Abstract
A goal of HIV-1 vaccine development is to elicit broadly neutralizing antibodies (BnAbs). Using a knock-in (KI) model of 2F5, a human HIV-1 gp41 MPER-specific BnAb, we previously demonstrated that a key obstacle to BnAb induction is clonal deletion of BnAb-expressing B-cells. Here, in this model, we provide a proof-of-principle that robust serum neutralizing IgG responses can be induced from pre-existing, residual self-reactive BnAb-expressing B-cells in vivo, using a structurally compatible gp41 MPER immunogen. Furthermore, in CD40L-deficient 2F5 KI mice, we demonstrate that these BnAb responses are elicited via a type II T-independent pathway, coinciding with expansion and activation of transitional splenic B-cells specific for 2F5's nominal gp41 MPER-binding epitope (containing the 2F5 neutralization domain ELDKWA). In contrast, constitutive production of non-neutralizing serum IgGs in 2F5 KI mice is T-dependent, and originates from a subset of splenic mature B2-cells that have lost their ability to bind 2F5's gp41 MPER epitope. These results suggest that residual, mature B-cells expressing autoreactive BnAbs like 2F5 as BCR, may be limited in their ability to participate in T-dependent responses, by purifying selection that selectively eliminates reactivity for neutralization epitope-containing/mimicked host antigens.
Introduction
One key to a protective HIV-1 vaccine will be the ability to elicit broadly neutralizing antibodies (BnAbs) against the HIV-1 envelope (Env) (1). Though Env contains multiple conserved sites, BnAbs cannot be elicited by any current vaccination regimens, although they can be made in a subset of chronically infected subjects (i.e., typically years after infection), which has thus allowed identification of one or more unusual characteristics shared by all BnAbs (2). These include high levels of somatic hypermutation, polyreactivity, and long heavy chain complementarity determining regions (HCDR3s)-the latter two features common in Abs controlled in their expression by tolerance mechanisms (3–5).
One highly conserved and well-studied vaccine target in Env is gp41 near the viral membrane (membrane proximal external region, MPER) where BnAbs 2F5, 4E10, and Z13 bind (6–10). Expression of the anti-MPER 2F5 BnAb's H chain V(D)J and L chain VJ mini-gene rearrangements in knock-in mice (2F5 “complete” (VH × VL) KI mice) results in profound deletion (~95%) of BnAb-expressing B-cells in BM at the first tolerance checkpoint when naïve B-cells express surface B-cell receptors (BCR) (11,12). Furthermore, residual 2F5 KI B-cells poorly express and flux calcium through, their BCRs (12,13), suggesting compromised signaling associated with functionally-silenced (anergic) B-cells (14, 15), and cultured 2F5 KI BM B-cells in the presence of IL-7 and BAFF extensively edit their 2F5 L chains when rescued from clonal deletion (12). Collectively, these data suggest that elimination of BnAb-expressing B-cells is profound but incomplete, and raise the question: can vaccines containing gp41 BnAb epitopes be effective in triggering residual, self-reactive B-cells expressing a pre-existing 2F5 BCR to produce plasma BnAbs? Moreover, the role of T cell help in any such responses are critical to evaluate.
Key to answering these questions is to determine adjuvant formulations that are required to optimally trigger residual anergic B-cell escapees of central tolerance. In this context, various Toll-like receptor (TLR) agonists, in combination with strong BCR cross-linking have been experimentally demonstrated in some instances to be effective in re-activating functionally silenced (anergic) peripheral B-cell clones ((16,17), reviewed in (18,19)). An even more critical issue with current immunization regimens is whether the inability to routinely elicit BnAbs by immunization, including those directed at the MPER, is impacted by structural hurdles attributable to lack of immunogen expression of native Env conformations (20,21). In this regard, we have recently developed an MPER peptide-liposome immunogen that binds at nM affinity to the 2F5 BnAb (22,23).
The 2F5 complete KI model provides a system in which to determine if an adjuvant-immunogen regimen designed to optimally bind to the 2F5 antibody can selectively target and/or reverse functional silencing of residual autoreactive B-cell subsets with BCR dual (i.e., lipid/MPER) reactivity required for 2F5's neutralization ability (22). The immunogen used in this study incorporates both the MPER in the context of lipids and TLR-4 and -9 agonists, and has been previously shown to elicit MPER-specific Ab responses focused on the 2F5 core (DKW) neutralizing residues (23) and that recognize the transient pre-hairpin intermediate (24) and MPER scaffolds stabilized in the 2F5-bound post-fusion conformation (25). In this current study, we demonstrate that this immunogen-adjuvant combination induces residual pre-existing 2F5-expressing B cells to clonally expand and produce clinically relevant titers of plasma BnAbs, thus providing proof-of-concept that self-reactive BnAb-expressing B-cells can be activated by a structurally compatible immunogen. Unexpectedly, our study has uncovered two pathways of HIV-1 serum IgG production in BnAb KI mice: T-independent (T-I) neutralizing serum IgG responses to immunization originating from MPER-binding, self-reactive B-cell pools, and constitutive production of non-neutralizing serum IgG originating from T-dependent (T-D) B-cells driven to lose 2F5 nominal MPER epitope-associated self-reactivity.
Materials and Methods
Immunogen formulations and mice
Immunogen components were produced, purified, formulated and used in immunizations based on previously described methods (26,27). Briefly, TLR agonist-containing MPER peptide-liposome conjugates were constructed using the adjuvant MPLA (Avanti Polar Lipids, Alabaster, AL), POPC/POPE/DMPA/CH-containing liposomes, and a version of the 2F5 epitope-containing MPER peptide 656 (NEQELLELDKWASLWNWNITNWLWIK) that was synthesized with the C-terminal hydrophobic membrane anchor tag GTH1 (YKRWIILGLNKIVRMYS). For all studies, a minimum of ≥3 mice per immunization group were used, and single-site injections were administered i.p. at d 0, wk 2, 4, 6, 8 and 10. Prior to all immunizations, either purified recombinant JRFL gp140 or the TLR agonist-MPER peptide-liposome conjugates, as described above, were formulated in 10% Emulsigen (MVP Technologies, Omaha, NE) and oCpG (Midland Certified Reagent Co., Midland, TX). Placebo immunization groups received 200 μl of 1X saline (for both priming and boosting injections), whereas experimental groups received 200 μl injection volumes of JRFL/ Emulsigen/ oCpG for priming (corresponding to 25 μg JRFL and 10 μg oCpG), and 200 μl injection volumes of TLR agonist-MPER peptide-liposome conjugate for boostings (corresponding to 25 μg MPER 656-GTH1 peptide, 10 μg MPLA, and 10 μg oCpG). Serum samples were collected 10 d after each immunization and stored at −80°C until further use.
CD154−/− (CD40L-deficient) mice (28) were obtained from The Jackson Laboratory and were crossbred with 2F5 complete KI mice to generate 2F5 complete KI × CD154−/− mice. All strains used in this study were housed in the MSRBII Vivarium at the Duke Human Vaccine Institute in a pathogen-free environment under AAALAC guidelines and all immunization and serum sample collection procedures were carried out in accordance with IACUC and the Duke University IBC-approved animal protocols.
ELISA serum Ig analysis
Total or MPER (nominal MPER epitope SP62 i.e., containing the 2F5 neutralization epitope)-specific serum Ig (all isotypes), IgM, or IgG endpoint titers were determined by ELISA using 10-point serum dilution curves, based on previously-published methods (11,12,29). Total serum Ab concentrations were quantitated by generating standard curves using reference standards with the following reagents: purified mouse IgM, λ isotype control (BD Pharmingen) to measure total IgM, and purified mouse IgG1, κ isotype (BD Pharmingen) to measure total IgG or kappa antibodies. MPER-specific serum Ab concentrations were determined by using m2F5 recombinant chimeric 2F5 mAb expressing human 2F5 VH/mouse Cγ1+human 2F5 Vκ+mouse Cκ (m2F5) (11) as a reference standard to measure either MPER-specific Ig of all isotypes (detected with alkaline phosphatase (AP)-labeled goat α-mouse κ L chain (Southern Biotech) or MPER-specific IgG (detected with AP-labeled goat α-mouse γ H chain (Southern Biotech), and using V3-1.4 (a m2F5 IgM+ mAb) (12) as a reference standard to measure MPER-specific IgM (detected with AP-labeled goat α-mouse μ H chain (Southern Biotech). Normalized amounts of MPER-specific Ig (all isotypes), IgM, or IgG were determined by dividing MPER-specific antibody concentrations with total serum Ab concentrations. To quantify MPER-specific IgG1, IgG3, IgG2b, and IgG2c concentrations, the same methodologies described above were used except that AP-labeled goat α-mouse γ1, γ3, γ2c and γ2b H chain isotype-specific detection reagents (Southern Biotech) were used for determining initial endpoint titers of MPER-specific IgG1, IgG3, IgG2b, and IgG2c, respectively, after which m2F5 (recombinant m2F5 IgG1)(11), V4-SE 6 (a 2F5 IgG2b mAb; Table 3), and V5-SP 3B11G (a 2F5 IgG2c mAb; Table 3), were then used as references for generating standard curves upon which serum endpoint titer values were then converted to concentrations (μg/ml) of MPER-specific IgG1, IgG3, IgG2b, and IgG2c, respectively. Total IgG isotype subclasses were measured by Luminex analysis using a MILLIPLEX mouse immunoglobulin isotyping kit (Millipore). Finally, half-maximum binding titers (OD50) of MPER+ total serum Ig in various samples were calculated by interpolation of mean OD50 values calculated from the V3-1.4 control, using the formula [(ODmax−ODmin)/2)+ODmin].
Table 3.
Summary of isotypic, neutralization, antigen reactivity, and Ig usage profiles of selected mAb hybridoma lines isolated from immunized 2F5 “complete” (VH+/+ × VL+/+) KI mice
| HIV-1 Neutralization IC50 (μg/ml)a | --Antigenic Reactivitiesb-- | ---------------------Ig usagec---------------------- | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone ID | Isotype | A. 92UG037.1 | B. MN.3 | B. SF162.LS | B. 6535.3 | B. BG1168.1 | MPER (2F5 epitope) | NIH-3T3 antigens | CL | Histones | VH rearrang. used | VH mutation type/aa location | VL rearrang. used | VL mutation type/aa location |
| V4-SE 2 CL1-1 | IgG2b | 0.12 | <0.01 | 0.34 | 7.61 | 0.78 | +++ | ++ | + | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SE 6 CL1-1 | IgG2b | 0.12 | <0.01 | 0.45 | 4.81 | 1.04 | +++ | + | + | ++ | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SE 8 CL1-1 | IgG2b | 0.14 | 0.01 | 0.54 | 5.6 | 1.1 | +++ | + | + | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SE 361 CL1-1 | IgG2b | 0.21 | 0.03 | 3.04 | 2.75 | 2.37 | +++ | + | + | ++ | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SP 1B5 CL1-1 | IgG2b | 0.32 | 0.06 | 1.18 | 17.3 | 1.53 | +++ | + | + | + | KI 2F5 VDJ | R:FWR1(T19M), CDR1(G38A) | KI 2F5 VJ | S:FWR2(P40), FWR3(E70) |
| V4-SE 18 CL1-2 | IgG2b | 0.06 | <0.01 | 0.23 | 2.81 | 0.64 | +++ | +++ | + | ++ | KI 2F5 VDJ | R:CDR3(T104I); S:FWR1(T3,T15), CDR1(G33) | KI 2F5 VJ | S:FWR1(G16), CDR1(V29), FWR3(E81) |
| V4-SE 372 CL1-1 | IgG2b | 0.14 | <0.01 | 0.38 | 7.19 | 1.04 | +++ | + | ++ | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SP 1G3 CL1-1 | IgG2b | 0.14 | 0.02 | 0.55 | 7.66 | 0.98 | +++ | +++ | ++ | ++ | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V5-SP 3H6 CL1-1 | IgG2b | 0.24 | 0.03 | 2.42 | 2.37 | 2.55 | +++ | + | + | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V5-SE 4 CL1-1 | IgG2b | 0.13 | <0.01 | 0.38 | 6.48 | 1.04 | +++ | + | + | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V5-SE 10 CL1-1 | IgG2b | 0.16 | 0.01 | 0.5 | 9.88 | 0.8 | +++ | + | + | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V5-SP 3B11G | IgG2c | 0.13 | <0.01 | 0.38 | 7.23 | 0.97 | +++ | ++ | ++ | + | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V4-SE 196 CL1-1 | IgM | 0.16 | 0.09 | 1.35 | 5.03 | 3.11 | +++ | +++ | ++ | ++ | KI 2F5 VDJ | none | KI 2F5 VJ | none |
| V5-SE 14 CL1-2 | IgM | 0.41 | 0.29 | 5.01 | 22.86 | 7.43 | +++ | ++ | ++ | +++ | KI 2F5 VDJ | none | KI 2F5 VJ | R:FWR2(L47P), CDR2 (S56R) |
| V5-SE1 CL1-1 | IgM | 0.64 | 0.28 | 2.86 | >25 | 9.59 | +++ | +++ | ++ | ++ | KI 2F5 VDJ | R:FWR1(L20V) | KI 2F5 VJ | R:CDR3(F98L) |
In addition to h2F5 (Table 2), m2F5 (recombinant mouse 2F5 IgG antibody) (11) was used as a neutralization control for IgG+ mAbs and had the following IC50 values (averaged from two experiments): 92UG037.1=0.96, B.MN.3=0.52, SF162.LS=8.11, B.6536.3=18.6, and B.BG1168.1=9.81. Similarly, V3-1.4 (a mouse 2F5 IgM mAb) (12) was used a neutralization control for the IgM+ mAbs and had the following IC50 values: 92UG037.1=0.25, B.MN.3=0.13, SF162.LS=2.35, B.6536.3=5.52, and B.BG1168.1=3.69.
Quantitative ELISAs to measure relative reactivities of purified mAbs to plate-bound SP62 peptide (encoding the nominal 2F5-specific MPER epitope), NIH-3T3 cytoplasmic and nuclear antigens, Cardiolipin (CL), and histones; criteria for relative positivity are described in Fig S6 and as previously described (12).
cDNAs from cloned hybridoma lines were amplified using a combination of either leader peptide-specific forward primers in the 2F5 KI expression cassette or degenerate forward HC or LC-specific forward primers (34), and reverse C region-specific degenerate primers.
PCR products were sequenced in both orientations, KI 2F5 VH and VL regions were analyzed for mutations using L-ALIGN software and endogenous LC usage was determined using the Ig-BLAST algorithm. Mutations in 2F5 VH and VL nucleotide sequences are represented for corresponding protein sequences as mutation type: R=replacement, S=substitution, N=Nonsense and mutation location; FRW1, FRW2, FRW3, FRW4=Framework Regions 1,2,3,4, respectively, CDR1, CDR2, CDR3=Complementarity Determining Regions 1,2, 3, respectively; information in brackets designate corresponding aa residue, number, and replacement (if applicable), with number nomenclature based on published 2F5 VH and VL aa sequences (9,52), and those in endogenous LCs, are annotated relative to Ig blast germline sequences.
ELISPOT assays
Total or MPER (2F5 epitope)-specific ASCs were detected based on published methods (30) with some minor modifications for optimizing assay sensitivity and quantitation. Briefly, for capture of MPER-specific spots, splenocytes were washed, plated and incubated all as previously described, except that plates were coated with streptavidin linking to a biotinylated version of the 2F5 epitope-containing peptide MPR.03 (KKKNEQELLELDKWASLWNWFDITNWLWYIRKKK-biotin-NH). Splenocytes were plated over a wide range of 3-fold dilutions, and incubated for 20h (37°C, 8% CO2). MPER-specific Ig+ (i.e. all isotypes), IgM+, IgG+ (i.e. all IgG isotypes), IgG1+, IgG3+, and IgG2b+ spots were detected using AP-labeled goat α-mouse κLC, μ, γ, γ1, γ3, or γ2b H chain-specific reagents (Southern Biotech), respectively. Total spots were captured and detected in the same fashion as described above for MPER-specific spots, except that plates were coated with goat α-mouse (H chain+L chain) reagent (Southern Biotech), splenocytes were incubated for 4h, and detection was performed using the AP-labeled goat α-mouse reagents described above. To quantitate ASC responses, input total B-cell numbers were estimated by determining percentages of live, CD19+ B-cells within singlet lymph gates by flow cytometry, using the same splenocytes used for ELISPOTs. Linear ranges of dilution curves from two independent experiments were then used to calculate total or MPER+ ASC numbers. Similar calculations were obtained using negatively purified or sorted total B-cells as input source.
Measuring HIV-1 neutralization of mouse serum and purified mAbs from vaccinated 2F5 KI mice
The TZM-b/l HIV-1 pseudo-virus infectivity neutralization assay (31) was adapted as an initial screening methodology for measuring HIV-1 neutralization by mouse serum antibodies. Given the high background of anti-viral activity reported in murine sera (32), achieving high-throughput reproducibility for individual mice at multiple time points was critical, and thus allowed us to only use limited serum volumes (typically, <25–50 μl residual volumes i.e. after ELISA assays), enough for one initial representative HIV-1 pseudo-virus isolate. We therefore first determined neutralization sensitivity of various Tier 1+2 isolates (33), including several known to be sensitive to 2F5 neutralization, by serial dilutions of serum from naïve WT C57BL/6 mice, spiked with 50, 100, or 1000 μg/ml of purified m2F5. Neutralization curves were constructed, and IC50 and IC80 neutralization values for m2F5-spiked serum were determined. Based on neutralization sensitivities of various isolates assayed to m2F5-spiked serum, we selected B.MN.3 for all screens with experimental serum samples, given our estimate that its use in this primary screening assay would require ≥25 μg/ml of MPER-specific serum Igs to reliably detect neutralization, well below concentrations elicited at peak responses in vaccinated 2F5 complete KI animals. This conservative estimate is based on the following calculation: (reciprocal of the lowest dilution at which non-specific neutralization activity in unspiked WT (B6) serum was eliminated for MN.3 (=60)) X (IC50 of m2F5-spiked WT(B6) serum to neutralize MN.3 (~0.4)).
To confirm neutralization of HIV-1 by serum Abs elicited in vaccinated 2F5 complete KI mice, individual mice at their peak titers of serum Ig MPER-specific concentrations and serum B.MN.3 neutralization potency were sacrificed, hybridoma lines from peripheral tissues were generated (described below), and culture supernatants were re-screened for neutralization with B.MN.3 using using >50% neutralization inhibition scores as the cutoff for positive neutralization activity, as previously described (12,29). Corresponding purified mAbs were then comprehensively assayed for neutralization breadth and potency using a larger panel of viral isolates in the standard TZM-b/l assay.
Generation/analysis of hybridomas and mAbs, and recombinant Abs
Hybridomas were generated by electrofusions using NSO myeloma fusion partner lines as per previously described methods (Verkoczy et al., 2011b). In addition to performing neutralization assays for primary screens, culture supernatants with secreting wells were identified by ELISA, using a general anti-mouse Ig H chain reagent, and isotypes and MPER reactivity of secreting clones were subsequently determined by ELISA using anti-mouse H chain γ, μ, and α-specific reagents and SP62 plate-bound peptides, as previously described (4,11,12). For certain subclones, purified mAbs were purified from supernatants, and their IgV regions were cloned/sequenced based on published methods (12) using either leader peptide-specific forward primers in the 2F5 KI expression cassette or degenerate forward H chain or L chain-specific forward primers (34), in combination with reverse C region-specific degenerate primers. Quantitative ELISAs to measure MPER, NIH-3T3 cytoplasmic nuclear Ag, cardiolipin, and histone reactivities of purified mAbs was also performed as previously described (12).
h2F5 Blocking assays
Measurements of the ability of neutralizing IgG mAbs derived from immunized 2F5 complete KI mice to block human 2F5 binding to the HIV-1 gp140 Env JRFL was based on previously described methods (35). Briefly, 384 well ELISA plates (Costar #3700) were coated with 30ng/well JRFL overnight at 4°C and blocked with assay diluent (PBS containing 4% (w/v) whey protein/ 15% Normal Goat Serum/ 0.5% Tween20/ 0.05% Sodium Azide) for 1 hour at room temp. All assay steps were conducted in assay diluent (except substrate step) and incubated for 1 hour at room temp followed by washing with PBS/0.1% Tween-20. 10 μl of a titration of experimental mAbs starting at 100 μg/ml along with h2F5 (Catalant) was added to the plates in duplicate. After washing, 10μl of biotinylated h2F5 was added at 0.125 μg/ml. Biotin-h2F5 binding was detected with streptavidin-alkaline phosphatase at 1:1000 (Promega V559C) followed by substrate (CBC buffer + 2mM MgCl2 +1mg/ml p-npp [4-Nitrophenyl phosphate di(2-amino-2-ethyl-1,3-propanediol) salt]). Plates were read with a plate reader at 405 nm at 45 minutes. Percent inhibition was calculated as follows: 100-(sample quadruplicate mean/no inhibition control mean) X100.
SPR Assays
SPR assays for MPER peptide binding, MPER peptide-liposome binding were carried as described earlier (26,35). The binding of GCN4-gp41 inter protein (22,24) to Abs were measured by flowing GCN4-gp41 inter (100 μg/ml) over Abs captured on immobilized anti-human IgG Fc (for 2F5 and Synagis) and anti-mouse IgG Fc (for 2F5 KI mice Abs) using a Biacore CM5 chip as before (36). Responses from captured Synagis surfaces were subtracted out to obtain specific binding to 2F5 and 2F5 KI mice Abs.
Results
Immunization with TLRs and MPER-lipid complexes induces potent MPER-specific humoral responses in 2F5 KI mice
To determine if immunization can trigger residual peripheral B-cells expressing the original (somatically mutated) 2F5 BnAb specificity, we immunized 2F5 VH+/+ × VL+/+ (complete) KI mice (and as controls, 2F5 VH KI and WT C57BL6 [B6] mice) i.p., using an HIV-1 Env gp140 immunogen as a general prime and an MPER-peptide-TLR agonist-liposome immunogen for boosting, a regimen previously shown in rhesus macaques to induce non-neutralizing antibodies focused to the MPER (Fig. 1A) (27). Toll-like receptor (TLR)-4 agonist monophosphoryl lipid A, and TLR-9 agonist cytidine-phosphate-guanine oligonucleotide (oCpG) 2006 [the latter which previously has been shown to break tolerance to DNA and generate autoantibody production in mice (35,37)], was formulated with both immunogens (Fig. 1A) (27,35).
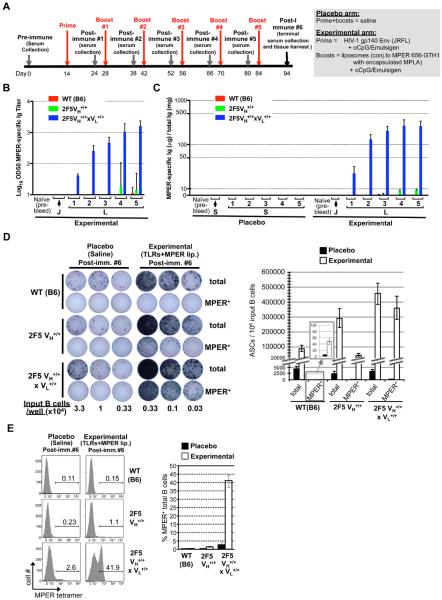
Figure 1. MPER+ splenic B-cell and serum Ig responses in immunized 2F5 KI strains.
A. Outline for experimental design for all immunization studies described in this report. Shown are both the placebo and experimental arms (prime+boosts) and study schedule, including timing of immunizations, serum collections, and harvests. Details of the experimental protocol and immunogen formulations are provided in Materials and Methods. B–C. ELISA measurements of MPER (2F5 epitope)-specific serum Ig responses (mean+SEM) in immunized WT, 2F5 VH+/+, and 2F5 complete KI mice represented either as half-maximum binding titers on a log scale (B), or quantitated in μg/mg total Ig (C), based on data shown in Fig. S1A, and as described in Materials and Methods. Post-immune serum collections (indicated by arrows) are indicated for each immunization group, annotated as follows: Placebo: (S)=Control (saline) injections (prime and boosts #1-4); Experimental: (J)=gp140 Env JRFL prime, and (L)=MPER peptide-TLR agonist-liposome boosts #1-4. D. Total and MPER-specific splenic ASC responses from above strains after 6th immunizations were detected by ELISPOT as previously described (30), and in Materials and Methods. Left: representative plates from dilution curves, with input B-cell numbers indicated at bottom. Right: graphical representation of ASC (mean+SD), calculated from linear range of dilution curves each of six individual mice per immunization group, taken from two comparable experiments. Inset shows that in WT (B6) mice, small numbers of MPER+ ASCs are induced by vaccine regimen. E. Surface reactivity profiles of above strains to the 2F5-specific MPER epitope, as determined by flow cytometry, with splenocytes gated on live, singlet, total (CD19+) B-cells and 2F5 epitope-specific tetramers (12,30).
2F5 complete KI mice had immunization-induced serum Ab responses specific for the MPER 2F5 nominal epitope SP62 (herein designated MPER+), with peak MPER+ Ab responses occurring by the fourth MPER peptide-TLR agonist-liposome boosts (Fig. 1B), and representing ~20–35% of all Ig elicited (Figs. 1C, S1A). We measured total Ig and MPER-specific Ig-secreting splenic B-cells by ELISPOT, as well as MPER tetramer+ B-cells by flow cytometry (30) to assay splenic B-cell BCR binding to the MPER 2F5 nominal epitope. In placebo-immunized 2F5 complete KI mice, the percentage of residual MPER+ sIg+ total splenic B-cells, although significantly higher than those from 2F5 VH KI mice, represented a minor population, due to editing of the 2F5 L chain and/or loss of sIg expression (12). In contrast, in experimentally-immunized 2F5 complete KI mice, >75% of all antibody-secreting cells (ASCs) were MPER+ Ig-secreting splenic B-cells (Fig. 1D), and the frequency of MPER-specific B-cells (Fig. 1E) relative to their placebo-immunized counterparts was significantly increased, demonstrating selective expansion/selection of MPER+ B-cell clones by the vaccine regimen. Experimentally-immunized 2F5 VH KI mice (relative to 2F5 complete KI mice), had comparable frequencies of total ASCs, but MPER-specific ASCs, although detectable, were considerably less frequent (Fig. 1D), confirming that only rare endogenous murine L chain homologues of the 2F5 L chain can complement 2F5 H chains for MPER reactivity (11,12). Interestingly, experimental immunization failed to elicit detectable MPER Ab responses in WT (C57/BL6) mice, and we are currently investigating the possibility that this non-responsiveness (i.e., relative to the robust MPER+, but non-neutralizing Ab responses observed in rhesus macaques) relates to MHC class II restriction as a result of inbred strain-specific haplotype expression.
MPER-specific serum Abs elicited in immunized 2F5 KI mice are predominantly IgG2b and potently neutralize HIV-1
To examine the quality of MPER+ serum Abs elicited by immunization in 2F5 KI strains, we first examined the serum Ig and splenic ASC isotypes by ELISA and ELISPOT, respectively. 2F5 complete KI and VH KI strains predominantly induced MPER+ IgG (Fig. S1B) with both MPER+ serum Ig and splenic Ig-secreting B-cells predominantly restricted to the IgG2b+ subclass (Figs. 2A–B, S2A). To determine if immunization-induced MPER-specific serum IgGs could neutralize HIV-1, we tested sera from complete 2F5 KI mouse in the TZM-b/l pseudovirus neutralization assay (Seaman et al., 2010). We found that serum from immunized 2F5 complete KI mice at peak responses exhibited potent neutralization of the HIV-1 B.MN.3 strain with IC50s of ~0.5 μg/ml or ~1800 geometric mean titer (GMT) of sera dilutions, comparable to neutralization mediated by sera from WT mice containing recombinant mouse 2F5 IgG antibody (m2F5) (11) as positive control sera (Fig. 2C, Table 1). Moreover, the kinetics of serum neutralizing antibody induction closely correlated with MPER+ IgG. Immunized WT mice had no detectable neutralization activity (Fig. 2D), nor did 2F5 VH KI mice, despite having low, but detectable MPER-specific serum Ig and ASC peak responses.
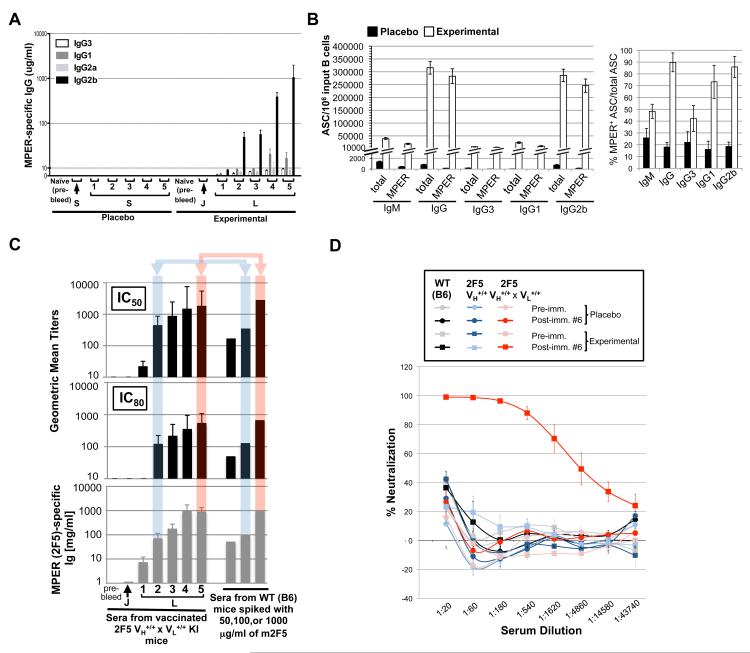
Figure 2. Specificity of MPER antibody responses in immunized 2F5 complete KI mice.
A. MPER (2F5 epitope)-specific serum IgG subclass levels in 2F5 complete KI mice from placebo (saline-injected) or experimental (JRFL primed, TLR agonist-MPER peptide-liposome conjugate boosted) immunization groups, calculated as described in Materials and Methods. B. Total or MPER-specific IgM and IgG subclass splenic ASC responses in immunization 2F5 complete KI groups. Shown is ELISPOT data calculated from dilution curve platings (as shown in Fig. S2A), from two independent experiments, each with ≥3 mice/group, graphically represented either as total ASCs for each isotype (left) or as MPER+ B-cells, normalized as frequency of all ASCs (right). C. Kinetics and potency of serum neutralizing antibody induction in vaccinated 2F5 complete KI mice. Top panels: GMTs of reciprocal dilutions of sera to inhibit HIV-1 B.MN.3 at 50 or 80% levels in the TZM-b/l neutralization assay (12,31), Bottom panel: corresponding MPER (2F5)-specific Ig levels from individual mice required. To demonstrate neutralization potency, GMTs of sera from WT mice spiked with 50,100, or 1000 μg/ml of m2F5 are also shown, with shaded boxes comparing control vs. vaccinated sera GMTs at similar MPER-specific Ig concentrations. Data are from two immunization studies, each with sera from ≥3 individual mice/time point assayed. D. Relative HIV-1 neutralization activity of serum Igs elicited by placebo or experimentally vaccinated WT, 2F5 VH+/+ KI, and 2F5 complete KI strains. Neutralization curves of serum were taken from vaccinated WT, 2F5 VH+/+ KI, and 2F5 complete KI groups (≥three mice/group) at peak MPER+ serum Ig induction (immunized 6 times), using the HIV-1 B.MN.3 isolate in the TZM-b/l neutralization assay.
Table 1.
Neutralization kinetics and potency of induced MPER-specific serum Abs from immunized 2F5 “complete” (VH+/+ × VL+/+) KI micea
| Source of sera | MPER-specific serum Ig IC50 and IC80 concentrations for neutralization of HIV-1 (μg/ml | |
|---|---|---|
| IC50 | IC80 | |
| Immunized 2F5 complete KI mice | ||
| Pre-immune | NAb | NA |
| Post-immune #1 | NA | NA |
| Post-immune #2 | 0.33 | NA |
| Post-immune #3 | 0.17 | 0.59 |
| Post-immune #4 | 0.21 | 0.81 |
| Post-immune #5 | 0.63 | 2.56 |
| Post-immune #6 | 0.48 | 1.62 |
MPER 2F5 nominal epitope-specific total Ig concentration values for experimental samples, based on values shown in Fig. 2D (and calculated by quantitative ELISA, as described in Materials and Methods) were used to convert GMT values to corresponding estimated concentrations (μg/ml) of MPER-specific serum Igs required to neutralize HIV-1 in the TZM-b/l assay.
NA=Not Applicable, i.e. no neutralization seen in serum.
Increasing B-cell survival does not impact MPER-specific serum Ab and ASC responses to immunization
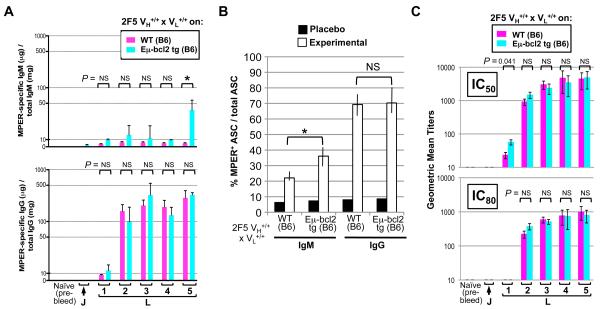
Previously, we showed that naïve 2F5 complete KI × Eμ-bcl2 tg mice (i.e., 2F5 complete KI mice crossed to mice expressing the anti-apoptotic gene Bcl-2 in the B-cell lineage (38)) resulted in elevated frequencies of both total and MPER-specific residual splenic B-cells, but uniformly express very low densities of surface Ig and are functionally analogous to anergic B-cells (12). Thus, increasing B-cell survival alone does not alleviate the anergic phenotype of residual 2F5-expressing B-cells, consistent with results in other autoreactive Ig transgenic models (39). To determine if increased B-cell survival impacted MPER-specific humoral responses induced by immunization, we immunized 2F5 complete KI mice on WT (B6) and Eμ-bcl2 tg backgrounds in parallel and compared their quantity and quality of MPER-specific Ig-secreting B-cells and serum Ig elicited. No significant differences in MPER-specific serum IgGs (Fig. 3A), MPER-specific IgG+ splenic ASCs (Figs. 3B, S2B), or neutralizing serum potency (Fig. 3C) were observed in 2F5 complete KI × Eμ-bcl2 tg mice compared to those on the WT background. However, modest increases in IgM responses, including increases in vaccine-induced MPER-specific serum IgM levels (Fig. 3A) and frequencies of vaccine-induced MPER-specific IgM+ splenic ASCs (Figs. 3B, S2B) were seen, as were increases in frequencies of total serum IgM and splenic IgM ASCs in both placebo and experimentally-immunized mice (Figs. 3B). Thus, increasing B-cell survival partially restored MPER+ IgM responses, but had no impact on MPER-specific, IgG+ B-cell or serum Ab responses. We interpret these results to reflect that the pool of residual MPER+ BnAb B-cells targeted for expansion and induced to produce IgG serum BnAbs by immunization either originate from a residual B-cell reservoir that is protected from clonal deletion, and/or their frequencies are not limiting to their induction by immunization.
Figure 3. Effect of constitutively increasing B cell survival on quantity/quality of MPER-specific serum Ab and splenic B-cell responses elicited by immunization in 2F5 complete KI mice.
A. ELISA measurements of normalized MPER-specific serum IgM and IgG Ab responses from 2F5 complete or 2F5 complete KI × Eμ-bcl2 tg mice, immunized with placebo or experimental regimens. B. ELISPOT analysis revealing total or MPER-specific IgM and IgG ASC responses in 2F5 complete KI groups on either WT (B6) or Eμ-bcl2 tg (B6) backgrounds (either placebo or experimentally immunized). Shown is graphical representation of ELISPOT data calculated from dilution curve platings (as shown in Fig. S2B), from ≥3 mice/group for two independent experiments, with MPER-specific B cells normalized as frequency of all ASCs. In all instances, significance values were determined using a two-tailed Student's test. *P ≤ 0.05, NS = Not Significant. C. Kinetics of serum neutralizing Ab induction in vaccinated 2F5 complete KI mice on WT (B6) or Eμ-bcl2 tg (B6) backgrounds. Shown are GMTs (Geometric Mean Titers) of reciprocal dilutions of sera to inhibit B.MN.3 at 50 or 80% levels in the TZM-b/l assay. Significance values were determined using a two-tailed Student's test. NS = Not Significant. Note the modestly elevated GMTs of IC50 neutralization in 2F5 complete KI × Eμ-bcl2 tg mice after the first boost, potentially reflective of the higher combined levels of MPER+ serum IgG+IgM observed at this early time point seen in Fig. 3A.
Characterization of hybridomas from peripheral tissues of immunized 2F5 KI mice
To definitively demonstrate that immunization induced class-switched BnAb-producing B-cells, we generated hybridomas by fusing NSO myeloma cells with B-cells from spleen and LNs of vaccinated 2F5 complete KI mice (on either WT or Eμ-bcl2 tg backgrounds) after 5th–6th boosts, and screened clonal culture supernatants from wells having cell growth, for isotype, MPER specificity and neutralization (Table 1). We found that >90% of all Ig-secreting lines, regardless of Ig isotype, were MPER-specific. Of 1910 MPER-reactive culture supernatants, 1866 (98%) neutralized HIV-1MN, confirming serum neutralization assays (Figs. 2C–D, 3C, Table 2). Of the combined neutralizing spleen and LN hybridoma supernatants from all immunized 2F5 complete KI mice, 1118 (61%) were IgG while 712 (38%) were IgM. Interestingly, 2F5 complete KI × Eμ-bcl2 tg mice had a greater fraction of IgG+ hybridomas than 2F5 complete KI mice on the WT background, a result we interpret as possibly reflecting the survival of a greater percentage of peripheral (mature B2 B-cells (12) in these mice, of which a substantial percentage retain an anergic phenotype, even after having undergone CSR. From the MPER-reactive, neutralizing hybridomas, we then cloned 15 representative hybridoma cell lines (12 IgG and 3 IgM), sequenced VH and VL regions, determined status of 2F5 VH/VL transgenes, and purified secreted mAbs to determine epitope reactivity and neutralization profiles. All 12 IgG hybridoma mAbs (of which 11/12 were IgG2b) had IC50 values and spectrums of neutralization comparable to m2F5 and the original human IgG mAb (h2F5) (Table 2), blocked binding of h2F5 to Env (Fig. S3A), and, like the parental 2F5 mAbs, were polyreactive in NIH-3T3, lipid and histone reactivity assays (Table 3, Fig S3C) and bound the 2F5-bound MPER epitope, MPER-lipid complexes and the transient gp41 fusion intermediate with comparable on/off-rate kinetics (Fig. S4). Furthermore, all three IgM mAbs also had similar neutralization, binding, and blocking characteristics as the parental 2F5 mAbs, as well as an IgM control mouse 2F5 mAb (12) (Table 3, Fig S3B, S3D).
Table 2.
Summary of isotypic distribution, MPER reactivities, and HIV-1 neutralization profiles of B cell hybridomas derived from peripheral immune tissues of immunized 2F5 complete KI mice
| A. Primary screens of hybridoma
clonesa | |||||
|---|---|---|---|---|---|
| Fusion name (method) | Mouse genotype/background | Tissue source of B-cells | Neutralizing clones/MPER±clones assayed b,c,d | ||
| IgM | IgG | Total (IgM+G) | |||
| V5 (electrofusion) | 2F5 VH+/+ × VL+/+/WT (B6) | Spleen | 342/344 | 149/153 | 491/497 |
| LN | 54/54 | 15/16 | 69/70 | ||
| Total % that neutralize | 396/398 (99.5%) | 164/169 (97.0%) | 560/567 (98.8%) | ||
| V6 (PEGe) | 2F5 VH+/+ × VL+/+/Eμ-bcl2 tg (B6) | Spleen | 39/45 | 46/48 | 85/93 |
| V6 (electrofusion) | Spleen | 263/271 | 907/926 | 1170/1197 | |
| LN | 14/15 | 37/38 | 51/53 | ||
| Total % that neutralize | 316/354 (89.3%) | 990/1012 (97.8%) | 1306/1343 (97.2%) | ||
| B. Neutralization profiles of
purified IgG+ mAbs selected from cloned hybridoma
linesf | |||||
|---|---|---|---|---|---|
| Clone ID | IC50 concentration (μg/ml) for neutralization of HIV-1 | ||||
| A.92UG037.1 | B.MN.3 | B.SF162.LS | B.6535.3 | B.BG1168.1 | |
| V4-SE 2 CL1-1 | 0.12 | <0.01 | 0.34 | 7.61 | 0.78 |
| V4-SE 6 CL1-1 | 0.12 | <0.01 | 0.45 | 4.81 | 1.04 |
| V4-SE 8 CL1-1 | 0.14 | 0.01 | 0.54 | 5.6 | 1.1 |
| V4-SE 361 CL1-1 | 0.21 | 0.03 | 3.04 | 2.75 | 2.37 |
| V4-SP 1B5 CL1-1 | 0.32 | 0.06 | 1.18 | 17.3 | 1.53 |
| V4-SE 18 CL1-2 | 0.06 | <0.01 | 0.23 | 2.81 | 0.64 |
| V4-SE 372 CL1-1 | 0.14 | <0.01 | 0.38 | 7.19 | 1.04 |
| V4-SP 1G3 CL1-1 | 0.14 | 0.02 | 0.55 | 7.66 | 0.98 |
| V5-SP 3H6 CL1-1 | 0.24 | 0.03 | 2.42 | 2.37 | 2.55 |
| V5-SE 4 CL1-1 | 0.13 | <0.01 | 0.38 | 6.48 | 1.04 |
| V5-SE 10 CL1-1 | 0.16 | 0.01 | 0.5 | 9.88 | 0.8 |
| V5-SP 3B11G | 0.13 | <0.01 | 0.38 | 7.23 | 0.97 |
| h2F5g | 0.28 | 0.03 | 1.49 | 6.93 | 1.93 |
Fusions were performed using NSO myeloma fusion partner lines using previously described methods (12) and represent separate experiments, each using individual immunized mice sacrificed 4 d after either fifth or sixth boosts. Data shown represent clones identified in primary screens of fusions in which total splenocytes or LN cells (lympholyte-M separated) were plated at limiting dilutions (1000 and 10000 cells/well for electrofusions and PEG fusions, respectively) in 96-well plates.
Non-clonal wells i.e. with>1 isotype, either as identified in ELISAs or which were subcloned for further analysis and had conflicting isotype identity (based on DNA sequence analysis) were excluded from this summary.
Secreting wells were identified by ELISA, using a general anti-mouse Ig H chain (G+M+A) reagent, and isotypes of secreting clones were subsequently determined by using anti-mouse H chain γ, μ, and α-specific reagents in ELISAs. MPER reactivity assays of culture supernatants were performed as previously described (4,11,12,26) using a plate-bound peptide SP62, which encodes the nominal 2F5-specific MPER epitope.
Neutralization in culture supernatants was determined using the TZM-bl HIV-1 Env pseudorvirus infectivity assay (Seaman et al., 2010), using the HIV-1 B.MN3 isolate as the test strain for primary screens and using >50% neutralization inhibition scores as the cutoff for positive neutralization activity, as previously described (12,31). The majority of non-neutralizing well supernatants were found not to neutralize due to low supernatant Ig concentration.
PEG=polyethylene glycol-mediated hybridoma fusion.
Shown are 50% neutralization concentrations (IC50) of affinity-purified mAbs required to neutralize each HIV-1 isolate listed in the TZM-bl cell assay.
Affinity-purified h2F5 (recombinant human 2F5) was used as a positive control; values shown are averages of three experiments.
Upon VH and VL sequencing, all MPER+ neutralizing clones also retained 2F5 VH and VL regions, and surprisingly, most had no additional somatic mutations in these regions i.e. other than ones already in the original (mutated) 2F5 mAb (Table 3). Conversely, four hybridoma lines we generated from MPER−/non-neutralizing clones had either extensive replacement mutations in their VH regions and/or replaced their 2F5 LCs, but interestingly, all retained at least partial NIH-3T3, lipid, and/or histone reactivities, similar to our previous studies of 2F5 KI BM B-cells cultured in IL-7 and BAFF, which edit their 2F5 L chains (12). Overall, these results extend our serum Ab analysis, by demonstrating that the majority of residual peripheral B-cell clones activated and expanded by immunization, retain characteristics of the original 2F5 mAb, including MPER specificity, self-/polyreactivities, neutralization potential, and bear 2F5 VH and VH regions with few additional somatic mutations.
Serum IgG BnAb responses in immunized 2F5 KI mice are elicited without cognate T-cell help, require dual engagement by TLR agonists and MPER peptide-lipid complexes, and coincide with expansion/activation of MPER+ transitional B-cells
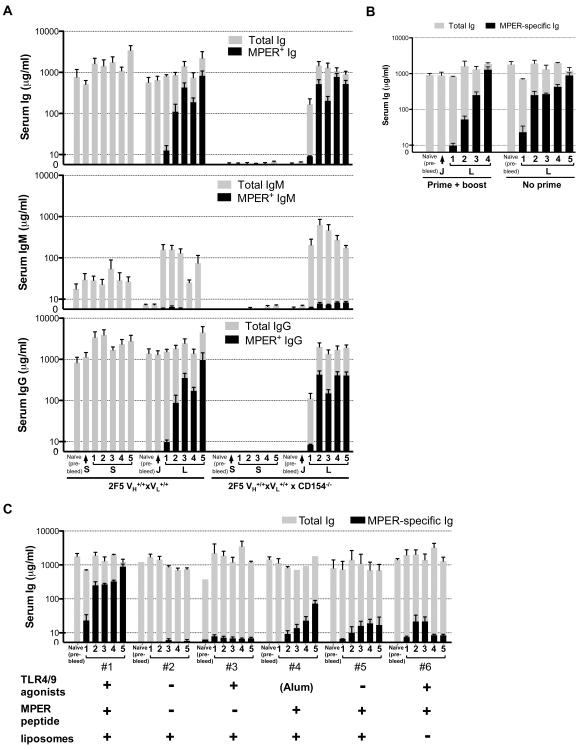
The scarcity of somatic mutations in 2F5 VH/VL regions of hybridoma B-cell lines from peripheral tissues of immunized 2F5 complete KI mice even after repeated boosting (Table 3), and the BnAb-specific Ab response lack of dependence on bcl2-mediated survival signals (Fig. 3), suggested that the induced BnAb response did not require cognate T-cell help. To formally test if BnAb responses in this model were elicited via a T-independent (T-I) pathway, we genetically removed CD40-CD40L interactions by crossing 2F5 complete KI mice with those on the same genetic background, but deficient in CD154 (CD40L) expression, and compared their MPER-specific serum Ab responses to immunization with those on the CD40L-sufficient background. Indeed, MPER-specific Ig titers elicited in immunized CD40L-deficient and CD40L-sufficient 2F5 complete KI mice were comparable, both in terms of titers, kinetics, and isotype (Fig. 4A), and, when normalized to total Ig levels, those in the former were actually slightly elevated (data not shown), confirming predominantly T-I MPER-specific serum Ig production.
Figure 4. T-independence of serum BnAb responses generated by immunization of 2F5 complete KI mice.
A. Comparison of total and MPER-specific serum Ig responses in CD154-deficient and sufficient 2F5 complete KI mice, both groups (≥3 mice/group) immunized with the standard (J)+(L) regimen. B. Effect of removing Env priming on MPER-specific serum Ab production. 2F5 complete KI mice (≥3 mice/group) shown were immunized IP either with the standard (J)+(L) immunization regimen (“prime+boost” group) or using the MPER peptide-TLR agonist-liposome boosting immunogen exclusively (“no prime” group). C. Analysis of immunogen components required to elicit serum MPER-specific Abs in 2F5 complete KI mice. Components of the boosting immunogen (L) were dissected into six immunization groups (≥3 mice/group) as follows: group #1=positive control (oCpG/Emulsigen+MPER peptide/MPLA-conjugated liposomes); group #2=”empty' liposomes (liposomes alone +Emulsigen); group #3= oCpG/Emulsigen+MPLA4-conjugated liposomes; groups #4 and #5=MPER peptide-conjugated liposomes formulated only with Alum or Emulsigen, respectively; group #6=oCpG/MPLA/Emulsigen+ unconjugated (free) MPER peptide. For all figure panels, total and MPER-specific serum Ig, IgM or IgG titers from immunized 2F5 complete KI mice were determined by ELISA.
T-I humoral responses can occur either via type I or type II B-cell activation mechanisms (40), the former involving polyclonal activation by TLR agonists alone, whereas the latter require strong BCR cross-linking by multimerically-presented Ags on APCs or as repetitive antigenic structures in pathogens or T-I immunogens i.e. polysaccharides, and frequently also involve dual (TLR+BCR)-mediated Ag-specific responses. To understand the mechanism of T-I BnAb induction in immunized 2F5 complete KI mice, we determined what components of the immunogen were critical. We first examined the relative role of priming and boosting components, and found acceleration of MPER-specific serum Ab response coinciding with removal of initial priming with the Env gp140 JRFL protein (Fig. 4B). That Env priming had no impact on BnAb production demonstrated involvement of one (or more) boosting components in this process.
We therefore further dissected the boosting regimen by immunizing 2F5 complete KI mice with individual and/or specific combinations of its components (Fig. 4C). We found that unconjugated MPER peptides could trigger low MPER+ serum Ab titers, but optimal Ab induction required MPER peptide conjugation to lipid complexes, suggesting that presentation of the MPER epitope in the correct context/orientation and/or as multimeric structures was required for Ag-specific activation of BnAb-expressing B-cells. Furthermore, TLR agonists alone failed to elicit any significant MPER+ serum Ab titers, excluding the possibility that mitogenic signals (including MPL, known to be a strong polyclonal B-cell activator) in the boosting formulation could have non-specifically/polyclonally elicited them in vivo. However, TLR agonists were required for optimal MPER-specific serum Ab responses to MPER peptide-liposome conjugates, suggesting they work in concert with MPER peptide-lipid complexes to trigger and/or expand BnAb-expressing B-cells. These results are therefore most consistent with BnAb induction in immunized 2F5 KI mice occurring via a type 2 T-I mechanism involving dual engagement of BnAb-expressing B-cells by TLR ligands and MPER-lipid complexes.
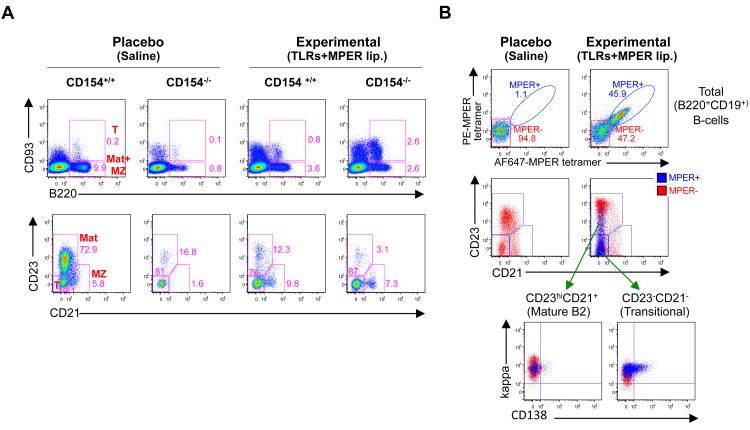
Finally, to obtain insight into the origins of the MPER+ IgG2b splenic ASCs induced by T-I immunization, we compared distributions of total and 2F5 epitope-specific MPER tetramer+ splenic B-cell subsets in naïve and immunized 2F5 complete KI mice by flow surface staining. Immunized 2F5 KI mice on either CD154-deficient or sufficient backgrounds had selectively increased frequencies of transitional (B220+CD21−CD23− or B220+CD93+) B-cells (Fig. 5A). Furthermore, the MPER-specific tetramer binding fraction of total (B220+CD19+) splenic 2F5 KI B-cells induced by immunization preferentially back-gated to the CD21−CD23− compartment, representing a major population of transitional (sIg+CD138−) B-cells, and a minor subset of (CD138+) short-lived plasmacytes (Fig. 5B) thus establishing a temporal relationship in the selective T-I induction/activation of MPER+ transitional B-cells, short-lived plasmacytes, and IgG2b+MPER+ ASCs. These results, together with the lack of somatic mutations observed in hybridomas of immunized 2F5 complete KI mice, are consistent with IgG2b+MPER+ ASCs in immunized 2F5 complete KI mice originating from newly formed/transitional splenic MPER+ B-cells recruited/expanded and triggered to undergo T-I CSR.
Figure 5. Distribution and gp41 MPER epitope specificity of splenic B-cells in immunized 2F5 complete KI mice.
A. FACS dot plot histograms (representative of two experiments) comparing the distribution of splenic B-cell subsets in 2F5 complete KI immunization groups (on either CD154−/− or CD154+/+ backgrounds), vaccinated with placebo (saline-injected) or experimental (JRFL primed, TLR agonist-MPER peptide-liposome conjugate boosted) regimens. Splenocytes were analyzed by flow cytometry 4d after 6th immunizations, either by sub-fractioning singlet, live lymphocytes with CD93 and B220-specific mAbs (upper panels), or fractioning total (CD19+B220+) B-cell subsets within singlet, live, lymphocyte-gated populations using CD21 and CD23-specific mAbs (lower panels). Numbers indicate percentages of B-cells in each gate, and B-cell subsets denoted in red lettering in top left panel are defined as: T=transitional (B220+CD93+), Mat+MZ (B220+CD93−), whereas those in the lower left panel are defined as: T=newly formed i.e., transitional (CD21−CD23−), MZ=marginal zone (CD23intCD21hi), and Mat=mature B2 (CD23hiCD21int). B. FACS dot plot histograms (representative of two experiments) of surface Ig reactivity to the 2F5 neutralization epitope. Top panels: live, singlet, lymphocyte-gated total (B220+CD19+) B-cells from placebo or experimentally-immunized 2F5 complete KI mice, as determined by flow cytometric bivariate fractionation using MPER epitope-specific tetramers (12,30) labeled with AF647 and PE. Lower panels: splenic B-cell subset distributions of MPER tetramer+ and MPER tetramer− subsets (denoted in blue and red dots, respectively), back-gated into mature B2, transitional, and short-lived plasmacyte B-cell subsets.
In contrast to T-I MPER-specific BnAb serum IgG responses to immunization, naïve 2F5 complete KI mice lose their ability to make MPER- serum IgGs on the CD154- deficient background, thus confirming that that their constitutive production of non-neutralizing serum IgGs occurs via a T-dependent (T-D) mechanism (Fig. 4A). Furthermore, placebo-immunized 2F5 complete KI mice on the CD154-sufficient background accumulate a substantial residual splenic mature B2 (B220+CD93− or CD19+B220+CD21+CD23+) B-cell subset, which are selectively reduced in those on the CD154-deficient background (Fig. 5A). Taken together, these results indicate that a minor IgG2b+ subset of T-D (B2) mature B-cells (i.e., distinct from the transitional B-cell pool triggered by immunization to undergo T-I CSR to IgG2b) have been selected for loss of 2F5 nominal MPER epitope reactivity, due to either receptor editing (i.e., 2° LC rearrangements, VH replacements) and/or somatic mutation.
Discussion
Our study makes several key observations relevant to inducing BnAbs with HIV-1 vaccine immunogens. First, we demonstrate that despite profound clonal deletion of B-cells expressing autoreactive BnAbs like 2F5 (12), residual anergic clones are competent to undergo class-switching, activation, and expansion in response to immunization, resulting in potent serum IgG neutralizing responses. Thus, in principle, this study shows that overcoming tolerization of self-reactive BnAb-expressing B-cells by immunization is a feasible part of an Ab-based HIV-1 vaccine strategy. One question relevant for vaccination aimed at triggering BnAbs specific for the 2F5 neutralization epitope is if the biology of re-activating residual anergic B-cells by immunization in our KI model, which expresses the original (somatically mutated) 2F5 BnAb as BCRs, would be similar in the context of naïve B-cells expressing unmutated 2F5 BCRs. We think this is likely, since KI mice expressing an inferred reverted (unmutated) form of the 2F5 Ab as BCR, exhibits at least as profound a developmental blockade in the BM (associated with efficient central B-cell deletion) as those carrying the original (mutated) 2F5 BnAb (L. Verkoczy, B.F. Haynes, unpublished results).
Secondly, this study demonstrates that all three components of the immunization regimen used in this study (i.e., TLR agonists, MPER peptides, and liposomes) are required for triggering and expanding preexisting, anergic 2F5-expressing B-cells to secrete serum BnAbs via a type 2 T-I pathway. This therefore strongly indicates that these components have critical roles in: i) recognizing the 2F5 nominal MPER epitope by B-cells expressing the 2F5 BnAb as BCR in vivo, and/or ii) circumventing tolerance (re-activating anergic B-cells). A key mechanistic question in this context is the relative role that lipid complexes have in these processes. Two possibilities are: they allow sufficient multimerization of the MPER epitope required for strong BCR cross-linking, and/or permit the 2F5 nominal MPER epitope to be presented in an orientation (and/or conformation) required for proper recognition by the 2F5 BCRs. Consistent with the former possibility is the fact that the combination of TLR agonists and multimeric Ag has previously been shown to be efficient in both re-activating anergic B-cells (16–19) and triggering T-I CSR (41). Experiments are currently underway to formally address these possibilities, including challenge of 2F5 KI mice with immunogenically “neutral” beads, coated with MPER peptides at similar densities and in a comparable orientation as those used to generate the MPER peptide-lipid conjugates described in this study.
Finally, by using KI mice expressing the original (mutated) 2F5 (thus making it possible to track MPER/neutralization specificity), we have uncovered distinct pathways of serum α-gp41 Env IgG production: a) T-I IgG BnAb induction by vaccination, originating from anergic self-reactive, MPER+ transitional B-cells, re-activated to become IgG2b+ MPER+ splenic ASCs, and b) constitutive T-D non-neutralizing serum IgGs, originating from a subset of splenic mature B2 cells selected for their loss of 2F5 nominal MPER epitope reactivity. A key question regarding the physiological relevance of immunization's failure to induce T-D serum BnAbs in the 2F5 KI model is what drives this loss of functional specificity (i.e. 2F5 affinity for the ELDKWA neutralization motif) in response to incidental Ag exposure. One formal possibility is that this represents a byproduct of general diversification of 2F5 monospecificity by V region modification mechanisms, as proposed to occur in certain instances where Ig receptor specificity is restricted, for example in quasi-monoclonal mice, which are diversified throughout B-cell differentiation via L chain editing and somatic mutation (42). However, since it is generally accepted that immune diversification primarily occurs early, in BM development, we do not think this explains loss of MPER binding in constitutively T-D serum IgG fractions, since most residual ex-vivo 2F5 KI B-cells (including mature B-cells) express uniformly low sIg densities, are functionally anergic, and retain dual MPER/lipid (i.e. neutralization) specificity (13), suggesting only a subset of mature B-cells undergo purifying selection against MPER binding. Furthermore, MPER neutralization epitope specificity itself is likely a key feature in the highly selective nature of 2F5 autoreactivity, as suggested by recent protein array screens which have identified candidate conserved mammalian autoantigen targets and compared polyreactivity profiles of 2F5 and 4E10 (43). In particular, 2F5 in this study was found to exhibit minimal polyreactivity and selectively bind two proteins, kynureninase (KYNU) and the XXX chemokine receptor (CMTM3), with domains that perfectly mimic the 2F5 complete (ELDKWA) and core (DKW) neutralizing epitopes, respectively, whereas 4E10 exhibited uncommonly high polyreactivity and its primary Ag target, splice factor 3B subunit 3 (SF3B3), had no obvious homology to the 4E10 neutralizing epitope NWFDIT. Finally, our recent comparative analysis of serum Igs from naïve 2F5 and 4E10 KI mice also mirror these data i.e., 2F5 KI IgG fractions stringently eliminate MPER neutralization epitope reactivity, but maintain residual low affinity lipid binding/basal polyreactivity, and conversely, 4E10 KI IgG fractions stringently lose their initially significant lipid reactivity but retain 4E10 neutralization epitope specificity (13), again implying that a 2F5-expressing mature B-cell subset escapes anergy by selective loss of neutralization epitope-binding. Collectively, these data suggest that peripheral 2F5-expressing B-cells, by eliminating ELDKWA-associated self-reactivity, also inadvertently remove a large fraction of overlapping HIV-1 neutralizing specificities, and raise the possibility that this may be a general limitation for generating T-D responses in BnAb lineages with selective autoreactivity (i.e., to host antigens mimicked by neutralizing epitopes).
Mechanistically, it will be interesting to understand how the selective elimination of putative MPER-associated self-reactivity identified here in mature B2 B-cell compartments, occurs. Although beyond the scope of this study, several lines of evidence suggest it is driven by multiple 2F5 VH-encoded self-reactive residues associated with ELDKWA binding, not all of which can be vetoed by L chain editing; these data include the following: i) B-cells which express 2F5 HCs paired with multiple endogenous mouse LCs in 2F5 VH+/+ mice stringently eliminate MPER binding, yet still undergo central deletion (11), and ii) most in vitro IL7/BAFF-cultured 2F5 complete KI BM B-cells also lose MPER binding due to editing of their 2F5 L chains, yet retain their anergic phenotype (12). Additionally, since the 2F5 VH is unusual in that it lacks a consensus cryptic RSS (12) required for H chain editing, this suggests an additional (and/or alternative) mechanism for purging autoreactivity may involve purifying selection for mutations at self-reactive VH residues associated with ELDKWA binding, as a last resort to rescue residual, anergic mature B-cells (like those expressing potentially “uneditable” H chain such as 2F5) from deletion during affinity maturation. Such purifying selection for mutations against self-reactivity and the resulting “tug-of-war” created with selection to acquire foreign Ag specificity has been previously proposed as a general process in the GC (44), and would be obvious of interest to understand further in the context of affinity maturation pathways that generate BnAbs, given the exceptionally high rates of somatic mutations observed in the VH regions of all BnAbs isolated to date from HIV-1 infected subjects (2,3,45). In particular, this unusual BnAb trait, in the context of a reaction trying to strike a balance between eliminating self-reactivity and acquiring functional specificity, could be further accentuated in chronically infected HIV-1 patients undergoing multiple rounds of mutation/selection.
The ultimate goal of developing an HIV-1 Ab-based vaccination strategy with a regimen such as the one used in this report will be to demonstrate its ability to appropriately drive BnAb clonal precursors to acquire their functional (neutralizing) specificity (46). In this context, it is noteworthy that vaccination studies in rhesus macaques, using the same regimen as in this study, induces Abs focused to the 2F5 core neutralization epitope DKW, but are “early” in a BnAb maturation pathway and are non-neutralizing (23). Thus, although the results in our 2F5 KI model provide insight for how residual, anergic B-cells can be targeted and activated via immunization, it does not address additional contributory factors that could limit induction of MPER+ BnAbs by immunization in the context of polyclonal, pre-immune repertoires of normal, outbred animals or healthy individuals (reviewed in (5, 46, 47, 48)). These potential issues include: i) triggering of naïve B-cells recognizing dominant, non-neutralizing MPER epitopes by existing immunogens in preference to those recognizing subdominant BnAb epitopes, ii) inability of MPER immunogens to sufficiently engage and/or activate reverted (unmutated) BnAb BCRs, and iii) the inability by current vaccine regimens to either: a) recapitulate complex affinity maturation pathways in the setting of chronic HIV-1 infection, in which many mutations are required to achieve neutralization breadth and potency (49), likely by acquiring the appropriate combination of desired FRW alterations (i.e., increased flexibility and/or Env binding yet maintaining structural integrity) (50), or, b) acquire somatic mutations that confer lipid reactivity, required for 2F5 and 4E10's initial “docking” to the viral membrane, and thus important for the neutralization mechanism of the MPER+ BnAbs in particular (22,26).
With respect to the issue of triggering non-neutralizing epitopes, we have shown that the MPER peptide-liposome conjugate used in this study not only elicits DKW-focused Ab responses in macaques (23), but in contrast to the MPER peptide alone, selectively binds the 4E10 and 2F5 BnAbs (i.e., and does not bind the non-neutralizing MPER+ mAb 13H11) thus suggesting the MPER epitope presented in lipids, favors a conformation that excludes MPER non-neutralizing epitopes (23,27). Furthermore, unlike most other experimentally-reverted BnAb unmutated common ancestors (UCAs) (that have undetectable affinity for their Env constructs), both inferred UCAs of BnAb 2F5 bind the core 2F5 neutralization epitope (51). Thus, the MPER-liposome conjugate used here arguably already has several features consistent with it addressing the first two hurdles mentioned above, although immunization studies using KI mice expressing reverted (unmutated) 2F5 BCRs either alone, or in the setting of adoptive co-transfer of different ratios of KI and WT BM B-cells into irradiated recipients, will be needed to formally confirm this notion. On the other hand, the third issue mentioned above: appropriately “guiding” the 2F5 BnAb lineage's affinity maturation pathway by immunization is a difficult issue that may require a strategy based on B-cell lineage immungen design (46). Such a strategy would propose to use the immunogen in this study (or optimized versions of it) to prime naïve B-cells expressing unmutated 2F5 BCRs, followed by serially boosting with other immunogens, optimized to target B-cells expressing the lineage's inferred intermediate BCRs. Again, immunizations of KI mice expressing reverted 2F5 BCRs, as well as complete Ig locus “humanized” mouse models with such immunogens will be critical testing platforms to rapidly evaluate the feasibility of such strategies.
Supplementary Material
Acknowledgements
We thank Krissey Lloyd and Celia LaBranche for assistance with mAb blocking and TZM-b/l/neutralization assays, respectively, and Kara Anasti and Lawrence Armand for help with production and quality control of TLR agonist-MPER peptide-liposomes and labeled, 2F5 nominal MPER epitope-specific tetramers, respectively. We are additionally grateful to the Duke Center for AIDS Research (CFAR) sequencing facility, and the DHVI Flow cytometry and Immune Reconstitution Facilities for expert assistance, as well as Garnett Kelsoe for discussions and critical review.
Footnotes
Supported by a Collaboration for AIDS Vaccine Discovery Vaccine Development Center grant (to BFH) from the Bill and Melinda Gates Foundation, the Center For HIV/AIDS Vaccine Immunology NIAID/NIH grant U19AI067854 (to BFH), and NIH grant R01AI087202 (to LV).
Abbreviations used: HIV-1, Env, gp41 MPER, 2F5, Immunogen, T-I and T-D immune responses.
References
- 1.Mascola JR, Montefiori DC. The role of antibodies in HIV vaccines. Annual Rev. Immunol. 2010;28:413–444. doi: 10.1146/annurev-immunol-030409-101256. [DOI] [PubMed] [Google Scholar]
- 2.McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type-1 by vaccination. Immunity. 2010;33:542–554. doi: 10.1016/j.immuni.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haynes BF, Moody MA, Verkoczy L, Kelsoe G, Alam SM. Antibody polyspecificity and neutralization of HIV-1: a hypothesis. Hum. Antibodies. 2005;14:59–67. [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, Ortel TL, Liao HX, Alam SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 5.Verkoczy L, Kelsoe G, Moody MA, Haynes BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Curr. Opin. Immunol. 2011;23:383–390. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiegler G, Kunert R, Purtscher M, Wolbank S, Voglauer R, Steindl F, Katinger H. A potent cross-clade neutralizing human monoclonal antibody against a novel epitope on gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses. 2001;17:1757–1765. doi: 10.1089/08892220152741450. [DOI] [PubMed] [Google Scholar]
- 7.Zwick MB, Labriijn AF, Wang M, Spenlehauer C, Sapphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 2001;75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muster T, Steindl F, Purtscher M, Trkola A, Klima A, Himmler G, Ruker F, Katinger H. A conserved neutralizing epitope on gp41 of human immunodeficiency virus type 1. J. Virol. 1993;67:6642–6647. doi: 10.1128/jvi.67.11.6642-6647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ofek G, Tang M, Sambor A, Katinger H, Mascola JR, Wyatt R, Kwong PD. Structure and mechanistic analysis of the anti-human immunodeficiency virus type 1 antibody 2F5 in complex with its gp41 epitope. J. Virol. 2004;78:10724–10737. doi: 10.1128/JVI.78.19.10724-10737.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardoso RM, Zwick MB, Stanfield RL, Kunert R, Binley JM, Katinger H, Burton DR, Wilson IA. Broadly neutralizing anti-HIV antibody 4E10 recognizes a helical conformation of a highly conserved fusion-associated motif in gp41. Immunity. 2005;22:163–173. doi: 10.1016/j.immuni.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Verkoczy L, Diaz M, Holl TM, Ouyang YB, Bouton-Verville H, Alam SM, Liao HX, Kelsoe G, Haynes BF. Autoreactivity in an HIV-1 broadly reactive neutralizing antibody variable region heavy chain induces immunologic tolerance. Proc. Natl. Acad. Sci. USA. 2010;107:181–186. doi: 10.1073/pnas.0912914107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verkoczy L, Chen Y, Bouton-Verville H, Zhang J, Diaz M, Hutchinson J, Ouyang YB, Alam SM, Holl TM, Hwang KK, Kelsoe G, Haynes BF. Rescue of HIV-1 broad neutralizing antibody-expressing B cells in 2F5 VH × VL knockin mice reveals multiple tolerance controls. J. Immunol. 2011;187:3785–3797. doi: 10.4049/jimmunol.1101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Zhang J, Hwang K-K, Bouton-Verville H, Xia S-M, Newman A, Ouyang Y-B, Haynes BF, Verkoczy L. Common tolerance mechanisms, but distinct cross-reactivities associated with gp41 and lipids, limit production of HIV-1 broad neutralizing antibodies 2F5 and 4E10. J. Immunol. doi: 10.4049/jimmunol.1300770. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Ann. Rev. Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 15.Cambier JC, Gauld SB, Merrell KT, Vilen BJ. B-cell anergy: from transgenic models to naturally occurring anergic B cells? Nat. Rev. Immunol. 2007;7:633–643. doi: 10.1038/nri2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–47. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 17.Uccellini MB, Busconi L, Green NM, Busto P, Christensen SR, Shlomchik MJ, Marshak-Rothstein A, Viglianti GA. Autoreactive B cells discriminate CpG-rich and CpG-poor DNA and this response is modulated by IFN-alpha. J. Immunol. 2009;181:5875–5884. doi: 10.4049/jimmunol.181.9.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Shlomchik MJ. Activating systemic autoimmunity: B's, T's, and tolls. Curr. Opin. Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montero M, van Houten NE, Wang X, Scott JK. The membrane-proximal external region of the human immunodeficiency virus type 1 envelope: dominant site of antibody neutralization and target for vaccine design. Microbiol. Mol. Biol. Rev. 2008;72:54–84. doi: 10.1128/MMBR.00020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montero M, Gulzar N, Klaric KA, Donald JE, Lepik C, Wu S, Tsai S, Julien JP, Hessell AJ, Wang S, Lu S, Burton DR, et al. Neutralizing epitopes in the membrane-proximal external region of HIV-1 gp41 are influenced by the transmembrane domain and the plasma membrane. J. Virol. 2012;86:2930–2941. doi: 10.1128/JVI.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam SM, Morelli M, Dennison SM, Liao HX, Zhang R, Xia SM, Rits-Volloch S, Sun L, Harrison SC, Haynes BF, Chen B. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 2009;106:20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennison SM, Sutherland LL, Jaeger FH, Anasti KM, Parks R, Stewart S, Bowman C, Xia SM, Zhang R, Shen X, Scearce RM, et al. Induction of antibodies in rhesus macaques that recognize a fusion-intermediate conformation of HIV-1 gp41. PLoS One. 2011;6:e27824. doi: 10.1371/journal.pone.0027824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frey G, Peng H, Rits-Volloch S, Morelli M, Cheng Y, Chen B. A fusion-intermediate state of HIV-1 gp41 targeted by broadly neutralizing antibodies. Proc. Natl. Acad. Sci. USA. 2008;105:3739–3744. doi: 10.1073/pnas.0800255105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofek G, Guenaga FJ, Schief WR, Skinner J, Baker D, Wyatt R, Kwong PD. Elicitation of structure-specific antibodies by epitope scaffolds. J. Virol. 2010;107:17880, 17887. doi: 10.1073/pnas.1004728107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alam SM, McAdams M, Boren D, Rak M, Scearce RM, Gao F, Camacho ZT, Gerwith D, Kelsoe G, Chen P, Haynes BF. The role of antibody specificity and lipid reactivity in binding of broadly neutralizing anti-HIV envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal epitopes. J. Immunol. 2007;178:4424–4435. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennison SM, Stewart SM, Stempel KC, Liao H-X, Haynes BF, Alam SM. Stable docking of neutralizing human immunodeficiency virus type1 gp41 membrane-proximal external region monoclonal antibodies 2F5 and 4E10 is dependent on the membrane immersion depth of their epitope regions. J. Virol. 2009;83:10211–10223. doi: 10.1128/JVI.00571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Renshaw BR, Fanslow WC, 3rd, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40-ligand-deficient mice. J. Exp. Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao H-X, Sutherland LL, Xia SM, Brock ME, Scearce RM, Vanleeuwen S, Alam SM, McAdams M, Weaver EA, Camacho Z, Ma BJ, et al. A group M consensus envelope glycoprotein induces antibodies that neutralize subsets of subtype B and C HIV-1 primary viruses. Virology. 2006;353:268–282. doi: 10.1016/j.virol.2006.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verkoczy L, Moody MA, Holl TM, Bouton-Verville H, Scearce RM, Hutchinson J, Alam SM, Kelsoe G, Haynes BF. Functional, non-clonal IgMa-restricted B cell receptor interactions with the HIV-1 envelope gp41 membrane proximal external region. PloS one. 2009;4:e7215. doi: 10.1371/journal.pone.0007215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, et al. Tiered categorization of a diverse panel of HIV-1 Env pseudovirus for assessment of neutralizing antibodies. J. Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montefiori DC. Evaluating neutralizing antibodies against HIV and SHIV in luciferase reporter gene assays. In Curr. Protocol. Immunol. 2004;chapt. 12(unit 12.11) doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 33.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J. Virol. 2005;79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiller T, Busse CE, Wardemann H. Cloning and expression of murine Ig genes from single B cells. J. Immunol. Methods. 2009;350:183–193. doi: 10.1016/j.jim.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Alam SM, Scearce RM, Parks RJ, Plonk K, Plonk SG, Sutherland LL, Gorny MK, Zolla-Pazner S, Vanleeuwen S, Moody MA, Xia SM, et al. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J. Virol. 2008;82:115–125. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicely NI, Dennison SM, Spicer L, Scearce RM, Kelsoe G, Ueda Y, Chen H, Liao HX, Alam SM, Haynes BF. Crystal structure of a non-neutralizing antibody to the HIV-1 gp41 membrane-proximal external region. Nat. Struct. Mol. Biol. 2010;17:1492–1498. doi: 10.1038/nsmb.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran TT, Reich CF, 3rd, Alam M, Pisetsky DS. Specificity and immunochemical properties of anti-DNA antibodies induced in normal mice by immunization with mammalian DNA with a CpG oligonucleotide as adjuvant. Clin. Immunol. 2003;109:278–287. doi: 10.1016/j.clim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 38.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc. Natl. Acad. Sci. USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartley SB, Cooke MP, Fulcher DA, Harris AW, Cory S, Basten A, Goodnow CC. Elimination of self-reactive B lymphocytes proceeds in two stages: arrested development and cell death. Cell. 1993;72:325–335. doi: 10.1016/0092-8674(93)90111-3. [DOI] [PubMed] [Google Scholar]
- 40.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Ann. Rev. Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 41.Pone EJ, Zhang J, Mai T, White CA, Li G, Sakakura JK, Patel PJ, Al-Qahtani A, Zan H, Casali P. BCR signaling synergizes with TLR signaling for induction of AID and immunoglobulin class-switching through the non-canonical NF-kB pathway. Nat. Commun. 2012;3:767–773. doi: 10.1038/ncomms1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascalho M, Ma A, Lee S, Masat L, Wabl M. A quasi-monoclonal mouse. Science. 1996;272:1649–1652. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 43.Yang G, Holl TM, Liu Y, Li X, Nicely N, Kepler TB, Alam SM, Liao H-X, Cain DW, Spicer L, VandeBerg JL, Haynes BF, Kelsoe G. Identification of autoantigens recognized by the 2F5 and 4E10 broadly neutralizing HIV-1 antibodies. J. Exp. Med. 2013;210:241–256. doi: 10.1084/jem.20121977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz M, Klinman NR. Relative roles of somatic and Darwinian evolution in shaping the antibody response. Immunol. Res. 2000;21:89–102. doi: 10.1385/IR:21:2-3:89. [DOI] [PubMed] [Google Scholar]
- 45.Haynes BF, Moody MA, Liao H-X, Verkoczy L, Tomaras GD. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends. Microbiol. 2011;17:108–116. doi: 10.1016/j.molmed.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haynes BF, Kelsoe G, Harrison SC, Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nature Biotech. 2012;30:423–433. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonsignori M, Alam SM, Liao H-X, Verkoczy L, Tomaras GD, Haynes BF, Moody MA. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol. 2012;20:532–539. doi: 10.1016/j.tim.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature's pathways. 2013 doi: 10.1111/imr.12075. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liao H-X, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. 2013 doi: 10.1038/nature12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancer M, Zhou T, Incesu R-H, Fu BZ, B.Z., et al. Somatic mutations of the immunoglobulin framework are generally required for broad and potent neutralization. Cell. 2013;153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alam SM, Liao HX, Dennison SM, Jaeger F, Parks R, Anasti K, Foulger A, Donathan M, Lucas J, Verkoczy L, Nicely N, et al. Differential reactivity germ line allelic variants of a broadly neutralizing HIV-1 antibody to a gp41 fusion intermediate conformation. J. Virol. 2011;85:11725–11731. doi: 10.1128/JVI.05680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zwick MB, Komori HK, Stanfield RL, Church S, Wang M, Parren PW, Kunert R, Katinger H, Wilson IA, Burton DR. The third complementarity-determining region of the heavy chain is important in the activity of the broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2F5. J. Virol. 2004;78:3155–3161. doi: 10.1128/JVI.78.6.3155-3161.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.