To the Editor of Biology of Blood and Marrow Transplantation
We previously reported that the infusion of ex vivo expanded umbilical cord blood (UCB)-derived natural regulatory T cells (nTregs) infused immediately after UCB transplantation was associated with a reduced incidence of acute graft-vs.-host disease (GVHD) relative to historical controls (1). We did not observe an increased incidence of opportunistic infections or relapse, suggesting that the transient nature of nTreg provided sufficient immune suppression to control GVHD without long-term deleterious effects. However, we hypothesized that early side effects might be heightened and evaluated at specific time points such as when adoptively transferred nTregs were detectable in the peripheral blood of patient. All but one patient, receiving nTreg dose of 10 × 106/kg on day +1 and 3 × 106/kg on day 15, have been reported (1). Patients eligibility, conditioning regimen and immune suppression, and supportive care have been reported (1). In this analysis the historical controls consisted of 65 patient subset of the 108 reported (1) for whom had post-UCB transplantation T cell subset phenotype data available using standard procedures.
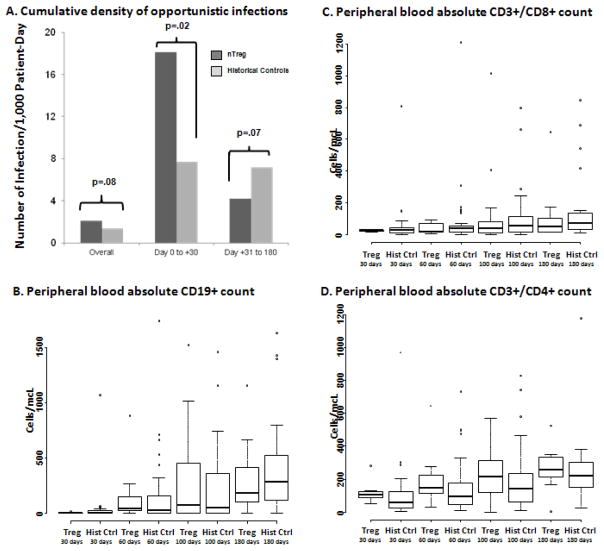
In contrast to prior analyses which compared the cumulative incidence of OIs, in this analysis we studied infection density that accounts for multiple infections in an individual patient. We calculated infection density per 1000 patient-days within 180 days by dividing total number of infections within the time period by total patient-day multiplied by 1000 (2). In our initial report we demonstrated that adoptively transferred nTregs were present in the peripheral blood of patients up to 14 days after the infusion of fresh and up to 4 days after the infusion of cryopreserved product (1). Notably, in the current study we found that in this early period (day 0 to +30) that nTregs are present, there was significantly higher cumulative density of OI in Treg (18.06 infections/1000 patient-days) as compared to historical controls (7.71 infections/1000 patient-days) patients (univariate RR 5.35, p=.02) (Figure 1A). These were essentially viral reactivations with no effect on the risk of fungal infections. Thus, it is possible that Treg can increase the risk of infection during the period of time they are detectable. This contrasts with our initial report in which we found no difference on the risk OIs as assessed by the cumulative incidence. In contrast to the first 30 days, between days +31 and +180 there was similar OIs density in the two groups (7.22/1000 vs. 4.22/1000, univariate RR 0.58, p=.07) (Figure 1A). Notably, the higher risk of early infection did not affect non-relapse mortality (18% [95%CI, 2–34%] vs. 10% [95%CI, 2–17%], p=.34) or progression-free survival (33% [95%CI, 16–52%] vs. 35% [95%CI, 24–47%], p=.93), as compared to historical controls.
Figure 1.
In panel (A) summarizes the cumulative density of infections per 1,000 patients years observed. The median and range of peripheral blood (B) CD19+, (C) CD3+/CD8+ and (D) CD3+/CD4+ lymphocytes at day +180 post-transplantation for nTreg vs. in historical controls.
The viral infections observed in nTreg patients through day +30 was human herpes virus-6 viremia (HHV6, n=9), cytomegalovirus (n=2) viremia and one case of parainflunenza upper respiratory infection. The corresponding viral infections observed in historical controls through day +30 were human herpes virus 6 (HHV-6) viremia (n=6), CMV reactivation (n=8), adenovirus gastroenteritis (n=4), polyoma virus in the urine (n=4), enterovirus gastroenteritis (n=1) and upper airway respiratory syncytial virus (n=1). Three of 9 the HHV-6 viremia episodes in the Treg group and 2 of 6 in the historical control group were treated with foscarnet. In our institution we observed 69% incidence reactivation of HHV-6 by 6 weeks after UCB transplantation, overall (3). Thus, while a high rate of HHV-6 infections in the first month is not unexpected, the higher density of this infection in the first month as compared to similarly treated patients could be the result of the Treg infusion. However, patients in a phase I clinical trial are monitored much closer than patients receiving the standard of care and we cannot rule out that our higher infection density is the result of an observation bias and/or the limited number of Treg recipients.
A potential benefit of not developing GVHD is the ability to taper immune suppression sooner, leading of potentially faster reconstitution of immune reconstitution, but we did not observe a significant difference between nTreg patients vs. historical controls. At day +180, the median absolute number of peripheral blood CD19+ cells was 179 (range, 0–1160)/mcL vs. 282 (range 1–1632)/mcL (p=.54), CD3+/CD8+ was 58 (range, 14–645)/mcL vs. 72 (range, 8–848)/mcL (p=.46), and CD3+/CD4+ cells was 255 (range, 6–527)/mcL vs. 219 (range, 27–1179)/mcL (p=.48), for nTreg vs. historical controls, respectively (Figure 1B–D). In contrast, data on the adoptive transfer of nTregs in combination with T effector cells in haploidentical related donor transplantation where antigen specific T-cell immunity was observed sooner than in historical controls (4). This may be secondary to biological differences between donor types as cord blood recipients may take longer to achieve adequate numbers virus specific T-cells (5). Clearly, longer follow-up and more detailed analysis are required for better understanding of lymphocyte subsets reconstitution.
In summary, there is potentially a higher viral infection risk within 30 days of UCB-derived nTregs infusion by suppression of the immune response; however, there was no adverse effect on the longer term outcomes, including later risk of OIs. While observation bias is a possibility, our data suggesting higher density of viral infections argues for close observation in studies of the adoptive transfer of nTregs to reduce GVHD in allogeneic HCT and in solid organ transplant.
Acknowledgments
This work was supported in part by grants from the National Cancer Institute P01 CA65493 (C.G.B, J.S.M, D.H.M, P.B.M, B.R.B, J.E.W.), , Leukemia and Lymphoma Society Scholar in Clinical Research Award # 2417-11(C.G.B.), the Children’s Cancer Research Fund (J.E.W., T.E.D.) and R01 CA105216 (C.H.J.), National Heart, Lung and Blood Institute N01 HB037164 (J.S.M., D.H.M., J.E.W.) and HHSN268201000008C (J.S.M., D.H.M., K.L.H., J.C., J.E.W.), Leukemia and Lymphoma Translational Research, grant R6029-07 (B.R.B.), and National Marrow Donor Program grant 13396 AM#2 (B.R.B., J.E.W.).
References
- 1.Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011 Jan 20;117(3):1061–70. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997 Apr 30;16(8):901–10. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 3.Betts BC, Young JA, Ustun C, Cao Q, Weisdorf DJ. Human herpesvirus 6 infection after hematopoietic cell transplantation: is routine surveillance necessary? Biol Blood Marrow Transplant. 2011 Oct;17(10):1562–8. doi: 10.1016/j.bbmt.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011 Apr 7;117(14):3921–8. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- 5.McGoldrick SM, Bleakley ME, Guerrero A, Turtle CJ, Yamamoto TN, Pereira SE, et al. Cytomegalovirus-specific T cells are primed early after cord blood transplant but fail to control virus in vivo. Blood. 2013 Feb 14; doi: 10.1182/blood-2012-09-453720. [DOI] [PMC free article] [PubMed] [Google Scholar]