Abstract
Neurocognitive aging studies have focused on age-related changes in neural activity or neural structure but few studies have focused on relationships between the two. The present study quantitatively reviewed 24 studies of age-related changes in fMRI activation across a broad spectrum of executive function tasks using activation likelihood estimation (ALE) and 22 separate studies of age-related changes in gray matter using voxel-based morphometry (VBM). Conjunction analyses between functional and structural alteration maps were constructed. Overlaps were only observed in the conjunction of dorsalateral prefrontal cortex (DLPFC) gray matter reduction and functional hyperactivation but not hypoactivation. It was not evident that the conjunctions between gray matter and activation were related to task performance. Theoretical implications of these results are discussed.
Keywords: aging, dorsolateral prefrontal cortex, efficiency, executive function, meta-analysis, plasticity
1. Introduction
As individuals age, many aspects of cognitive function become less efficient most notably working memory, inhibitory function, and long-term memory (e.g., Rypma, Eldreth & Rebbechi, 2007; Hasher, et al., 1991; Gazzaley et al., 2008; Craik & McDowd, 1987; Nyberg et al., 2003; see Nyberg & Backman, 2010). Gray matter (GM) reductions have been reported in regions associated with these functions most notably prefrontal cortex, caudate, cerebellum, and hippocampus (Raz & Rodrigue, 2006; Dennis & Cabeza, 2008). To confront these increased endogenous challenges (i.e., those brought on by changes to neural anatomy and physiology), as well as exogenous challenges (i.e., those brought on by changes to the environment), older adults must flexibly adapt. Changes in neural activity associated with neuroanatomic changes could be thought of as manifestations of this “neural plasticity” (i.e., adaptation-related skill reacquisition; Greenwood, 2007; Park & Reuter-Lorenz, 2009; Reuter-Lorenz & Park, 2010; Park & Bischoff, 2010; Schneider-Garces et al., 2010) if it were observed (1) that age-related GM changes corresponded spatially with age-related neural activation (as measured by fMRI) and (2) that these age-related structure-function changes corresponded to improvements in performance (Grady, 2012; Rypma & D'Esposito, 2001).
Studies of brain function in older adults using positron emission tomography (PET) and functional magnetic resonance imaging (fMRI) have demonstrated consistent patterns of neural activity alterations (Davis et al., 2008; Spreng et al. 2010. but see Nyberg et al., 2010). These alterations generally take the form of age-related increases in frontal activity (i.e., hyperactivation). These hyperactivations have been interpreted as reflecting compensation, (i.e., adaptation to the decline of some cognitive functions; Grady 1998), de-differentiation of cognitive processes (Baltes & Lindenberger, 1997), and reduced efficiency of cognitive processes (Motes, Biswal & Rypma, 2010; Rypma et al., 2005, Rypma & D'Esposito, 2000).
Age-related neural increases in activity might be related to anatomic degeneration (e.g., Bennett et al., 2012). Specifically, it might be that local anatomic deficits lead to neural inefficiency as reflected by enhanced functional responses (e.g., Greenwood; 2007, Bennett et al., 2012). Structural alterations have been extensively investigated in previous work using manual volumetric measurement (e.g., Raz et al., 2005), voxel-based morphometry (VBM; Good et al., 2001), and cortical thickness techniques (e.g., Salat et al., 2004). Age-related gray matter reductions occur over the entire cortex, but disproportionately in regions associated with age-related functional deficits (i.e., prefrontal cortex, caudate, cerebellum, and hippocampus, Raz & Rodrigue, 2006; Dennis & Cabeza, 2008).
In the present study we sought to characterize relationships between age-related neuroanatomic changes and functional activity changes. We focused on age-related activation changes related to general cognitive processes of executive function drawn from studies in the literature. Activation likelihood estimation (ALE, Turkeltaub et al., 2002) was used to identify age-related activation changes over a range of different types of executive function tasks (e.g. working memory, executive control, and delayed response task). Based on similar consideration, Spreng et al. (2010) quantitatively reviewed 77 neuroimaging studies of aging effects using the ALE technique. Their results showed age-related increases in prefrontal activity and performance-dependent age differences in activation laterality. In contrast, we analyzed data only from articles that directly compared activity differences between older and younger groups. In addition, another ALE analysis was conducted to examine consistent anatomical alterations using VBM analysis (Ashburner & Friston, 2000; Di et al., 2009; Chan et al., 2011). Conjunction analyses were then conducted to examine age-related structural and functional correspondence.
Four patterns of structure-function associations could be expected. First, age-related GM decreases would correspond with reductions in functional activity. This result would suggest that, with aging, neural loss is associated with reductions in the neural metabolic activity that gives rise to the BOLD signal. Second, age-related GM decreases would be associated with increases in functional activity. This result would suggest that neural loss is associated with increases in neural metabolic activity. Third, GM preservation would be associated with decreases in functional activity. Finally, age-related GM preservation might be associated with increases in functional activity. These latter outcomes would suggest more complex relationships between age-related GM change and changes in neural metabolic activity. Interpretation of these results would be contingent upon their relationships to performance. Based on plasticity theories of neurocognitive aging (Greenwood, 2007; Park & Reuter-Lorenz, 2009), we predicted that regions that showed consistent hyperactivation but not hypoactivation in older group would overlap with regions that showed consistent GM reductions. In addition, observations of overlap between age-related activation changes and GM changes would be associated with age-related changes in performance.
2. Methods
2.1 Article selection
2.1.1 Functional imaging studies
Studies were searched in the PubMed database using “aging” combined with task keywords and imaging modality keywords (functional magnetic resonance imaging, fMRI or PET). The task keywords included delayed match-to-sample, delayed response, go/no-go, mental arithmetic, N-back, oddball, sequence recall, Stroop, Wisconsin Card Sort, and word generation task, which was consistent with a previous meta-analysis on executive function of patients with schizophrenia (Minzenberg et al., 2009). In addition, we searched the reference lists of the studies identified and recent ALE studies (Spreng et al., 2010; Turner & Spreng, 2012) for potential inclusion. The inclusion criteria were as follows: 1) they were research articles; 2) they studied linear correlations between the age and task related activations, or compared differences in activations between a group of older subjects and a group of younger subjects; 3) the results were normalized to a stereotactic standardized space such as the Montreal Neurological Institute (MNI) space or Talairach space (Talairach & Tournoux, 1988), and the coordinates of the activation areas were explicitly reported.
Twenty four articles with a total of 860 subjects were included in the fMRI meta-analysis (Table 1). Paxton et al. (2008) reported two experiments with independent subject samples, so the two experiments were treated as independent. Esposito et al. (1999) and Nagels et al. (2012) examined linear correlation between task related activation and age, while the other experiments directly compared the task related activations between the older and younger groups. All of the included studies but Prakash et al. (2012) reported hyperactivation for the older group, and fifteen studies also reported hypoactivation. The task used in each experiment was listed in Table 1. Task performance was determined based on accuracy but not reaction time, consistent with a previous meta-analysis (Spreng et al., 2010). Equivalent performance describes experiments where the accuracy of a given task performance was not statistically significant between young and old group. Twelve experiments did not report significant different performance between young and old groups (denoted as ‘=’ in Table 1), whereas 13 experiments reported significantly poorer performance in old adults (denoted as ‘≠’ in Table 1).
Table 1.
List of fMRI and PET studies on executive functions that are included in the fMRI ALE analysis.
| Study # | First author & year | Task | Category | Performance | Modality | Effect of age | Young | Old | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| N | Age | N | Age | |||||||||||
| 1 | Anguera 2011 | Spatial working memory task | WM | ≠ | fMRI | ↑↓ | 18 | 21.1 | 18 | 71.4 | ||||
| 2 | Cabeza 2004 | Delayed-response | WM | = | fMRI | ↑↓ | 20 | 22.6 | 20 | 70.3 | ||||
| 3 | Colcombe 2005 | Flanker task | Inhibition | = | fMRI | ↑ | 20 | 23.5 | 40 | 67.5 | ||||
| 4 | Esposito 1999 | Wisconsin card sorting task | Other | ≠ | PET | ↑↓ | n = 41; range:18-80 | |||||||
| 5 | Freo 2005 | Delayed match to sample | WM | = | PET | ↑↓ | 13 | 27 | 13 | 65 | ||||
| 6 | Grady 1998 | Delayed match to sample | WM | ≠ | PET | ↑↓ | 13 | 25 | 16 | 66 | ||||
| 7 | Grady 2008 | N-back task | WM | ≠ | fMRI | ↑ | 16 | 26.1 | 18 | 65.8 | ||||
| 8 | Grossman 2002 | Sentence comprehension task | WM | = | fMRI | ↑↓ | 13 | 22.6 | 11 | 63.5 | ||||
| 9 | Huang 2012 | Stroop-like Task | Inhibition | = | fMRI | ↑ | 15 | 25.5 | 18 | 66.1 | ||||
| 10 | Hubert 2009 | Tower of Toronto task | Other | ≠ | PET | ↑ | 12 | 22.4 | 12 | 65 | ||||
| 11 | Lamar 2004 | Delayed match to sample | WM | ≠ | fMRI | ↑↓ | 16 | 27.9 | 16 | 69.1 | ||||
| 12 | Lee 2006 | Response regulation task | Inhibition | ≠ | fMRI | ↑ | 12 | 29.8 | 9 | 65.2 | ||||
| 13 | Madden 2010 | Task switching | Other | ≠ | fMRI | ↑↓ | 20 | 22.4 | 20 | 69.6 | ||||
| 14 | Mathis 2009 | Stroop task | Inhibition | ≠ | fMRI | ↑ | 12 | 26.8 | 24 | 51.7 | ||||
| 15 | Mell 2009 | Probabilistic object reversal task | Inhibition | = | fMRI | ↑↓ | 14 | 26.5 | 14 | 67.8 | ||||
| 16 | Nagels 2012 | Word generation | Other | = | fMRI | ↑ | n = 56; range:22-56 | |||||||
| 17 | Onur 2011 | Stroop task | Inhibition | ≠ | fMRI | ↑↓ | 15 | 24.2 | 13 | 63.8 | ||||
| 18 | O'Connell 2012 | Oddball task | Other | = | fMRI | ↑ | 15 | 22 | 14 | 70.6 | ||||
| 19a | Paxton 2008 | AX Continuous performance task | WM | = | fMRI | ↑↓ | 21 | 22.8 | 20 | 73 | ||||
| 19b | Paxton 2008 | AX Continuous performance task | Inhibition | ≠ | fMRI | ↑↓ | 16 | 21.6 | 16 | 72.4 | ||||
| 20 | Prakash 2012 | N-back task | WM | ≠ | fMRI | ↓ | 25 | 23.4 | 25 | 72.2 | ||||
| 21 | Ricciardi 2009 | Delayed match to sample | WM | = | PET | ↑↓ | 10 | 26.2 | 10 | 68.4 | ||||
| 22 | Rypma 2001 | Item-recognition task | WM | = | fMRI | ↑↓ | 6 | 25.3 | 6 | 68.6 | ||||
| 23 | Van Impe 2011 | Mental arithmetics | Other | ≠ | fMRI | ↑ | 20 | 25.2 | 21 | 68.0 | ||||
| 24 | Zysset 2006 | Stroop task | Inhibition | = | fMRI | ↑ | 23 | 26.6 | 24 | 57.1 | ||||
‘↑’ represents that the paper reported higher activations in older group compared with younger group, whereas ‘↓’ denotes that older group demonstrated lower activations compared with younger group. ‘↑↓’ represents that the paper reported both higher and lower activations in older group compared with younger group. WM represents working memory.
2.1.2 VBM studies
Pubmed search used the key words “Voxel Based Morphometry” and “aging,” or “VBM” and “aging,” respectively. In addition, we searched the reference lists of the studies identified for potential inclusion. From the about 150 resultant articles, we included the studies considering the following criteria: 1) they were empirical articles; 2) they used the voxel-based morphometry analysis to investigate the GM concentration or volume changes of MRI dataset; 3) they studied linear correlations between the GM alterations and age, or compared GM differences between the older and younger individuals; 4) the results were normalized to a stereotactic standardized space such as the MNI space or Talairach space (Talairach & Tournoux, 1988), and the coordinates of the activation areas were explicitly reported.
Twenty-two articles with a total of 2657 subjects were included in the VBM meta-analysis (Table 2). One paper by Takahashi et al. (2011) reported separately the male and female results, so the two results were treated as two independent experiments. These studies used different software such as (SPM99, SPM2, SPM5, and SPM8. http://www.fil.ion.ucl.ac.uk/spm/), FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/), and in house software (Tisserand et al., 2002; 2004) to conduct VBM analyses. In addition, different algorithms were used, including traditional VBM (Ashburner & Friston, 2000), optimized segmentation (Good et al., 2001), unified segmentation (Ashburner & Friston, 2005), and DARTEL (Ashburner, 2007). Sixteen studies compared age-related differences using modulated GM images (i.e. GMV, gray matter volume), while seven studies used unmodulated GM images (i.e., GMC gray matter concentration). Good et al., (2001) used both GMV and GMC images, but we only included the GMV results in the current analysis. All of the included studies reported a GM reduction across aging, while ten studies also reported relative GM preservation after controlling for global GM loss. Seventeen studies examined the linear correlation between the GM volume/concentration and age, and the other six studies directly compare GM measures between older and younger groups. There was no overlap of subject samples between the fMRI meta-analysis and the VBM meta-analysis.
Table 2.
List of VBM studies included in the ALE analysis.
| Study # | First author & year | No. of subjects a | male | female | Mean age | Age range b | Software | Algorithm | Modulation | Measure | Effect of age |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Abe 2008 | 73 | 73 | 39.2 | 22-70 | SPM2 | Optimized | GMV | Linear | ↓↑ | |
| 2 | Alexander 2006 | 26 | 15 | 11 | 50.7 | 22-77 | SPM2 | Optimized | GMV | Linear | ↓↑ |
| 3 | Antonova 2009 | 10o/10y | 20 | 47.9 | 23.6-72.1 | SPM2 | Optimized | GMV | group difference | ↓ | |
| 4 | Bauer 2012 | 18o/18y | N.A | 42.3 | 24.4-60.2 | SPM8 | DARTEL | GMV | group difference | ↓ | |
| 5 | Bergfield 2010 | 29 | 11 | 18 | 47.7 | 23-84 | SPM5 | Unified | GMV | Linear | ↓↑ |
| 6 | Berlingeri 2010 | 24o/24y | 24 | 24 | 44.3 | 26.5-62 | SPM2 | Optimized | GMV | group difference | ↓ |
| 7 | Curiati 2009 | 45 | 45 | 70.1 | ∼67-75 | SPM2 | Optimized | GMV | Linear | ↓↑ | |
| 8 | Giorgio 2010 | 66 | 31 | 35 | 36.7 | 23.0-81.6 | FSL | Optimized | GMV | Linear | ↓ |
| 9 | Good 2001 | 465 | 265 | 200 | ∼30 | 17-79 | SPM99 | Optimized | GMV c | Linear | ↓↑ |
| 10 | Grieve 2005 | 223 | 117 | 106 | 34.5 | Aug-79 | SPM2 | Optimized | GMV | Linear | ↓↑ |
| 11 | Kalpouzos 2009 | 45 | 21 | 24 | 49.4 | 20-83 | SPM2 | Optimized | GMV | Linear | ↓↑ |
| 12 | Kalpouzos 2012 | 20o/16y | 8 | 28 | 45.2 | 25-61.3 | SPM5 | Unified | GMV | group difference | ↓ |
| 13 | Kennedy 2009 | 200 | 81 | 119 | 46.9 | 18-81 | FSL | Optimized | GMV | Linear | ↓ |
| 14 | Lehmbeck 2006 | 17o/17y | 34 | 46.5 | 25.9-67.1 | SPM2 | Optimized | GMC | group difference | ↓ | |
| 15 | Lemaître 2005 | 662 | 331 | 331 | 69.5 | 63.7-75.6 | SPM99 | Optimized | GMV | Linear | ↓ |
| 16 | Maguire 2003 | 12o/12y | 12 | 12 | 53.6 | 32.4-74.8 | SPM99 | Traditional | GMC | group difference | ↓ |
| 17 | Nunnemann 2007 | 133 | 60 | 73 | 55 | 29-80 | SPM2 | Optimized | GMV | Linear | ↓↑ |
| 18a | Takahashi 2011 | 111 | 111 | 48.3 | ∼20-79 | SPM8 | Optimized | GMC | Linear | ↓ | |
| 18b | Takahashi 2011 | 116 | 116 | 55.4 | ∼20-79 | SPM8 | Optimized | GMC | Linear | ↓ | |
| 19 | Terribilli 2011 | 89 | 48 | 41 | 30.2 | ∼18-50 | SPM2 | Optimized | GMV | Linear | ↓↑ |
| 20 | Tisserand 2002 | 57 | 34 | 23 | 55.7 | 21-81 | In house | Traditional | GMC | Linear | ↓ |
| 21 | Tisserand 2004 | 38 | 18 | 20 | 71.8 | 52-82 | In house | Traditional | GMC | Linear | ↓ |
| 22 | Van Laere 2001 | 81 | 40 | 41 | 44.2 | 20-81 | SPM99 | Traditional | GMC | Linear | ↓↑ |
‘↓’ represents that the paper reported decreased gray matter volume / concentration in older group compared with younger group, whereas ‘↑’ denotes that the paper reported relative preservation of gray matter volume / concentration with age. ‘↑↓’ represents that the paper reported both decreased and relative preservation of gray matter volume / concentration in older group compared with younger group. GMV, gray matter volume; GMC, gray matter concentration.
For studies that examined linear trend of aging, number of subjects for each groups are reported separately. O represents old group, while y represents young group.
For studies that examined linear trend of aging, age range represents minimum and maximum of the whole sample, whereas for the studies that directly compared between two groups of old young subjects, the age range represents the mean age of each group. ∼ denotes that the studies did not explicitly report the age range in their papers, we made an approximation of the age range based on the description of the original paper.
Good et al., (2001) reported both GMV and GMC in the paper. We only used the GMV results in the current analysis.
2.2 Activation likelihood estimation analysis
Because most of the studies reported results in MNI space, the ALE analyses were also conducted in MNI space. For papers whose results had been converted from MNI to Talairach space using Brett's transformation (Brett, 1999), or a simple affine transformation (e.g. in Lamar et al., 2004), results were converted back to MNI space using the corresponding method. For the studies whose results were originally in Talairach space, anatomical coordinates were converted into MNI space using the Lancaster transform (Lancaster et al., 2007).
The Activation Likelihood Estimation meta-analysis (Turkeltaub et al., 2002) was carried out using GingerALE 2.1.1 software with revised random effect algorithm (Eickhoff et al., 2009), and non-additive method (Turkeltaub et al., 2012). The idea behind ALE analysis is that the peak coordinates reported in VBM studies should be viewed as probability distributions around these coordinates (Turkeltaub et al., 2002). Accordingly, the coordinates were convolved with a three-dimensional Gaussian kernel, whose full width at half maximum (FWHM) was a function of the sample size of a particular study. An algorithm was used to model the spatial uncertainty of each focus using an estimation of the spatial variability. For the correlation studies that calculate correlations between the imaging variables and subjects' age, the study N was set as the total number of subjects. Study Ns were set as the number of the smaller group when studies reported group differences between the older and younger groups. After obtaining the activation map for each study, the convergence of activations across experiments was assessed quantitatively.
Four ALE maps were constructed. First, an fMRI hyperactivation map was constructed based on 159 foci from 24 independent comparisons. Second, an fMRI hypoactivation map was constructed based on 84 foci from 15 independent comparisons. Third, the GM reduction map was constructed according to 312 coordinates from 23 independent comparisons. And last, the GM relative preservation map was constructed according to 77 coordinates from ten studies. The resultant ALE maps were thresholded using a false discovery rate (FDR)-corrected threshold of p<0.05, with a recommended cluster extent threshold obtained from the FDR-correction procedure. Results-clusters were identified according to the peak locations using an anatomical label assigned by the Talairach Daemon (Lancaster et al., 2000).
We first binarized the thresholded ALE maps and then performed conjunction analysis on these maps. Four conjunction analyses were conducted: (1) between GM reductions and functional hyperactivations; (2) between GM reductions and functional hypoactivations; (3) between GM relative increases and functional hyperactivations; and (4) between GM relative increases and functional hypoactivations. An AND operation was performed to find voxels that were commonly activated in both ALE maps. Number of voxels and mean coordinates of the resulting clusters were calculated. It is noteworthy that the purpose of conjunction analysis is to find common activations of two statistical maps, thus the number of subjects, foci and studies of the two maps will not affect the results of conjunction analysis.
Finally, we examined the characteristics of studies contributing to clusters of significant conjunction effects. The variables of interests included the effects of task performance (equal vs. unequal), executive function components (working memory, inhibition and others) and imaging modality (fMRI vs. PET) for functional studies. The studies that contributed to these two clusters were pooled together (10 studies). For each variable, the number of contributing studies of each category was calculated and compared with the expected number of studies of each category, which were calculated from the whole studies sample of the current meta-analysis. Chi square was calculated to determine statistical significance (Laird et al., 2009).
3. Results
3.1 ALE analyses of functional imaging studies
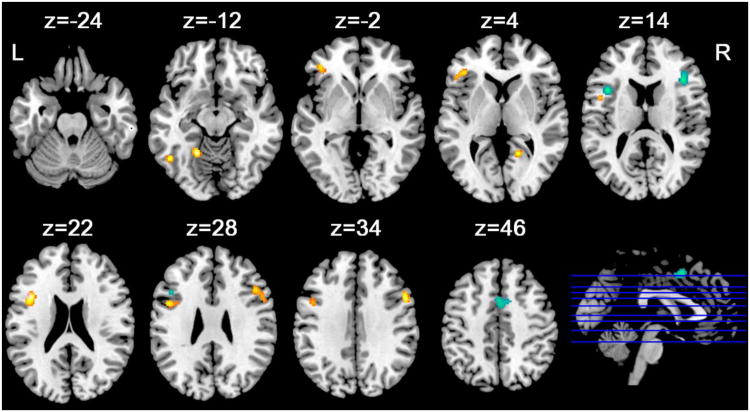
As illustrated in Figure 1 and Table 3, the older group showed consistent enhanced activation related to executive function than the younger group in distributed networks, including the bilateral dorsalateral prefrontal cortex (DLPFC) (BA 6/9), anterior cerebellum, and left inferior frontal gyrus (BA 13) (cluster extent threshold was 432 mm3 for FDR correction). In contrast, the younger group conveyed consistent greater activation related to executive function than the older group in the bilateral insula (BA 13), medial frontal gyrus/cingulate gyrus (BA 32/24), and cuneus (BA 18) (cluster extent threshold was 296 mm3 for FDR correction).
Figure 1.
Regions show consistent greater (hot) and smaller (cold) activations of executive function tasks in older subjects as compared to younger subjects. Clusters were displayed using a threshold at p<0.05 (FDR corrected). Z represents z coordinates in MNI space. L, left; R, right.
Table 3.
Regions reveal consistent age differences of executive function related activations.
| Volume (mm3) | Label | MNI coordinates | Extrema Value | Contributed studies # | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| Old > Young | ||||||
| 2032 | L. Inferior Frontal Gyrus, BA 9 | − 40 | 12 | 22 | 0.0132 | 3, 5, 8, 13, |
| L. Inferior Frontal Gyrus, BA 6 | −46 | 6 | 30 | 0.0129 | 16, 17, 23, 24 | |
| L. Inferior Frontal Gyrus, BA 44 | −48 | 6 | 14 | 0.0109 | ||
| L. Middle Frontal Gyrus, BA 9 | −46 | 12 | 36 | 0.0104 | ||
| 1240 | R. Inferior Frontal Gyrus, BA 9 | 54 | 10 | 32 | 0.0137 | 2, 3, 13, 15 |
| R. Middle Frontal Gyrus, BA 9 | 46 | 20 | 28 | 0.0115 | ||
| 864 | L. Inferior Frontal Gyrus, BA 13 | −40 | 34 | 2 | 0.0120 | 5, 7, 14, 16, |
| L. Inferior Frontal Gyrus, BA 13 | −48 | 28 | 4 | 0.0103 | 24 | |
| 832 | L. Fusiform Gyrus, BA 37 | −48 | −58 | −16 | 0.0160 | 7, 16, 21 |
| 592 | L. Cerebellum, Anterior Lobe, Culmen | −20 | −52 | −12 | 0.0152 | 1, 5, 13 |
| 584 | R. Parahippocampal Gyrus, BA 30 | 16 | −52 | 6 | 0.0147 | 11, 18, 24 |
|
| ||||||
| Young > Old | ||||||
| 1056 | L. Insula, BA 13 | −40 | 14 | 14 | 0.0123 | 6, 20, 21, 22 |
| L. Middle Frontal Gyrus, BA 9 | −44 | 18 | 28 | 0.0095 | ||
| 976 | R. Insula, BA 13 | 40 | 24 | 12 | 0.0124 | 6, 19a, 22 |
| 960 | L. Medial Frontal Gyrus, BA 32 | 0 | 10 | 48 | 0.0113 | 4, 11, 19b, 22 |
| R. Cingulate Gyrus, BA 24 | 10 | 8 | 44 | 0.0103 | ||
The clusters in bold represent the two clusters that overlap with consistent gray matter reductions. Contributed studies # refers to the study # in Table 1. L, left; R, right; BA, Brodmann's Area.
3.2 ALE analyses of VBM studies
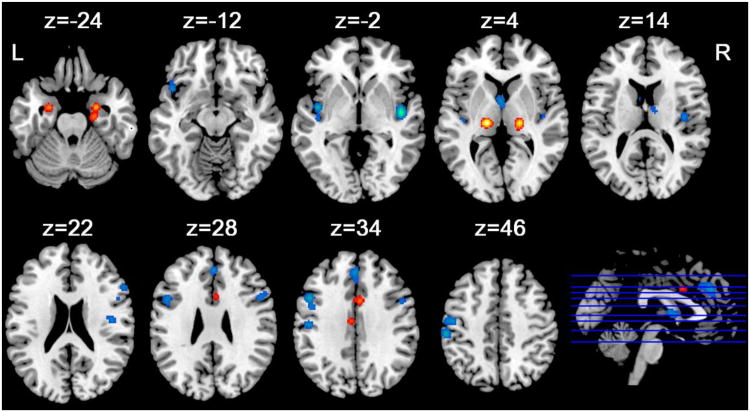
As illustrated in Figure 2 and Table 4, there were consistent age related GM reductions in the left sensorimotor cortex (BA 1/2/3/4), bilateral insula (BA 13), medial frontal gyrus (BA 6) caudate/thalamus, bilateral dorsolateral prefrontal cortex (BA 6/9), and left ventrolateral prefrontal cortex (BA 47) (cluster extent threshold was 912 mm3 for FDR correction). There was also consistent age related relative GM preservation in the bilateral parahippocampal gyrus/amygdala, bilateral thalamus, and cingulate gyrus (BA 24) (cluster extent threshold was 320 mm3 for FDR correction).
Figure 2.
Thresholded ALE maps of gray matter reduction (hot) and relative preservation (cold) in aging. Clusters are displayed using a threshold at p<0.05 (FDR corrected). Z represents z coordinates in MNI space. L, left; R, right.
Table 4.
Regions show consistent gray matter reduction and relative preservation in the old group relative to the young group.
| Volume (mm3) | Label | MNI coordinates | Extrema Value | Contributed studies # | ||
|---|---|---|---|---|---|---|
|
| ||||||
| x | y | z | ||||
| Gray matter reduction | ||||||
| 2936 | L. Postcentral Gyrus, BA 2 | −56 | −26 | 46 | 0.0241 | 2, 3, 5, 9, 10, 11, |
| L. Postcentral Gyrus, BA 3 | −50 | −16 | 36 | 0.0232 | 14, 15, 16, 17 | |
| L. Precentral Gyrus, BA 4 | −48 | −14 | 44 | 0.0200 | ||
| 1720 | L. Inferior Frontal Gyrus, BA 9 | −48 | 12 | 32 | 0.0243 | 2, 4, 6, 12, 13, 15, |
| L. Precentral Gyrus, BA 6 | −46 | 2 | 32 | 0.0188 | 17 | |
| 1360 | L. Insula, BA 13 | −44 | −4 | −4 | 0.0275 | 5, 13, 15, 16, 17, |
| L. Insula, BA 13 | −44 | −16 | 0 | 0.0155 | 18a, 18b, 22 | |
| L. Insula, BA 13 | −40 | −22 | 8 | 0.0152 | ||
| 1304 | R. Insula | 44 | −10 | −2 | 0.0355 | 5, 13, 17, 18a, 18b, 22 |
| 1296 | L. Medial Frontal Gyrus, BA 6 | 0 | 40 | 32 | 0.0210 | 2, 5, 9, 16, 20, 21, 22 |
| 1232 | R. Insula, BA 13 | 42 | −16 | 12 | 0.0185 | 2, 6, 11, 15, 16, 17 |
| R. Insula, BA 13 | 44 | −12 | 20 | 0.0177 | ||
| 1224 | L. Caudate Body | −2 | 4 | 6 | 0.0188 | 1, 3, 6, 11, 17, 18a, |
| L. Thalamus | 0 | −2 | 6 | 0.0185 | 18b | |
| R. Thalamus | 8 | −6 | 14 | 0.0139 | ||
| 1136 | R. Inferior Frontal Gyrus, BA 9 | 58 | 24 | 20 | 0.0176 | 2, 5, 10, 17, 20, 21 |
| R. Inferior Frontal Gyrus, BA 9 | 50 | 12 | 28 | 0.0173 | ||
| R. Precentral Gyrus, BA 6 | 50 | 8 | 34 | 0.0150 | ||
| 944 | L. Inferior Frontal Gyrus, BA 47 | −46 | 16 | −10 | 0.0205 | 5, 10, 20, 21, 22 |
| L. Inferior Frontal Gyrus, BA 47 | −48 | 24 | −8 | 0.0193 | ||
|
| ||||||
| Gray matter relative preservation | ||||||
| 1368 | R. Parahippocampal Gyrus, Amygdala | 24 | −4 | −22 | 0.0197 | 9, 11, 17, 19 |
| R. Uncus, BA 28 | 20 | −8 | −30 | 0.0194 | ||
| R. Parahippocampal Gyrus, BA 34 | 22 | −14 | −24 | 0.0164 | ||
| 1040 | L. Thalamus, Ventral Posterior Medial Nucleus | −16 | −20 | 6 | 0.0297 | 5, 10, 11, 17 |
| 1000 | L. Parahippocampal Gyrus, Amygdala | −26 | −4 | −22 | 0.0205 | 9, 17, 19 |
| 992 | R. Thalamus, Ventral Posterior Medial Nucleus | 18 | −20 | 6 | 0.0282 | 5, 10, 11, 17 |
| 696 | R. Cingulate Gyrus, BA 24 | 4 | 10 | 32 | 0.0160 | 1, 10, 19 |
| 336 | L. Cingulate Gyrus, BA 24 | −6 | −14 | 36 | 0.0163 | 1, 5 |
The clusters in bold represent the clusters which overlap with executive function related hyper-activations in the old individuals. Contributed studies # refers to the study # in Table 2. L, left; R, right; BA, Brodmann's Area.
3.3 Conjunction analysis
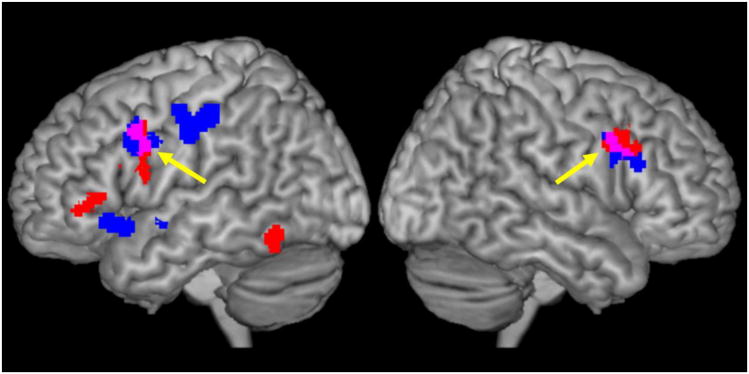
As illustrated in Figure 3, conjunction analysis of fMRI hyperactivation and GM reduction in the old group revealed two clusters located in the bilateral dorsolateral prefrontal cortex (BA6/9; centered coordinates:− 47, 7, 32, 408 mm3 for the left cluster, and at 52, 12, 30, 216 mm3 for the right cluster). No overlap was observed in the other three conjunction analyses.
Figure 3.
Illustration of overlap between hyperactivation of executive function tasks and gray matter reduction in the older group than younger group. Clusters in red represent hyper-activation of executive function tasks, and clusters in blue represent gray matter reduction. The yellow arrows highlight the overlaps of the hyperactivation and gray matter reduction (in violate).
3.4 Regions of interest analysis
For the two clusters of hyperactivation that overlap with GM reduction clusters, totally 10 studies were identified that contribute to these two clusters (shown in bold in Table 3). The number of equal and unequal performance studies from the contributed studies were not significantly different from the expected number of studies with different task performance from the whole study sample (Chi square = 1.94, p = 0.16). The number of working memory, inhibition, and other studies from the contributing studies were not significantly different from the expected number of studies of each executive function component from the whole study sample (Chi square = 0.80, p = 0.67). The number of PET and fMRI studies from the contributed studies were not significantly different from the expected number of studies from different imaging modality from the whole study sample (Chi square = 0.63, p = 0.43).
4 Discussion
The present study suggested that regions with disproportionate age-related GM loss overlapped with regions wherein older adults showed greater activation than younger adults during performance of executive function tasks. Thus, neural loss in DLPFC was associated with increases in neural metabolic or BOLD activity. Additional analyses did not indicate that DLPFC hyperactivation was biased to specific PET or fMRI modalities. These overlaps highlight a central role for bilateral DLPFC in the process of neurocognitive aging.
A central question in neurocognitive aging is whether age-related increases in activation reflect processes in the service of optimizing performance or whether they reflect deterioration. Although cortical volume decrease is broad-spread in aging (Good et al., 2001; Raz et al., 2005), the present study revealed consistent regions of disproportionate GM loss. Importantly, the most impaired GM regions overlapped with regions of age-related activation increases, but not decreases, during executive task performance. These results suggest on one hand, that age-related activation increases might be associated more with deterioration than with performance optimization. On the other hand, the increased neural activity in regions of neural atrophy could reflect a number of changes in cognitive function aimed at optimizing performance.
Age-related increases in frontal activity have been interpreted as support for the idea that older adults cognitively compensate for loss of function, due to neuroanatomic loss either within the region showing increased activity or in a region distal to that showing increased activity (Reuter-Lorenz & Cappell, 2008; Park & Reuter-Lorenz, 2009). DLPFC has been posited as the locus of compensation in the neurocognitive aging process. In these theories, hyperactivation in DLPFC reflects the erection of temporary skill-acquisition mechanisms (i.e., “scaffolds”) to compensate for anatomical deficits that develop with age and maintain cognitive performance. The effectiveness of such scaffolds might be limited by older adults' reduced cognitive capacity leading ultimately to age-related reductions in DLPFC activity when tasks are sufficiently difficult (Cappell et al., 2010; but see Bennett et al., 2012). Evaluation of the extent to which the present results reflect such compensatory processes would require assessment of performance-related changes associated with phenomena such as those we have observed here. Such tests of association were not significant in the present study. Thus the hypothesis that the relationships we observed between GM and activation represented any form of compensation was not supported.
When considering how the relationships between structural and functional measures might reflect cognitive function, the relationships between these measures and task performance is a vital factor in assessing whether or not one could attribute the functional hyperactivation we observed to cognitive constructs like compensation or de-differentiation (Rypma & D'Esposito, 2001; Berlingeri et al., 2010; Grady, 2012). Some studies have suggested a pattern of “hemispheric asymmetry reduction in older adults” (Cabeza, 2002). Better-performing older adults sometimes activate bilateral frontal regions, while poor performing elderly only activate the right frontal region (Cabeza et al., 2002). Such a pattern was observed by Spreng et al. (2010). They observed right DLPFC hyperactivation in older subjects who performed similar to young subjects, but not for those whose performance was poorer. This pattern, however, was not observed in the present study (Figure 4A/B). The effect of task performance on age-related activation changes requires further meta-analytic investigation to resolve these empirical ambiguities.
Figure 4.
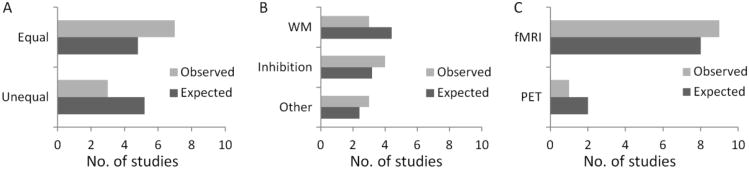
Results of regions of interest analysis for the bilateral DLPFC that consistent hyperactivations overlap with consistent GM reductions. Numbers of studies of task performance (A), executive function category (B), and imaging modality (C) from the two hyperactivation clusters were not significantly different from the number of studies from all the functional studies included in the meta-analysis.
It is possible to speculate that processing deficits due to regional atrophy might drive neuronal plasticity through strategy changes and training similar to that observed as patients performance improves in the process of the performance improvements that accompany development of skilled performance (Greenwood 2007). FMRI studies of the neural basis of cognitive training indicate that prefrontal cortex activity changes in the training process. Some studies have shown training-related activation increases in PFC (e.g., Olesen et al., 2004; Westerberg & Klingberg, 2007; Callan et al., 2003) but others have shown training-related decreases (e.g., Gobel, Parrish & Reber, 2011; Babiloni et al., 2009; Del Percio et al., 2009; Wartenburger et al., 2009). The role of prefrontal cortex is not yet well-understood but its versatility suggests that it probably supports a number of plasticity-related processes associated with training-related performance improvements (e.g., Fuster, 2002). The present results, however, while indicating relationships between age-related structural changes and activation changes, did not indicate any consequence of these relationships to performance.
Age-related activation increases have been posited to reflect de-differentiation (Baltes & Lindenberger, 1997). However, even though a causal relationship of structural alteration and functional hyperactivation seems reasonable, most of the evidence at hand (like the present results) are only correlational. It is also possible that structural and functional alterations are independent processes during aging, and only show epiphenomenal overlap (e.g., Steffener et al., 2012). As with other studies, we cannot rule out the possibility of some third factor that contributes to both of functional and structural alterations, such as hypertension or diabetes (D'Esposito et al., 2003). Several reports have demonstrated age-related coupling changes between cerebral blood flow (CBF) and cerebral-metabolic rate of oxygen consumption (CMRO2). Regional reductions in grey matter could lead to CMRO2 decreases that could, combined with age-related CBF increases, lead to apparent increases in BOLD signal (e.g., Restom et al., 2007; Ances et al., 2009; Hutchison et al., 2012). Further studies using longitudinal designs and pharmacologic manipulations will be required to provide the kind of direct evidence required to infer causal structure-function relationships.
In terms of function, bilateral DLPFC is not the only part of the distributed network that supports executive function (Smith &Jonides, 1999: Minzenberg et al., 2009), bilateral DLPFC is also involved in a broad range of tasks including perception (Spreng et al., 2010) and memory (Grady et al., 2003; Spreng et al., 2010). A parsimonious explanation of this age-related activation increase in DLPFC is that it provides some general task functions that provide support for cognition (e.g., Zarahn et al., 2007).
In terms of connectivity, the DLPFC is intensively connected to other brain regions. The DLPFC constitutes part of a task positive network (Fox et al., 2005; Toro et al., 2008), which includes distributed brain regions such as DLPFC, ventrolateral prefrontal cortex (VLPFC), supplementary motor area (SMA), inferior parietal lobule (IPL), ventral occipital cortex, and middle temporal region. The regions within the task positive network are extensively interconnected between each other. In contrast to the DLPFC, however, the posterior task-positive network regions, such as the ventral occipital cortex and middle temporal regions, generally have shown decreased activation in perceptual tasks in older adults (Spreng et al., 2010). The scaffolding theory proposes that age-related hyperactivation of DLPFC reflects compensation for functional deficits in these posterior regions. Evidence to support this speculation includes that increased PFC activation was correlated with the extent of deficient ventral visual and sensory activations (Davis et al., 2008). Greater connectivity has also been observed between DLPFC and hippocampus in older subject during memory task performance (Grady et al., 2003). Thus it is possible that, in the face of age-related processing deficits, older individuals might rely on more controlled processing, supported mainly by prefrontal brain regions, rather than on more automatic processing, supported mainly by posterior brain regions (cf. Shiffrin & Schneider, 1984; Rypma & Prabhakaran, 2009).
Although the present study focused on general processes of executive function, recent studies have considered executive function to be comprised of three independent components: working memory (updating), inhibition, and task-switching (Miyake et al., 2000). Turner & Spreng (2012) have shown a dissociation of working memory and inhibition related hyperactivation in aging in the anterior and posterior part of the DLPFC. The present analyses, however, failed to show any selective association between the hyperactivation results and either working memory or inhibition processes. Although a parsimonious explanation is that the DLPFC clusters observed in the present study involve general processes of executive function, further research is certainly needed to understand the functional significance of age-related prefrontal hyperactivation.
It has been demonstrated that GM volume generally declines with aging (Raz & Rodrigue, 2006; Kennedy et al., 2009). Regional specific alterations of the GM structure, however, can provide insight to relatively independent neural mechanisms of cognitive aging. The ALE analysis of VBM studies identified distributed networks, which were generally consistent with other types of structural measures such as cortical thickness (Salat et al., 2004), and longitudinal volumetric studies (Raz et al., 2005). The most consistent GM reduction across the studies considered here was in the left sensorimotor area (BA1/2/3/4), which has also been reported using cortical thickness measures (Salat et al., 2004). However, regional atrophy of left sensorimotor cortex has also been observed (Salat et al., 2004) but has not drawn much attention. Parallel to the anatomical studies, functional imaging studies of motor function have revealed hyperactivation in contralateral sensorimotor area (Mattay et al., 2002; Ward & Frackowiak, 2003). Consistent with these studies, we could hypothesize that the hyperactivation in left SMC might reflect compensatory processes to account for reduced motor function (Ward, 2006), driven by focal anatomical deficits in the same area. The absence of performance changes associated with this hyperactivation suggests that it might also reflect age-related CBF/CMRO2 coupling dysregulation. More research is required to understand the relations between the age-related SMC activation increases we observed here and performance.
It is possible that updates to improve VBM algorithm (e.g. optimized segmentation (Good et al., 2001), unified segmentation (Ashburner & Friston, 2005), and DARTEL (Ashburner, 2007)) may introduce variance across all studies. Differences in other processing steps such as carrying out GM modulation or not that result in GMC or GMV, respectively, are other possible source of variance across these studies. For example, a meta-analysis have reported discrepancies of structural alterations in schizophrenia patients when measuring with GMV and GMV (Fornito et al., 2009). Although their effects on age-related structural changes need further exploration, we didn't observe systematic bias of VBM algorithms and GM modulation in the current data.
There are some limitations in the current study. First, brain activation patterns differ across various task domains (e.g. memory and perception (Biswal et al., 2010; Spreng et al. 2010)); therefore, it is highly possible that age-related alteration in brain activation patterns and thus, the structure-function correspondence may differ depending on the task domain at hand. Conceivably, the structure-function correspondence in the DLPFC may be specific to executive function tasks. Secondly, our result is based on a spatial overlap between structural and functional alterations; thus, a solid evidence of structure-function relationship may be gained from directly examining individual differences between the brain activations and regional gray matter. Further studies with a large number of subjects are needed to explore this direct relationship.
5 Conclusion
The present study illustrated the correspondence of the functional hyperactivation in the executive function and the GM reduction in the bilateral DLPFC. Many of the studies that contributed to the DLPFC clusters showed age-equivalent behavioral performance. Taken together, the results suggest that intrinsic age-related anatomical deficits in DLPFC are associated with increases in activation. Further research will be required to understand the relationship between these age related structure-function relationship changes and cognitive function.
Highlights.
A series of meta-analyses were conducted to study age related brain alterations.
Functional alterations related to executive function were examined.
Anatomical reductions and relative preservations of gray matter were examined.
Only hyperactivations and gray matter reductions overlapped in the bilateral DLPFC.
Acknowledgments
This work was supported by grants 5R01AG032088 (BB) and 1R01AG029523 (BR) from the NIH. These funding agents had no further role in the study design, the collection, analysis and interpretation of the data, the writing of the manuscript or the decision to submit this paper for publication.
Abbreviations
- ALE
activation likelihood extimation
- CBF
cerebral blood flow
- CMRO2
cerebral-metabolic oxygen rate of oxygen
- DLPFC
dorsalateral prefrontal cortex
- FDR
false discovery rate
- fMRI
functional magnetic resonance imaging
- FWHM
full width at half maximum
- GM
gray matter
- IPL
inferior parietal lobule
- MNI
Montreal Neurological Institute
- PET
positron emission tomography
- SMA
supplementary motor area
- VBM
voxel-based morphometry
- VLPFC
ventrolateral prefrontal cortex
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe O, Yamasue H, Aoki S, Suga M, Yamada H, Kasai K, Masutani Y, Kato N, Kato N, Ohtomo K. Aging in the CNS: comparison of gray/white matter volume and diffusion tensor data. Neurobiol Aging. 2008;29(1):102–16. doi: 10.1016/j.neurobiolaging.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, Hampel H, Rapoport SI, Moeller JR. Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport. 2006;17(10):951–6. doi: 10.1097/01.wnr.0000220135.16844.b6. [DOI] [PubMed] [Google Scholar]
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2011;23(1):11–25. doi: 10.1162/jocn.2010.21451. [DOI] [PubMed] [Google Scholar]
- Antonova E, Parslow D, Brammer M, Dawson GR, Jackson SH, Morris RG. Age-related neural activity during allocentric spatial memory. Memory. 2009;17(2):125–43. doi: 10.1080/09658210802077348. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Rossini PM, Marzano N, Iacoboni M, Infarinato F, Lizio R, Piazza M, Pirritano M, Berlutti G, Cibelli G, Eusebi F. Judgment of actions in experts: a high-resolution EEG study in elite athletes. Neuroimage. 2009;45:512–521. doi: 10.1016/j.neuroimage.2008.11.035. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychol Aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Bauer E, Gebhardt H, Gruppe H, Gallhofer B, Sammer G. Altered negative priming in older subjects: first evidence from behavioral and neural level. Front Hum Neurosci. 2012;6:270. doi: 10.3389/fnhum.2012.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Motes MA, Rao NK, Rypma B. White matter tract integrity predicts visual search performance in young and older adults. Neurobiol Aging. 2012;33(2):433.e21–31. doi: 10.1016/j.neurobiolaging.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. Neuroimage. 2010;49(2):1750–9. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlingeri M, Bottini G, Danelli L, Ferri F, Traficante D, Sacheli L, Colombo N, Sberna M, Sterzi R, Scialfa G, Paulesu E. With time on our side? Task-dependent compensatory processes in graceful aging. Exp Brain Res. 2010;205(3):307–24. doi: 10.1007/s00221-010-2363-7. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Eldreth DA, Motes MA, Rypma B. Task-dependent individual differences in prefrontal connectivity. Cereb Cortex. 2010;20(9):2188–97. doi: 10.1093/cercor/bhp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas, Cambridge Imagers. 1999 http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach. Last visit on May 8th, 2013.
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol. 2001;42(3):277–86. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17(3):1394–402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14(4):364–75. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Callan DE, Tajima K, Callan AM, Kubo R, Masaki S, Akahane-Yamada R. Learning-induced neural plasticity associated with improved identification performance after training of a difficult second-language phonetic contrast. Neuroimage. 2003;19(1):113–24. doi: 10.1016/s1053-8119(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex. 2010;46(4):462–73. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2009;37(1):177–88. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P. The implications of cortical recruitment and brain morphology for individual differences in inhibitory function in aging humans. Psychol Aging. 2005;20(3):363–75. doi: 10.1037/0882-7974.20.3.363. [DOI] [PubMed] [Google Scholar]
- Craik FI, McDowd JM. Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13(3):474–479. [Google Scholar]
- Curiati PK, Tamashiro JH, Squarzoni P, Duran FL, Santos LC, Wajngarten M, Leite CC, Vallada H, Menezes PR, Scazufca M, Busatto GF, Alves TC. Brain structural variability due to aging and gender in cognitively healthy Elders: results from the Sao Paulo Ageing and Health study. AJNR Am J Neuroradiol. 2009;30(10):1850–6. doi: 10.3174/ajnr.A1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18(5):1201–9. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Percio C, Babiloni C, Bertollo M, Rossini P, Marzano N, Iacobini M, Infarinato F, Lizio R, Stocchi M, Robazza C, Cibelli G, Comani S, Eusebi F. Visuo-attentional and sensorimotor alpha rhythms are related to visuo-motor performance in athletes. Hum Brain Mapp. 2009;30:3527–3540. doi: 10.1002/hbm.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. Handbook of aging and cognition. Third. Mahwah, NJ: Erlbaum; 2008. pp. 1–54. [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4(11):863–72. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Di X, Chan RC, Gong QY. White matter reduction in patients with schizophrenia as revealed by voxel-based morphometry: an activation likelihood estimation meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1390–4. doi: 10.1016/j.pnpbp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Kirkby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122(Pt 5):963–79. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108(1-3):104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freo U, Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Pharmacological modulation of prefrontal cortical activity during a working memory task in young and older humans: a PET study with physostigmine. Am J Psychiatry. 2005;162(11):2061–70. doi: 10.1176/appi.ajp.162.11.2061. [DOI] [PubMed] [Google Scholar]
- Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31(3-5):373–85. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D'Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proc Natl Acad Sci U S A. 2008;105(35):13122–6. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio A, Santelli L, Tomassini V, Bosnell R, Smith S, De Stefano N, Johansen-Berg H. Age-related changes in grey and white matter structure throughout adulthood. Neuroimage. 2010;51(3):943–51. doi: 10.1016/j.neuroimage.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobel EW, Parrish TB, Reber PJ. Neural correlates of skill acquisition: Decreased cortical activity during a serial interception sequence learning task. Neuroimage. 2011;58:1150–1157. doi: 10.1016/j.neuroimage.2011.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL. Brain imaging and age-related changes in cognition. Exp Gerontol. 1998;33(7-8):661–73. doi: 10.1016/s0531-5565(98)00022-9. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Bookstein F, Horwitz B, Rapoport SI, Haxby JV. Age-related changes in regional cerebral blood flow during working memory for faces. Neuroimage. 1998;8(4):409–25. doi: 10.1006/nimg.1998.0376. [DOI] [PubMed] [Google Scholar]
- Grady CL. The cognitive neuroscience of aging. Nat Rev Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13(5):572–86. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, Yu H, Alain C. Age-related differences in brain activity underlying working memory for spatial and nonspatial auditory information. Cereb Cortex. 2008;18(1):189–99. doi: 10.1093/cercor/bhm045. [DOI] [PubMed] [Google Scholar]
- Greenwood PM. Functional plasticity in cognitive aging: review and hypothesis. Neuropsychology. 2007;21(6):657–73. doi: 10.1037/0894-4105.21.6.657. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M, Cooke A, DeVita C, Alsop D, Detre J, Chen W, Gee J. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15(2):302–17. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. J Exp Psychol Learn Mem Cogn. 1991;17(1):163–9. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Huang CM, Polk TA, Goh JO, Park DC. Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia. 2012;50(1):55–66. doi: 10.1016/j.neuropsychologia.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert V, Beaunieux H, Chételat G, Platel H, Landeau B, Viader F, Desgranges B, Eustache F. Age-related changes in the cerebral substrates of cognitive procedural learning. Hum Brain Mapp. 2009;30(4):1374–86. doi: 10.1002/hbm.20605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison JL, Lu H, Rypma B. Neural mechanisms of age-related slowing: The ΔCBF/ΔCMRO2 ratio mediates age-differences in BOLD signal and human performance. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpouzos G, Chételat G, Baron JC, Landeau B, Mevel K, Godeau C, Barré L, Constans JM, Viader F, Eustache F, Desgranges B. Voxel-based mapping of brain gray matter volume and glucose metabolism profiles in normal aging. Neurobiol Aging. 2009;30(1):112–24. doi: 10.1016/j.neurobiolaging.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Kalpouzos G, Persson J, Nyberg L. Local brain atrophy accounts for functional activity differences in normal aging. Neurobiol Aging. 2012;33(3):623.e1–623.e13. doi: 10.1016/j.neurobiolaging.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiol Aging. 2009;30(10):1657–76. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the functional heterogeneity of the default mode network using coordinate-based meta-analytic modeling. J Neurosci. 2009;29(46):14496–505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamar M, Yousem DM, Resnick SM. Age differences in orbitofrontal activation: an fMRI investigation of delayed match and nonmatch to sample. Neuroimage. 2004;21(4):1368–76. doi: 10.1016/j.neuroimage.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutiérrez D, Martinez M, Salinas F, Evans A, Zilles K, Mazziotta JC, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28(11):1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM, Zhang JX, Chan CC, Yuen KS, Chu LW, Cheung RT, Chan YS, Fox PT, Gao JH. Age-related differences in response regulation as revealed by functional MRI. Brain Res. 2006;1076(1):171–6. doi: 10.1016/j.brainres.2005.12.124. [DOI] [PubMed] [Google Scholar]
- Lehmbeck JT, Brassen S, Weber-Fahr W, Braus DF. Combining voxel-based morphometry and diffusion tensor imaging to detect age-related brain changes. Neuroreport. 2006;17(5):467–70. doi: 10.1097/01.wnr.0000209012.24341.7f. [DOI] [PubMed] [Google Scholar]
- Lemaître H, Crivello F, Grassiot B, Alpérovitch A, Tzourio C, Mazoyer B. Age- and sex-related effects on the neuroanatomy of healthy elderly. Neuroimage. 2005;26(3):900–11. doi: 10.1016/j.neuroimage.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. Adult age differences in functional connectivity during executive control. Neuroimage. 2010;52(2):643–57. doi: 10.1016/j.neuroimage.2010.04.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD. Aging affects the engagement of the hippocampus during autobiographical memory retrieval. Brain. 2003;126(Pt 7):1511–23. doi: 10.1093/brain/awg157. [DOI] [PubMed] [Google Scholar]
- Mathis A, Schunck T, Erb G, Namer IJ, Luthringer R. The effect of aging on the inhibitory function in middle-aged subjects: a functional MRI study coupled with a color-matched Stroop task. Int J Geriatr Psychiatry. 2009;24(10):1062–71. doi: 10.1002/gps.2222. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58(4):630–5. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- Mell T, Wartenburger I, Marschner A, Villringer A, Reischies FM, Heekeren HR. Altered function of ventral striatum during reward-based decision making in old age. Front Hum Neurosci. 2009;3:34. doi: 10.3389/neuro.09.034.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Motes MA, Biswal BB, Rypma B. Age-dependent relationships between prefrontal cortex activation and processing efficiency. Cog Neurosci. 2010;2(1):1–10. doi: 10.1080/17588928.2010.512974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels A, Kircher T, Dietsche B, Backes H, Marquetand J, Krug A. Neural processing of overt word generation in healthy individuals: the effect of age and word knowledge. Neuroimage. 2012;61(4):832–40. doi: 10.1016/j.neuroimage.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Nunnemann S, Wohlschläger AM, Ilg R, Gaser C, Etgen T, Conrad B, Zimmer C, Mühlau M. Accelerated aging of the putamen in men but not in women. Neurobiol Aging. 2009;30(1):147–51. doi: 10.1016/j.neurobiolaging.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Maitland SB, Rönnlund M, Bäckman L, Dixon RA, Wahlin Å, Nilsson L. Selective adult age differences in an age-invariant multifactor model of declarative memory. Psychology and Aging. 2003;18(1):149–160. doi: 10.1037/0882-7974.18.1.149. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Salami A, Andersson M, Eriksson J, Kalpouzos G, Kauppi K, Lind J, Pudas S, Persson J, Nilsson LG. Longitudinal evidence for diminished frontal cortex function in aging. Proc Natl Acad Sci U S A. 2010;107(52):22682–6. doi: 10.1073/pnas.1012651108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Bäckman L. Memory changes and the aging brain: a multimodal imaging approach. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. San Diego, CA: Academic Press; 2010. pp. 121–132. [Google Scholar]
- Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7(1):75–9. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
- Onur OA, Piefke M, Lie CH, Thiel CM, Fink GR. Modulatory Effects of Levodopa on Cognitive Control in Young, but not in Older Subjects: A Pharmacological fMRI Study. J Cogn Neurosci. 2011;23(10):2797–810. doi: 10.1162/jocn.2011.21603. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Balsters JH, Kilcullen SM, Campbell W, Bokde AW, Lai R, Upton N, Robertson IH. A simultaneous ERP/fMRI investigation of the P300 aging effect. Neurobiol Aging. 2012;33(10):2448–61. doi: 10.1016/j.neurobiolaging.2011.12.021. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–96. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Bischof GN. Neuroplasticity, aging, and cognitive function. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. San Diego, CA: Academic Press; 2010. pp. 109–119. [Google Scholar]
- Paxton JL, Barch DM, Racine CA, Braver TS. Cognitive control, goal maintenance, and prefrontal function in healthy aging. Cereb Cortex. 2008;18(5):1010–28. doi: 10.1093/cercor/bhm135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash RS, Heo S, Voss MW, Patterson B, Kramer AF. Age-related differences in cortical recruitment and suppression: implications for cognitive performance. Behav Brain Res. 2012;230(1):192–200. doi: 10.1016/j.bbr.2012.01.058. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–89. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30(6):730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restom K, Bangen KJ, Bondi MW, Perthen JE, Liu TT. Cerebral blood flow and BOLD responses to a memory encoding task: a comparison between healthy young and elderly adults. Neuroimage. 2007;37:430–439. doi: 10.1016/j.neuroimage.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. Neurocognitive Aging and the Compensation Hypothesis. Curr Dir Psychol Sci. 2008 Jun;17(3):177–182. [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. J Gerontol B Psychol Sci Soc Sci. 2010;65(4):405–15. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi E, Pietrini P, Schapiro MB, Rapoport SI, Furey ML. Cholinergic modulation of visual working memory during aging: a parametric PET study. Brain Res Bull. 2009;79(5):322–32. doi: 10.1016/j.brainresbull.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3(5):509–15. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Rypma B, D'Esposito M. Age-related changes in brain-behaviour relationships: Evidence from event-related functional MRI studies. Eur J Cogn Psychol. 2001;13(1-2):235–256. [Google Scholar]
- Rypma B, Prabhakaran V, Desmond JE, Gabrieli JD. Age differences in prefrontal cortical activity in working memory. Psychol Aging. 2001;16(3):371–84. doi: 10.1037//0882-7974.16.3.371. [DOI] [PubMed] [Google Scholar]
- Rypma B, Berger JS, Genova HM, Rebbechi D, D'Esposito M. Dissociating age-related changes in cognitive strategy and neural efficiency using event-related fMRI. Cortex. 2005;41(4):582–94. doi: 10.1016/s0010-9452(08)70198-9. [DOI] [PubMed] [Google Scholar]
- Rypma B, Eldreth DA, Rebbechi D. Age-related differences in activation-performance relations in delayed-response tasks: a multiple component analysis. Cortex. 2007;43(1):65–76. doi: 10.1016/s0010-9452(08)70446-5. [DOI] [PubMed] [Google Scholar]
- Rypma B, Prabhakaran V. When less is more and when more is more: The mediating roles of capacity and speed in brain-behavior efficiency. Intelligence. 2009;37(2):207–222. doi: 10.1016/j.intell.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14(7):721–30. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 2010;22(4):655–69. doi: 10.1162/jocn.2009.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffrin RM, Schneider W. Automatic and controlled processing revisited. Psychol Rev. 1984;91(2):269–76. [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283(5408):1657–61. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 2010;34(8):1178–94. doi: 10.1016/j.neubiorev.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Ishii K, Kakigi T, Yokoyama K. Gender and age differences in normal adult human brain: Voxel-based morphometric study. Hum Brain Mapp. 2011;32(7):1050–8. doi: 10.1002/hbm.21088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotactic Atlas of the Human Brain: 3-Dimensional Proportional System—An Approach to Cerebral Imaging. Thieme Medical Publishers; New York, NY: 1988. [Google Scholar]
- Terribilli D, Schaufelberger MS, Duran FL, Zanetti MV, Curiati PK, Menezes PR, Scazufca M, Amaro E, Jr, Leite CC, Busatto GF. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol Aging. 2011;32(2):354–68. doi: 10.1016/j.neurobiolaging.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MP, Evans AC, Jolles J, Uylings HB. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17(2):657–69. [PubMed] [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14(9):966–73. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Toro R, Fox PT, Paus T. Functional Coactivation Map of the Human Brain. Cerebral Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox P. Minimizing Within-Experiment and Within-Group Effects in Activation Likelihood Estimation Meta-Analyses. Human Brain Mapping. 2012;33(1):1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner GR, Spreng RN. Executive functions and neurocognitive aging: dissociable patterns of brain activity. Neurobiol Aging. 2012;33(4):826.e1–13. doi: 10.1016/j.neurobiolaging.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Van Impe A, Coxon JP, Goble DJ, Wenderoth N, Swinnen SP. Age-related changes in brain activation underlying single- and dual-task performance: Visuomanual drawing and mental arithmetic. Neuropsychologia. 2011;49(9):2400–9. doi: 10.1016/j.neuropsychologia.2011.04.016. [DOI] [PubMed] [Google Scholar]
- Van Laere KJ, Dierckx RA. Brain perfusion SPECT: age- and sex-related effects correlated with voxel-based morphometric findings in healthy adults. Radiology. 2001;221(3):810–7. doi: 10.1148/radiol.2213010295. [DOI] [PubMed] [Google Scholar]
- Ward NS, Frackowiak RS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126(Pt 4):873–88. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Compensatory mechanisms in the aging motor system. Ageing Res Rev. 2006;5(3):239–54. doi: 10.1016/j.arr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Wartenburger I, Heekeren HR, Preusse F, Kramer J, van de Meer E. Cerebral correlates of analogical processing and their modulation by training. Neuroimage. 2009;48:291–302. doi: 10.1016/j.neuroimage.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Westerberg H, Klingberg T. Changes in cortical activity after training of working memory--a single-subject analysis. Physiol Behav. 2007;92(1-2):186–92. doi: 10.1016/j.physbeh.2007.05.041. [DOI] [PubMed] [Google Scholar]
- Zysset S, Schroeter ML, Neumann J, Yves von Cramon D. Stroop interference, hemodynamic response and aging. An event-related fMRI study. Neurobiol Aging. 2006;28:937–946. doi: 10.1016/j.neurobiolaging.2006.05.008. [DOI] [PubMed] [Google Scholar]