Abstract
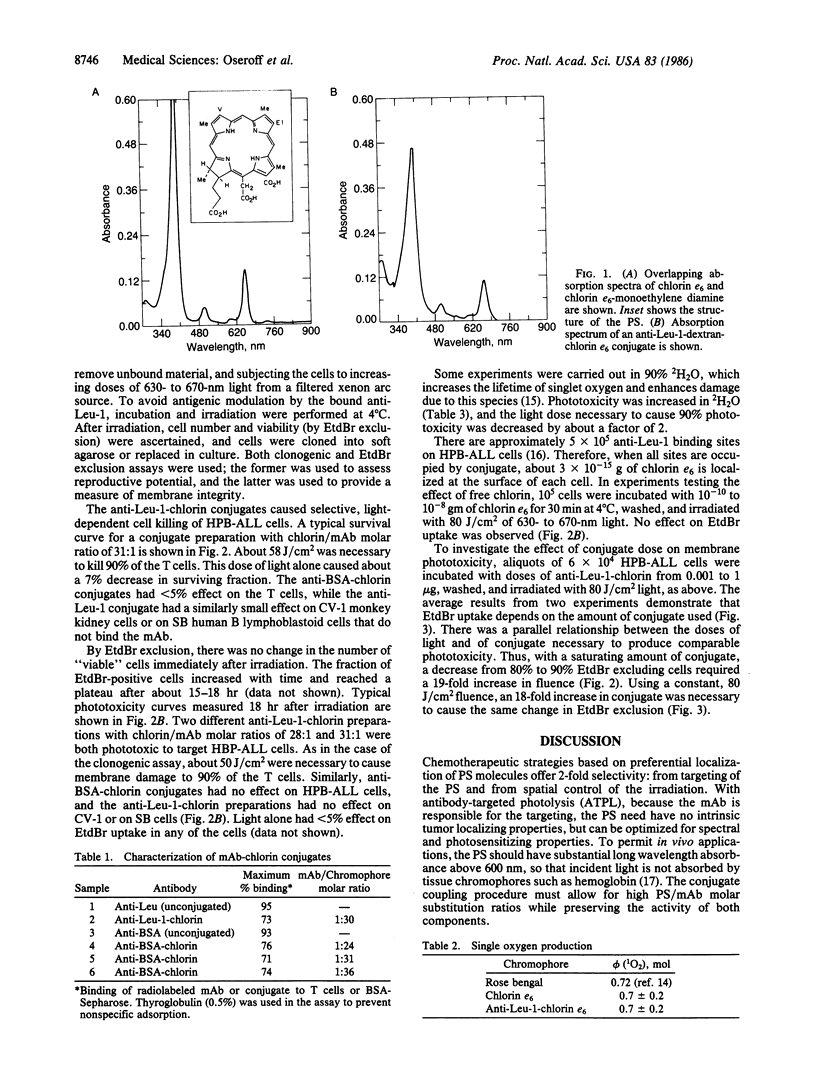
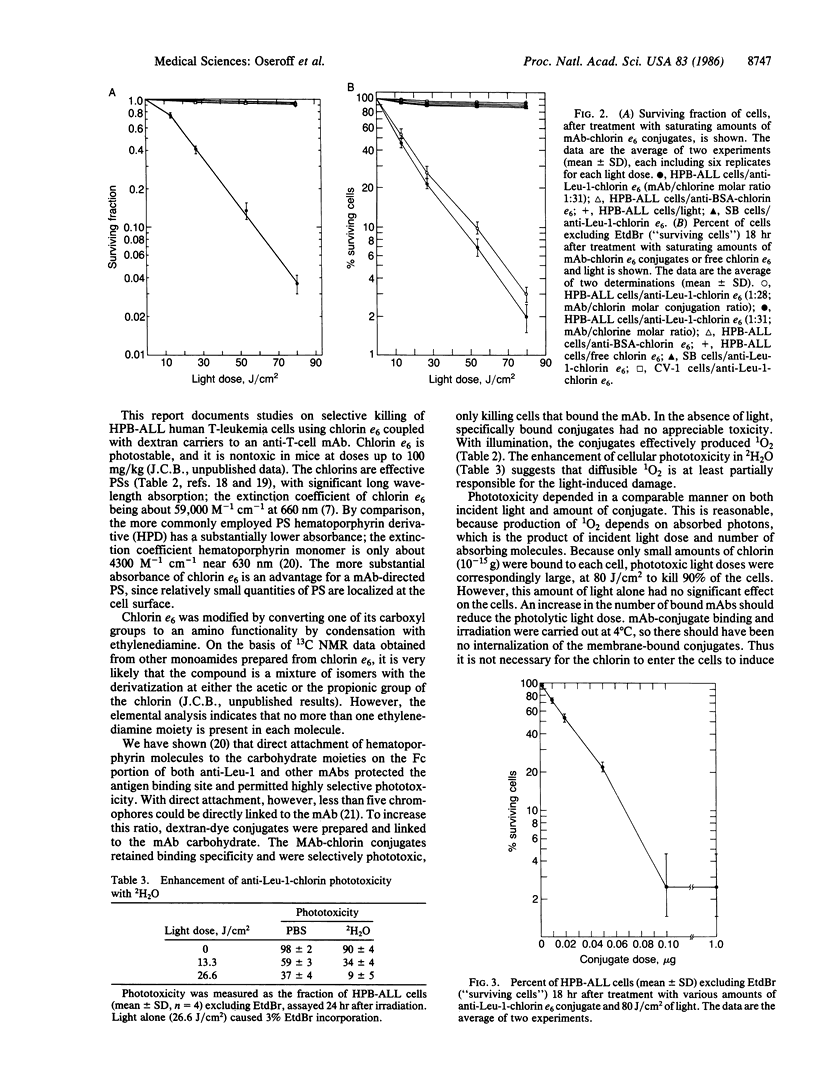
Selective in vitro photodestruction of HPB-ALL human T-cell leukemia cells was accomplished using the photosensitizer chlorin e6 coupled through dextran molecules to an anti-T-cell monoclonal antibody (mAb), anti-Leu-1. Conjugates with mAb/chlorin molar ratios as high as 1:36 retained mAb binding activity, and the absorption spectrum and quantum efficiency for singlet oxygen production of bound chlorin (0.7 +/- 0.2) were unchanged from that of the free photosensitizer. Phototoxicity, as measured by a clonogenic assay and by uptake of ethidium bromide, was dependent on the doses of both mAb-chlorin and 630- to 670-nm light, was enhanced by 2H2O, and was observed only in target populations that bound the mAb. Similarly, free chlorin e6 in solution had no photodynamic effect in amounts 100 times more than that carried by the mAb. For this antibody-targeted system, approximately 10(10) molecules of singlet oxygen were necessary to kill a cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellnier D. A., Dougherty T. J. Membrane lysis in Chinese hamster ovary cells treated with hemtoporphyrin derivative plus light. Photochem Photobiol. 1982 Jul;36(1):43–47. doi: 10.1111/j.1751-1097.1982.tb04338.x. [DOI] [PubMed] [Google Scholar]
- Berns M. W., Dahlman A., Johnson F. M., Burns R., Sperling D., Guiltinan M., Siemens A., Walter R., Wright W., Hammer-Wilson M. In vitro cellular effects of hematoporphyrin derivative. Cancer Res. 1982 Jun;42(6):2325–2329. [PubMed] [Google Scholar]
- Blair A. H., Ghose T. I. Linkage of cytotoxic agents to immunoglobulins. J Immunol Methods. 1983 Apr 29;59(2):129–143. doi: 10.1016/0022-1759(83)90024-8. [DOI] [PubMed] [Google Scholar]
- GRANICK S., BOGORAD L., JAFFE H. Hematoporphyrin IX, a probable precursor of protoporphyrin in the biosynthetic chain of heme and chlorophyll. J Biol Chem. 1953 Jun;202(2):801–813. [PubMed] [Google Scholar]
- Garnett M. C., Embleton M. J., Jacobs E., Baldwin R. W. Preparation and properties of a drug-carrier-antibody conjugate showing selective antibody-directed cytotoxicity in vitro. Int J Cancer. 1983 May 15;31(5):661–670. doi: 10.1002/ijc.2910310520. [DOI] [PubMed] [Google Scholar]
- Ghose T. I., Blair A. H., Kulkarni P. N. Preparation of antibody-linked cytotoxic agents. Methods Enzymol. 1983;93:280–333. doi: 10.1016/s0076-6879(83)93050-1. [DOI] [PubMed] [Google Scholar]
- Gomer C. J., Smith D. M. Photoinactivation of Chinese hamster cells by hematoporphyrin derivative and red light. Photochem Photobiol. 1980 Sep;32(3):341–348. doi: 10.1111/j.1751-1097.1980.tb03772.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Seide R. K., Kindt T. J. Quantitative determination of allotypic and idiotypic markers with antisera coupled to N-hydroxysuccinimide-activated sepharose. J Immunol. 1975 Jan;114(1 Pt 1):51–54. [PubMed] [Google Scholar]
- Kessel D., Dutton C. J. Photodynamic effects: porphyrin vs chlorin. Photochem Photobiol. 1984 Sep;40(3):403–405. doi: 10.1111/j.1751-1097.1984.tb04607.x. [DOI] [PubMed] [Google Scholar]
- Mew D., Lum V., Wat C. K., Towers G. H., Sun C. H., Walter R. J., Wright W., Berns M. W., Levy J. G. Ability of specific monoclonal antibodies and conventional antisera conjugated to hematoporphyrin to label and kill selected cell lines subsequent to light activation. Cancer Res. 1985 Sep;45(9):4380–4386. [PubMed] [Google Scholar]
- Mew D., Wat C. K., Towers G. H., Levy J. G. Photoimmunotherapy: treatment of animal tumors with tumor-specific monoclonal antibody-hematoporphyrin conjugates. J Immunol. 1983 Mar;130(3):1473–1477. [PubMed] [Google Scholar]
- Moolten F. L., Schreiber B. M., Zajdel S. H. Antibodies conjugated to potent cytotoxins as specific antitumor agents. Immunol Rev. 1982;62:47–73. doi: 10.1111/j.1600-065x.1982.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Oldham R. K. Monoclonal antibodies in cancer therapy. J Clin Oncol. 1983 Sep;1(9):582–590. doi: 10.1200/JCO.1983.1.9.582. [DOI] [PubMed] [Google Scholar]
- Parrish J. A. New concepts in therapeutic photomedicine: photochemistry, optical targeting and the therapeutic window. J Invest Dermatol. 1981 Jul;77(1):45–50. doi: 10.1111/1523-1747.ep12479235. [DOI] [PubMed] [Google Scholar]
- Roper P. R., Drewinko B. Comparison of in vitro methods to determine drug-induced cell lethality. Cancer Res. 1976 Jul;36(7 Pt 1):2182–2188. [PubMed] [Google Scholar]
- Thorpe P. E., Ross W. C. The preparation and cytotoxic properties of antibody-toxin conjugates. Immunol Rev. 1982;62:119–158. doi: 10.1111/j.1600-065x.1982.tb00392.x. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S., Krolick K. A., Uhr J. W. Neoplastic B cells as targets for antibody-ricin A chain immunotoxins. Immunol Rev. 1982;62:159–183. doi: 10.1111/j.1600-065x.1982.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Willan K. J., Golding B., Givol D., Dwek R. A. Specific spin labelling of the Fc region of immunoglobulins. FEBS Lett. 1977 Aug 1;80(1):133–136. doi: 10.1016/0014-5793(77)80423-7. [DOI] [PubMed] [Google Scholar]
- Yuhas J. M., Toya R. E., Pazmiño N. H. Neuraminidase and cell viability: failure to detect cytotoxic effects with dye-exclusion techniques. J Natl Cancer Inst. 1974 Aug;53(2):465–468. doi: 10.1093/jnci/53.2.465. [DOI] [PubMed] [Google Scholar]



