinar cells induced a PDX1+ metaplasia where 10.8% of the epithelium was comprised of DCLK1+ tuft cells, mimicking a pancreatobiliary progenitor cell phenotype and confirming a role for SOX17 in tuft cell genesis. The robust stromal reaction included innate and adaptive inflammatory responses (Figure 4) and fibrosis, consistent with a chronic pancreatitis phenotype, but no PanIN formation as determined by Muc5aC IHC (Figure S4). Taken together, our data indicate that epithelial overexpression of SOX17 was sufficient to drive a stable phenotype recapitulating all major aspects of chronic pancreatitis, a known risk factor for pancreatic tumorigenesis, in the absence of overt tissue damage.
We then hypothesized that SOX17-induced ADM would collaborate with KrasG12D expression, accelerating the relatively inefficient metaplastic process induced by KrasG12D alone. To test this, we tamoxifen treated ROSAtTa/+;Kras LSL−G12D/+;Ptf1aCreERTM/+;tetO-SOX17 mice (KrasG12D;Ptf1aCreERTM/+;SOX17OE), 5–10 weeks of age, which initiates co-expression of KrasG12D and SOX17 in adult acinar cells, and allowed for them to recover for 6 weeks. Compared to expression of KrasG12D alone, which ranged from 5–12% of total tissue replacement by PanIN-containing regions, concomitant SOX17 expression led to a virtually complete replacement of the normal acinar cell compartment with PanIN together with reactive epithelia and stroma. Tumors were characterized by PDX1 expression and DCLK1+ tuft cells (Figure 5). IHC for the PanIN marker, Muc5aC, confirmed that SOX17 expression did not affect the type of tumors formed in these mice (Figure S4) and pathology showed no indication of advanced progression, with the majority of tumors being early PanIN1/2 stage. These data indicate that SOX17-induced biliary metaplasia is highly susceptible to KrasG12D transformation and, as such, can drive pancreatic disease initiation.
Figure 5. SOX17expression facilitates KrasG12D-driven tumorigenesis.
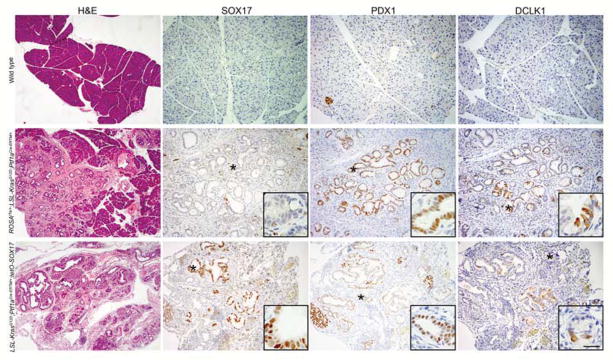
Immunohistochemical comparison of the pancreata from a wild type mouse to that of a KrasG12D;Ptf1aCreERTM/+;ROSAtTa/+mouse and a KrasG12D;Ptf1aCreERTM/+;SOX17OE mouse treated with tamoxifen and allowed to recover for 6 weeks. In concert with KrasG12D, overexpression of SOX17 leads to a greater degree of transformed tissue, as identified in H&E, characterized by PDX1 expression and DCLK1+ tuft cells. Scale bars for H&E=200μm, all IHC=100μm for all panels and all insets=25μm.
Human pancreatic disease assumes a biliary-like phenotype
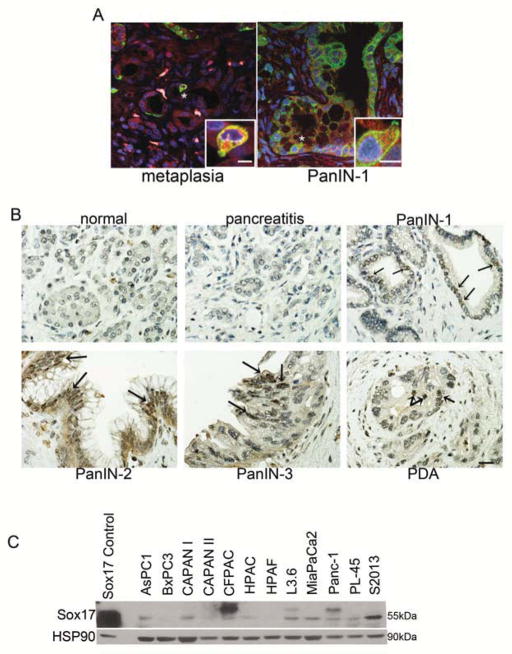
To determine if human pancreatic disease phenocopies progression seen in murine models, we assessed human pancreatic tissue and cell lines for biliary markers. To analyze the possible association of tuft cells, human CP, PanIN, and PDA samples were assessed by IHC for tuft cell markers. Finding that DCLK1 IHC was not robust in positive control human intestinal tissue, we examined factors whose co-expression was also definitive for tuft cells in the mouse: COX-1 and phospho-EGFR. Using co-IF in human pancreatic tissue microarrays we found that tuft cells were associated with PanINs. We found 58% (29/50) PanIN1s, 40% (12/30) PanIN2s and 33% (9/27) PanIN3s contained at least one tuft cell. Unlike the murine model, 30% of PDA samples (24/79) contained tuft cells (Figure 6A). Also distinct from the murine model, tuft cells were associated with ducts in 25% (20/79) of normal pancreas samples and 24% (21/78) of pancreatitis samples.
Figure 6. Human pancreatic disease assumes a biliary phenotype.
(A) Co-immunofluorescence for phospho-EGFR (Y1068) (green) and COX1 (red) on human pancreatic tissue arrays reveals the presence of tuft cells in metaplasia and early PanIN lesions. Nuclei are stained with DAPI. Scale bars = 20μm for both panels, 80μm for insets. (B) IHC for SOX17 in human pancreatic tissue microarray. Staining was found from PanIN1 to PDA, with arrows indicating regions with positive staining in PanIN-3 and PDA. Scale bar = 20 μm. (C) Western blot analysis of a panel of human PDA cell lines reveals detectable levels of SOX17 in 9/12 cell lines. SOX17 control is lysate from MiaPaCa2 cells transfected with mSox17(164/623)IRESGFP-pTRE.
SOX17 expression was also examined in these pancreas tissue microarrays. Though staining in general was less intense compared to murine tissue, 78% (39/50) of PanIN1, 80% (24/30) of PanIN2, 93% (25/27) of PanIN3 and 30% of PDA (14/47) expressed SOX17, though the number of cells in PDA samples were relatively few (Figure 6B). 31% of chronic pancreatitis samples had SOX17 positive cells and 6% (5/79) of normal pancreas showed positivity, confined to large normal ducts.
To confirm SOX17 expression in human PDA, a panel of PDA cell lines was assessed for SOX17 expression by western blot. While expression levels of the isoforms varied, 9/12 PDAC cell lines expressed detectable levels of SOX17 (Figure 6C).
DISCUSSION
Pancreatic metaplastic ductal lesions represent a transdifferentiation event and are hypothesized to be pre-neoplastic. Our work demonstrates that KrasG12D-induced ADM presents as an epithelium that assumes several characteristics of the developmentally related bile duct, marked by the presence of numerous tuft cells and SOX17 expression. This tissue-switching phenomenon, a previously unrecognized process in the pancreas, reinforces the well-established concept that metaplasia is composed of cell types phenotypically similar to those normally restricted to tissues derived from a neighboring region of the embryo4. We find that SOX17 expression alone, limited to adult acinar cells, was sufficient to induce a stable metaplastic change that included genesis of tuft cells and co-expression of PDX1, the latter being suggestive of reprogramming akin to a pancreatobiliary progenitor cell. SOX17 overexpression combined with expression of oncogenic KrasG12D, leads an enhancement of tumor initiation compared to KrasG12D expression alone, consistent with the metaplastic change being a prerequisite for pancreatic tumorigenesis.
While enhanced metaplasia is one explanation for accelerated tumorigenesis, SOX17 expression also induced a dramatic, concurrent stromal response reminiscent of chronic pancreatitis. Chronic pancreatitis is a known risk factor for PDA and several studies have shown that the desmoplastic and inflammatory responses can contribute to pancreatic tumor progression32. The ability of SOX17 overexpression to induce this stromal reaction may contribute to the initial epithelial response. However, we favor a model where the stromal response and susceptibility to transformation is a consequence of biliary reprogramming, rather than the converse. During examination of KrasG12D or SOX17 induced metaplasia for proinflammatory signals, for instance, we found key components of the prostaglandin synthesis pathway, including HPGDS, COX-1, and COX2, the latter being a known contributor to pancreatic disease33, were expressed at very high levels in metaplastic tuft cells. The identification of these cells as chemosensory is suggestive of a role in detection of injury and initiation of a repair response. Consistent with this, tuft cells also express progenitor cell markers, such as LGR5 and DCLK1 (34 and data not shown), which could aid in tissue repair. Interestingly, pancreatic tuft cells have been proposed to act as tumor-initiating cells when they are engineered to express oncogenic KRAS, which may usurp their putative regenerative function (Steven Leach, personal communication).
The role of SOX17 in pancreatic metaplasia is somewhat reminiscent of what was recently observed in mice overexpressing SOX9, a related transcription factor2. In the previous study, SOX9 is not capable of hijacking acinar cell differentiation when expressed during organogenesis, as is SOX17, suggesting a unique reprogramming activity for SOX17 in pancreatic progenitors. Conversely, while SOX9 appears to be critical for stabilization of pancreatic metaplasia, we find that administration of doxycycline to turn off SOX17 expression after metaplasia forms did not lead to collapse of metaplastic ducts (data not shown). Thus SOX17 and SOX9 appear to have similar, but distinct, functions in pancreatic disease.
We conclude that SOX17 expression itself is sufficient to drive formation of tuft cell-containing, pro-inflammatory, ductal reprogramming of pancreatic acinar cells in vivo. We find that this metaplasia is accompanied by a dramatic stromal response in the absence of further manipulation. Finally, when combined with KrasG12D expression, SOX17 overexpression enhances tumorigenesis, suggesting that SOX17 expression and the ensuing biliary transdifferentiation promotes tumor formation.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by a VA merit award, the Knapp Chair for Pancreatic Cancer Research and NIH grants R01CA100126 and R01CA136754 to HCC; P30DK058404 for the Vanderbilt Digestive Diseases Research Center Tissue Morphology Subcore: GI SPORE Tissue Core; NIH P50CA095103 and NIH P50 CA102701 for the Mayo Clinic SPORE in Pancreatic Cancer and by 1F32CA123939 to KPO; NIH NIDDK U01DK089570 to CVEW; JDRF scholar award #3-2008-118 to FCP
The authors would like to thank Lesley Scudder for assistance with animal husbandry procedures, Brandy Edenfield for immunohistochemical analysis, Megan Hoffman and Louise Peverley for assistance with manuscript preparation and the Central Microscopy Imaging Center (C-MIC) at Stony Brook University, Stony Brook, New York 11794 for assistance with electron microscopy. Special thanks to Steven Leach and Jennifer Bailey for sharing their work prior to publication.
Abbreviations
- ADM
acinar-to-ductal metaplasia
- CNS
central nervous system
- CP
chronic pancreatitis
- DCLK1
doublecortin-like kinase 1
- DCS
diffuse chemosensory system
- EGFR
epidermal growth factor receptor
- IF
immunofluorescence
- IHC
immunohistochemistry
- mPanIN
murine pancreatic intraepithelial neoplasia
- PanIN
pancreatic intraepithelial neoplasia
- PBG
peribiliary gland
- PDA
pancreatic ductal adenocarcinoma
- PDG
pancreatic duct gland
- PDX1
pancreatic and duodenal homeobox 1
- SCC
solitary chemosensory cell
- SOX17
sex determining region Y-box 17
- YFP
yellow fluorescent protein
Footnotes
Disclosures: None
Writing assistance: None
Author contributions:
Kathleen E. DelGiorno: study concept and design; acquisition of data; analysis and interpretation of data; drafting of the manuscript
Jason C. Hall: acquisition of data; analysis and interpretation of data
Kenneth K. Takeuchi: acquisition of data; analysis and interpretation of data
Fong Cheng Pan: material support
Christopher J. Halbrook: acquisition of data
M. Kay Washington: analysis and interpretation of data; technical support; material support
Kenneth P. Olive: material support
Jason Spence: material support
Bence Sipos: material support
Christopher V. E. Wright: material support
James M. Wells: critical revision of the manuscript for important intellectual content; material support
Howard C. Crawford: study concept and design; analysis and interpretation of data; drafting of the manuscript; obtained funding; administrative, study supervision
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JPt, Pan FC, Akiyama H, Wright CV, Jensen K, Hebrok M, Sander M. Identification of Sox9-Dependent Acinar-to-Ductal Reprogramming as the Principal Mechanism for Initiation of Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2012;22:737–50. doi: 10.1016/j.ccr.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ardito CM, Gruner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, Pal D, Briel T, Herner A, Trajkovic-Arsic M, Sipos B, Liou GY, Storz P, Murray NR, Threadgill DW, Sibilia M, Washington MK, Wilson CL, Schmid RM, Raines EW, Crawford HC, Siveke JT. EGF Receptor Is Required for KRAS-Induced Pancreatic Tumorigenesis. Cancer Cell. 2012;22:304–17. doi: 10.1016/j.ccr.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8:369–78. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 5.Sbarbati A, Bramanti P, Benati D, Merigo F. The diffuse chemosensory system: exploring the iceberg toward the definition of functional roles. Prog Neurobiol. 2010;91:77–89. doi: 10.1016/j.pneurobio.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Spence JR, Lange AW, Lin SC, Kaestner KH, Lowy AM, Kim I, Whitsett JA, Wells JM. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandgren EP, Luetteke NC, Palmiter RD, Brinster RL, Lee DC. Overexpression of TGF alpha in transgenic mice: induction of epithelial hyperplasia, pancreatic metaplasia, and carcinoma of the breast. Cell. 1990;61:1121–35. doi: 10.1016/0092-8674(90)90075-p. [DOI] [PubMed] [Google Scholar]
- 8.Pan FC, Bankaitis ED, Boyer D, Xu X, Van de Casteele M, Magnuson MA, Heimberg H, Wright CV. Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development. 2013;140:751–64. doi: 10.1242/dev.090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, Jacks T, Tuveson DA. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–8. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–34. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 11.Park KS, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006;294:192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 12.Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol. 2012;362:57–64. doi: 10.1016/j.ydbio.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–44. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bombardelli L, Carpenter ES, Wu AP, Alston N, DelGiorno KE, Crawford HC. Pancreas-specific ablation of beta1 integrin induces tissue degeneration by disrupting acinar cell polarity. Gastroenterology. 2010;138:2531–40. 2540 e1–4. doi: 10.1053/j.gastro.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navas C, Hernandez-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318–30. doi: 10.1016/j.ccr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerbe F, van Es JH, Makrini L, Brulin B, Mellitzer G, Robine S, Romagnolo B, Shroyer NF, Bourgaux JF, Pignodel C, Clevers H, Jay P. Distinct ATOH1 and Neurog3 requirements define tuft cells as a new secretory cell type in the intestinal epithelium. J Cell Biol. 2011;192:767–80. doi: 10.1083/jcb.201010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.May R, Sureban SM, Lightfoot SA, Hoskins AB, Brackett DJ, Postier RG, Ramanujam R, Rao CV, Wyche JH, Anant S, Houchen CW. Identification of a novel putative pancreatic stem/progenitor cell marker DCAMKL-1 in normal mouse pancreas. Am J Physiol Gastrointest Liver Physiol. 2010;299:G303–10. doi: 10.1152/ajpgi.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saqui-Salces M, Keeley TM, Grosse AS, Qiao XT, El-Zaatari M, Gumucio DL, Samuelson LC, Merchant JL. Gastric tuft cells express DCLK1 and are expanded in hyperplasia. Histochem Cell Biol. 2011;136:191–204. doi: 10.1007/s00418-011-0831-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bezencon C, Furholz A, Raymond F, Mansourian R, Metairon S, Le Coutre J, Damak S. Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol. 2008;509:514–25. doi: 10.1002/cne.21768. [DOI] [PubMed] [Google Scholar]
- 20.Bezencon C, le Coutre J, Damak S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem Senses. 2007;32:41–9. doi: 10.1093/chemse/bjl034. [DOI] [PubMed] [Google Scholar]
- 21.Bjerknes M, Khandanpour C, Moroy T, Fujiyama T, Hoshino M, Klisch TJ, Ding Q, Gan L, Wang J, Martin MG, Cheng H. Origin of the brush cell lineage in the mouse intestinal epithelium. Dev Biol. 2012;362:194–218. doi: 10.1016/j.ydbio.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luciano L, Reale E. Brush cells of the mouse gallbladder. A correlative light- and electron-microscopical study. Cell Tissue Res. 1990;262:339–49. doi: 10.1007/BF00309889. [DOI] [PubMed] [Google Scholar]
- 23.Carpino G, Cardinale V, Onori P, Franchitto A, Berloco PB, Rossi M, Wang Y, Semeraro R, Anceschi M, Brunelli R, Alvaro D, Reid LM, Gaudio E. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat. 2012;220:186–99. doi: 10.1111/j.1469-7580.2011.01462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strobel O, Rosow DE, Rakhlin EY, Lauwers GY, Trainor AG, Alsina J, Fernandez-Del Castillo C, Warshaw AL, Thayer SP. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology. 2010;138:1166–77. doi: 10.1053/j.gastro.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuttler AS, LeClair RJ, Stohn JP, Wang Q, Sorenson CM, Liaw L, Lindner V. Characterization of Pdgfrb-Cre transgenic mice reveals reduction of ROSA26 reporter activity in remodeling arteries. Genesis. 2011;49:673–80. doi: 10.1002/dvg.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong SM, Omura N, Vincent A, Li A, Knight S, Yu J, Hruban RH, Goggins M. Genome-wide CpG island profiling of intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res. 2012;18:700–12. doi: 10.1158/1078-0432.CCR-11-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salnikov AV, Groth A, Apel A, Kallifatidis G, Beckermann BM, Khamidjanov A, Ryschich E, Buchler MW, Herr I, Moldenhauer G. Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. J Cell Mol Med. 2009;13:4023–33. doi: 10.1111/j.1582-4934.2009.00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wen J, Park JY, Park KH, Chung HW, Bang S, Park SW, Song SY. Oct4 and Nanog expression is associated with early stages of pancreatic carcinogenesis. Pancreas. 2010;39:622–6. doi: 10.1097/MPA.0b013e3181c75f5e. [DOI] [PubMed] [Google Scholar]
- 29.Wang Z, Ma Q, Liu Q, Yu H, Zhao L, Shen S, Yao J. Blockade of SDF-1/CXCR4 signalling inhibits pancreatic cancer progression in vitro via inactivation of canonical Wnt pathway. Br J Cancer. 2008;99:1695–703. doi: 10.1038/sj.bjc.6604745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyatsuka T, Kaneto H, Shiraiwa T, Matsuoka TA, Yamamoto K, Kato K, Nakamura Y, Akira S, Takeda K, Kajimoto Y, Yamasaki Y, Sandgren EP, Kawaguchi Y, Wright CV, Fujitani Y. Persistent expression of PDX-1 in the pancreas causes acinar-to-ductal metaplasia through Stat3 activation. Genes Dev. 2006;20:1435–40. doi: 10.1101/gad.1412806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Washington MK, Crawford HC. Loss of FOXA1/2 is essential for the epithelial-to-mesenchymal transition in pancreatic cancer. Cancer Res. 2010;70:2115–25. doi: 10.1158/0008-5472.CAN-09-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pandol S, Edderkaoui M, Gukovsky I, Lugea A, Gukovskaya A. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2009;7:S44–7. doi: 10.1016/j.cgh.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–28. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Itzkovitz S, Lyubimova A, Blat IC, Maynard M, van Es J, Lees J, Jacks T, Clevers H, van Oudenaarden A. Single-molecule transcript counting of stem-cell markers in the mouse intestine. Nat Cell Biol. 2012;14:106–14. doi: 10.1038/ncb2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.