Abstract
This study was carried out to evaluate the adverse effects of exposure to prallethrin on oxidant/antioxidant status and liver dysfunction biomarkers and the protective role of Origanum majorana essential oil (EO) in rat. Male rats were divided into 4 groups: (i) received only olive oil (ii) treated with 64.0 mg/kg body weight prallethrin (1/10 LD50) in olive oil via oral route daily for 28 days, (iii) treated with 64.0 mg/kg body weight prallethrin (1/10 LD50) and EO (160 μL/kg b.wt.) in olive oil and (iv) received EO (160 μL/kg b.wt.) in olive oil via oral route twice daily for 28 days. Prallethrin treatment caused decrease in body weight gain and increase in relative liver weight. There was a significant increase in the activity of serum marker enzymes, aspartate transaminase, alanine transaminase, and alkaline phosphatase. It caused increase in thiobarbituric acid reactive substances and reduction in the activities of superoxide dismutase, catalase, and glutathione-S-transferase in liver. Consistent histological changes were found in the liver of prallethrin treatment. EO showed significant protection with the depletion of serum marker enzymes and replenishment of antioxidant status and brought all the values to near normal, indicating the protective effect of EO. We can conclude that prallethrin caused oxidative damage and liver injury in male rat and co-administration of EO attenuated the toxic effect of prallethrin. These results demonstrate that administration of EO may be useful, easy, and economical to protect human against pyrethroids toxic effects.
1. Introduction
Synthetic pyrethroids are the newest major class of broad-spectrum organic insecticides used in agricultural, domestic, and veterinary applications. They are widely applied in view of the fact that they have shown to possess a high insecticidal activity as well as a broad spectrum of high initial toxic action on several types of pests [1]. Although they were not used commercially until 1980, pyrethroid insecticides accounted for more than 25% of the world market [2, 3]. Therefore, the widespread use of pyrethroid insecticides consequently leads to the exposure of manufacturing workers, field applicators, the ecosystem, and finally the public to the possible toxic effects of pyrethroid insecticides.
Pyrethroid insecticides are acute neurotoxicants [4], modulate the function of voltage-gated sodium channels [5]; specifically, they alter the permeability of excited nerve cells to sodium ions and cause repetitive nerve impulses [4, 6]. They also have other neurobiologic actions, including effects on central γ-amino butyric acid, noradrenergic, dopaminergic, and cholinergic neurotransmission [7]. However, the toxicity of pyrethroid insecticides to mammals has received much attention in recent years because animals exposed to these insecticides showed changes in their physiological activities besides other pathological features [8, 9]. Due to lipophilic nature of pyrethroid insecticides [10], they easily cross biological membranes but accumulate in biological membranes leading to stimulate the production of reactive oxygen species (ROS) and result in oxidative damage in mammals [11, 12] and aquatic organisms [13]. Oxidative stress and resulting damage to essential cell components caused by oxygen-free radicals are generally considered a serious mechanism. Previous studies suggested that some effects directly related to pesticide toxicity could be due to changes in membrane fluidity [14–16], in lipid composition [17], and inhibition of enzyme activities [18–20].
Prallethrin is the most popular Type I synthetic pyrethroid that produces a rapid knockdown in household insect pests such as mosquitoes, houseflies, and cockroaches [21]. It has prevalent household presence in the form of mosquito repellant mats, coils, liquid vaporizers, and so forth and therefore there could be direct and indirect exposure in pets and humans through accidental continued contamination of food and water [22].
Currently, there is an increased demand for using medicinal plants in therapy, in both developing and developed countries due to growing recognition of natural products, the “back to nature” slogan, instead of using synthetic drugs which might have adverse effects. Origanum majorana L. (O. majorana) is a member of the mint family Lamiaceae. In folk medicine, marjoram is used for cramps, depression, dizziness, gastrointestinal disorders, migraine, nervous headaches, and paroxysmal coughs and as a diuretic [23]. It contains phenolic terpenoids (thymol, carvacrol), flavonoids (diosmetin, luteolin, and apigenin), tannins, hydroquinone, phenolic glycosides (arbutin, methyl arbutin, vitexin, and orientinthymonin), triacontane, sitosterol, acids (oleanolic acid), and cis-sabinene hydrate [24, 25].
There are several reports on oils indicating that it results in alterations of pharmacologic responses to drugs [26]. In our previous study [27], O. majorana essential oil (EO) was analyzed by gas chromatography-mass spectrometry (GC-MS) and gas chromatography-flame ionization detector (GC-FID) and evaluated for free radical scavenging activities. GC-MS analysis revealed the presence of 4-terpineol (29.97%), γ-terpinene (15.40%), trans-sabinene hydrate (10.93%), α-terpinene (6.86%), and 3-cycolohexene-1-1 methanal,a,a4-trimethyl-,(S)-(CAS) (6.54%) as main constituents. It exhibited concentration-dependent inhibitory effects on DPPH∙, hydroxyl radical, hydrogen peroxide, reducing power, and lipid peroxidation [27].
At this time, a very little, unsatisfactory information is available in literature on oxidative stress and hepatotoxicity of prallethrin in mammals. In addition, the use of O. majorana EO to alleviate the oxidative damage and hepatotoxicity induced by pesticides has not been previously examined. Therefore, this study was interested first in evaluating the adverse effects of exposure to prallethrin on oxidant/antioxidant status and liver dysfunction biomarkers and second in the protective role of O. majorana EO against prallethrin-induced oxidative damage and hepatotoxicity in rat.
2. Materials and Methods
2.1. Materials
Prallethrin (96.2%) was obtained from Jiangsu Yangnong Chemical Co., Ltd, China. The assay kits used for biochemical measurements of catalase (EC 1.11.1.6), superoxide dismutase (EC 1.15.1.1), glutathione-S-transferase (EC 2.5.1.13), aspartate aminotransferases (EC 2.6.1.1.), alanine aminotransferases (EC 2.6.1.2), alkaline phosphatase (EC 3.1.3.1), and lipid peroxidation were purchased from Biodiagnostic Company, 29 Tahrir Street, Dokki, Giza, Egypt. Kit of protein was obtained from Stanbio Laboratory, Texas, USA. All other chemicals were of reagent grades and obtained from the local scientific distributors in Egypt.
2.2. Preparation of Essential Oil
O. majorana EO was obtained from leaves by hydrodistillation in a Clevenger apparatus. The distillation continued until no more condensing oil could be seen. The oil was permitted to stand undisturbed so that a good separation from water could be obtained. The essential oil was separated from the aqueous solution, dried over anhydrous sodium sulfate, transferred into an amber glass flask, and kept at a temperature of −20°C until used. O. majorana EO was identified by GC (THERMO TRACE 2000) equipped with a MS (FINNI-GAN SSQ 7000) GC-MS system (Central Laboratory of the National Research Centre, Cairo, Egypt) as described in our previous study [27].
2.3. Animals
Healthy male Wistar rats were obtained from Animal Breeding House of the National Research Centre (NRC), Dokki, Cairo, Egypt. Rats were housed in clean plastic cages with free access to food (standard pellet diet) and tap water ad libitum, under standardized housing conditions (12 h light/dark cycle, the temperature was 23 ± 2°C, and a minimum relative humidity of 44%) in the laboratory animal room. Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals.” The Local Ethics Committee at the National Research Centre (NRC), Dokki, Cairo, Egypt, approved the experimental protocols and procedures. The rats attained a body weight range of 145–155 g before being used for this study.
2.4. Experimental Design
Dosages of prallethrin and O. majorana EO were freshly prepared in olive oil, given via oral route for 28 consecutive days, and adjusted weekly for body weight changes. The animals were acclimatized for a minimum of 7 days before treatment and randomly assigned into four groups of seven rats each. Rats in group one received olive oil and served as control. Group two received prallethrin at a dose 64.0 mg/kg b.wt. (1/10 LD50). Group three received prallethrin at a dose 64.0 mg/kg b.wt. and O. majorana EO at 160 μL/kg b.wt. twice daily. Group four received O. majorana EO at a dose 160 μL/kg b.wt. twice daily.
The selective dose of prallethrin is based on published LD50 (640 mg/kg b.wt.) [28], and dose of O. majorana EO (160 μL/kg b.wt. twice daily) is based on El-Ashmawy et al. [29]. At the end of the administration, the animals were fasted for 12 hours and sacrificed by ether anesthesia with cervical dislocation on 29th day.
2.5. Body Weight and Samples Preparation
Body weights were recorded weekly during the experimental period (28 days). At the end of this period, blood samples were withdrawn from the animals under light ether anaesthesia by puncturing the retero-orbital venous plexus of the animals with a fine sterilized glass capillary. Blood samples were taken and left to clot in clean dry tubes and then centrifuged at 3000 rpm (600 g) for 10 minutes using Heraeus Labofuge 400R, Kendro Laboratory Products GmbH, Germany, to obtain the sera. The sera was then stored frozen at −20°C for the biochemical analysis (ALT, AST, and ALP). After blood collection, rats were then killed by decapitation, and livers were dissected out, cleaned, weighed. Small pieces of liver were cut and kept in 10% formalin solution for histological studies. Other portions of liver washed with saline solution, weighed, cut in small parts, homogenized in 10% (w/v) ice cold 100 mM phosphate buffer (pH 7.4) and centrifugation at 10,000 ×g for 15 minutes at 4°C, and then the supernatant was obtained and used for antioxidant enzyme measurements (CAT, SOD, and GST) and total protein.
2.6. Serum Liver Dysfunction Marker Enzymes
Serum aminotransferases (AST and ALT) and ALP were measured spectrophotometrically as described by Reitman and Frankel [30] and Young et al. [31], respectively, using Shimadzu UV-VIS Recording 2401 PC (Japan), performed according to the details given in the kit's instructions and were expressed in terms of U/L.
2.7. Liver Lipid Peroxidation and Antioxidant Enzymes
Antioxidant enzyme activities and lipid peroxidation were determined in liver homogenate. A centrifugation was carried out and thus, antioxidants were measured in the isolated cell fraction using a spectrophotometer Shimadzu UV-VIS Recording 2401 PC (Japan). It was performed according to the details given in the kit's instructions. The principals below of different methods are given for each concerned biochemical parameter.
2.8. Lipid Peroxidation
Lipid peroxidation was estimated by measuring thiobarbituric acid reactive substances (TBARS) and was expressed in terms of malondialdehyde (MDA) content by a colorimetric method according to Satoh [32]. The MDA values were expressed as nmoles of MDA/mL.
2.9. Antioxidant Enzymes
Superoxide dismutase activity was determined according to the method of Nishikimi et al. [33]. The method is based on the ability of SOD enzyme to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye (NTB). Briefly, 0.05 mL sample was mixed with 1.0 mL buffer (pH 8.5), 0.1 mL nitroblue tetrazolium (NBT), and 0.1 mL NADH. The reaction was initiated by adding 0.01 mL phenazine methosulphate (PMs), and then increase in absorbance was read at 560 nm for five minutes. SOD activity was expressed in μmol/mg protein.
Catalase activity was determined according to the method of Aebi [34]. The method is based on the decomposition of H2O2 by catalase. The sample containing catalase is incubated in the presence of a known concentration of H2O2. After incubation for exactly one minute, the reaction is quenched with sodium azide. The amount of H2O2 remaining in the reaction mixture is then determined by the oxidative coupling reaction of 4-aminophenazone (4-aminoantipyrene, AAP) and 3,5-dichloro-2-hydroxybenzenesulfonic acid (DHBS) in the presence of H2O2 and catalyzed by horseradish peroxidase (HRP). The resulting quinoneimine dye (N-(4-antipyrl)-3-chloro-5-sulfonate-p-benzoquinonemonoimine) is measured at 510 nm. The catalase activity was expressed in μmol/mg protein.
Glutathione-s-transferase activity in the liver was assessed spectrophotometrically according to the method of Habig et al. [35]. The method was based on the conjugation of 1-chloro-2 4-dinitrobenzene (CDNB) with reduced Glutathione (GSH) in a reaction catalyzed by GST. Increase in absorbance was monitored for 3 min at 30 sec intervals at wavelength of 340 nm. Results were expressed as nmol/mg protein.
2.10. Protein Concentration
The total protein concentrations in homogenate were determined spectrophotometrically based on the colorimetric biuret method according to Gornall et al. [36].
2.11. Histological Study
After the end of the treatment period, rats were killed, and liver samples were dissected and fixed in 10% neutral formalin, dehydrated in ascending grades of alcohol, and imbedded in paraffin wax. Paraffin sections (5 μm thick) were stained for routine histological study using haematoxylin and eosin (H&E). Two slides were prepared for each rat; each slide contains two sections. Ten field areas for each section were selected and examined for histopathological changes (x160) under light microscope. The liver fields were scored as follows: normal appearance (−), minimal cellular disruption in less than 1% of field area (+), mild cellular disruption of 1–30% of field area (++), moderate cellular disruption of 31–60% of field area (+++), severe cell disruption of 61–90% of field area (++++), and very severe cellular disruption of 91–100% of field area (++++). Previous investigators have performed such quantitative assessment of histopathological injury [37].
2.12. Statistical Analysis
The results were expressed as means ± S.E. All data were done with the Statistical Package for Social Sciences (SPSS 17.0 for windows). The results were analyzed using one way analysis of variance (ANOVA) followed by Duncan's test for comparison between different treatment groups. Statistical significance was set at P ≤ 0.05.
3. Results
3.1. Signs of Toxicity
No clinical signs of prallethrin poisoning were observed among rats of treated groups such as diarrhea, hair loss, nasal hemorrhage, and bloated abdomen. Moreover, death was not observed during experimental period (28 days).
3.2. Body and Relative Liver Weights
Compared to controls, a statistically significant decrease in body weights gain (%) of prallethrin-treated group (8.13% versus 13.53%) and prallethrin + EO-treated group (11.63% versus 13.53%) was recorded (Figure 1). The relative liver weight of prallethrin-treated animals showed a significant increase compared to control (3.95% versus 2.84%). While insignificant changes were observed in relative liver weights of prallethrin + EO and EO-treated groups compared to control (Figure 1).
Figure 1.
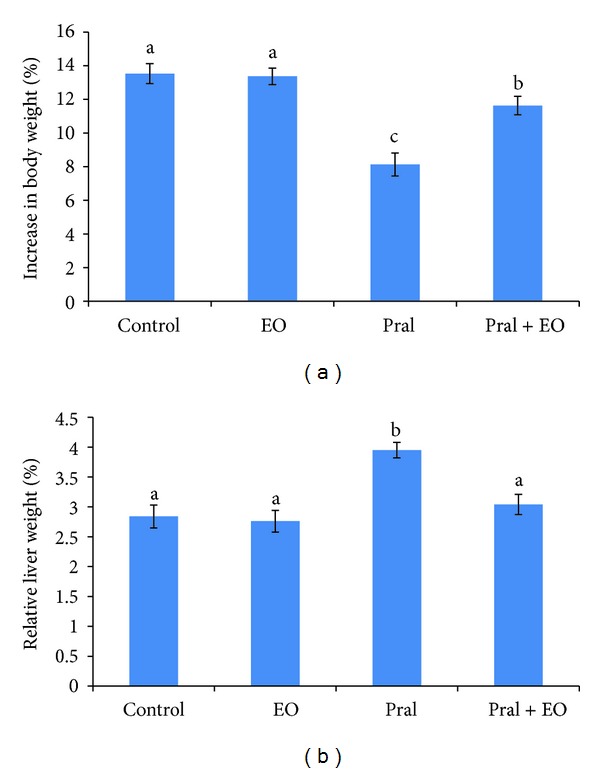
Body (a) and relative liver (b) weights of rats exposed to prallethrin (Pral) and the protective effect of Origanum majorana essential oil (EO). Each value is a mean of 7 rats ± SE; a,b,c values are not sharing superscripts letters (a, b, c) differ significantly at P ≤ 0.05. Increase in body weight (%) = ((final b.wt. − initial b.wt.)/initial b.wt.) × 100. Relative liver weight (%) = (liver weight/body weight) × 100.
3.3. Hepatic Function Enzymes
As shown in Figure 2, prallethrin-induced hepatotoxicity reflected by elevated serum ALT, AST, and ALP levels (P ≤ 0.05). Insignificant changes were observed after EO treatment. Coadministration of EO with prallethrin comparatively and markedly significantly reduced the activities of ALT, AST, and ALP compared with prallethrin group. Compared to controls, AST (45.70 U/L versus 41.72 U/L) and ALT (53.94 U/L versus 33.49 U/L) returned to control values of EO + prallethrin-treated group, while the increase of ALP was significant (122.39 U/L versus 107.34 U/L).
Figure 2.
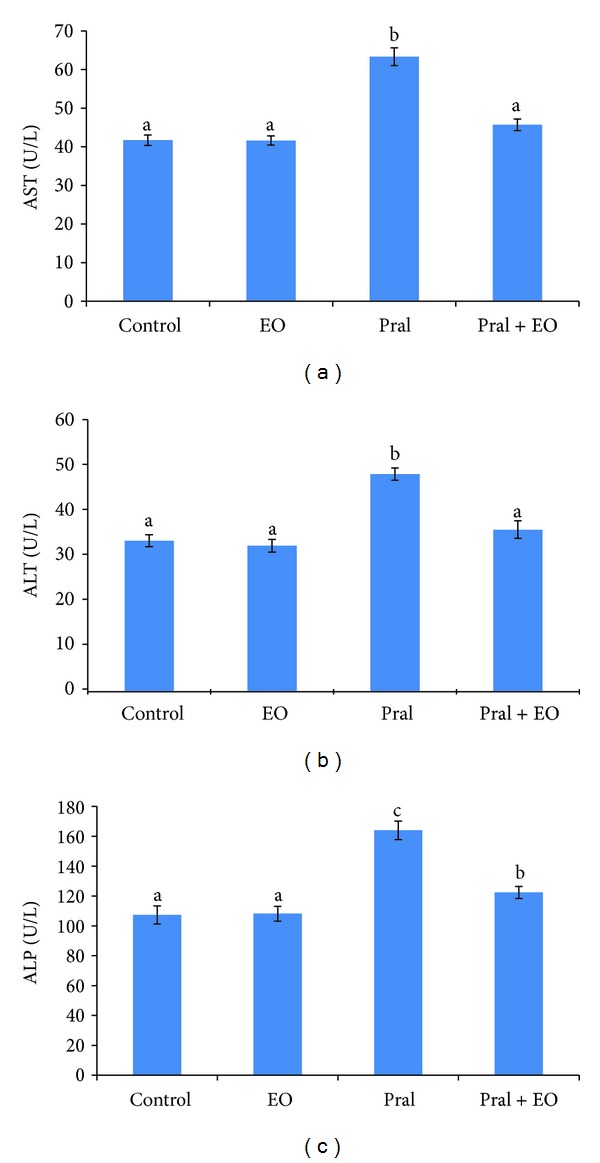
AST (a), ALT (b), and ALP (c) activities in the sera of rats exposed to prallethrin (Pral) and the protective effect of Origanum majorana essential oil (EO). Each value is a mean of 7 rats ± SE; a,b,c values are not sharing superscripts letters (a, b, c) differ significantly at P ≤ 0.05.
3.4. Effect on Lipid Peroxidation
Liver MDA level was markedly increased by prallethrin administration as compared to control group. The difference between the two groups was statistically significant (152.07 nmoles of MDA/g tissue versus 102.77 nmoles of MDA/g tissue). EO administered to rats of prallethrin + EO group alleviated lipid peroxidation induced by prallethrin treatment and modulated significantly (116.46 nmoles of MDA/g tissue versus 102.77 nmoles of MDA/g tissue) the levels of MDA in liver compared to control. Results indicated that treatment with EO produced a significant reduction in MDA in prallethrin-treated rats; however EO per se did not alter MDA (Figure 3).
Figure 3.
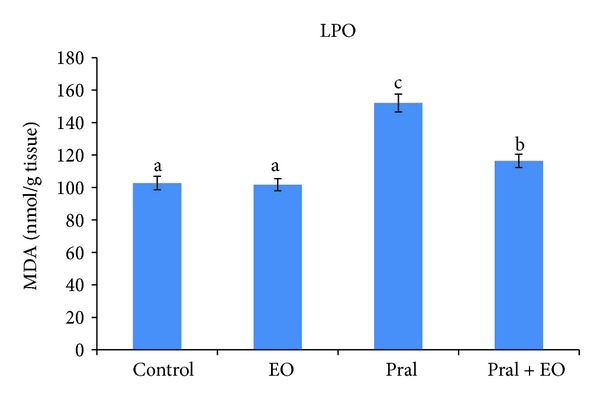
Effect of prallethrin (Pral) and Origanum majorana essential oil (EO) coadministered with Pral on the MDA levels in rat liver. Each value is a mean of 7 rats ± SE; a,b,c values are not sharing superscripts letters (a, b, c) differ significantly at P ≤ 0.05.
3.5. Effect on Antioxidant Enzymes
The effects of prallethrin treatment on the activities of SOD, CAT, and GST in liver tissue are shown in Figure 4. Activities of CAT (13.90 μmol/mg protein versus 30.22 μmol/mg protein) and GST (452.64 μmol/mg protein versus 643.01 μmol/mg protein) in liver were significantly decreased compared to control group. EO administrated in prallethrin-treated rats improved significantly the activities of CAT, SOD, and GST in liver compared with control values. The activity of CAT and SOD was returned to control values in prallethrin + EO-treated group, while the decrease of GST was significant compared with untreated group (Figure 4).
Figure 4.
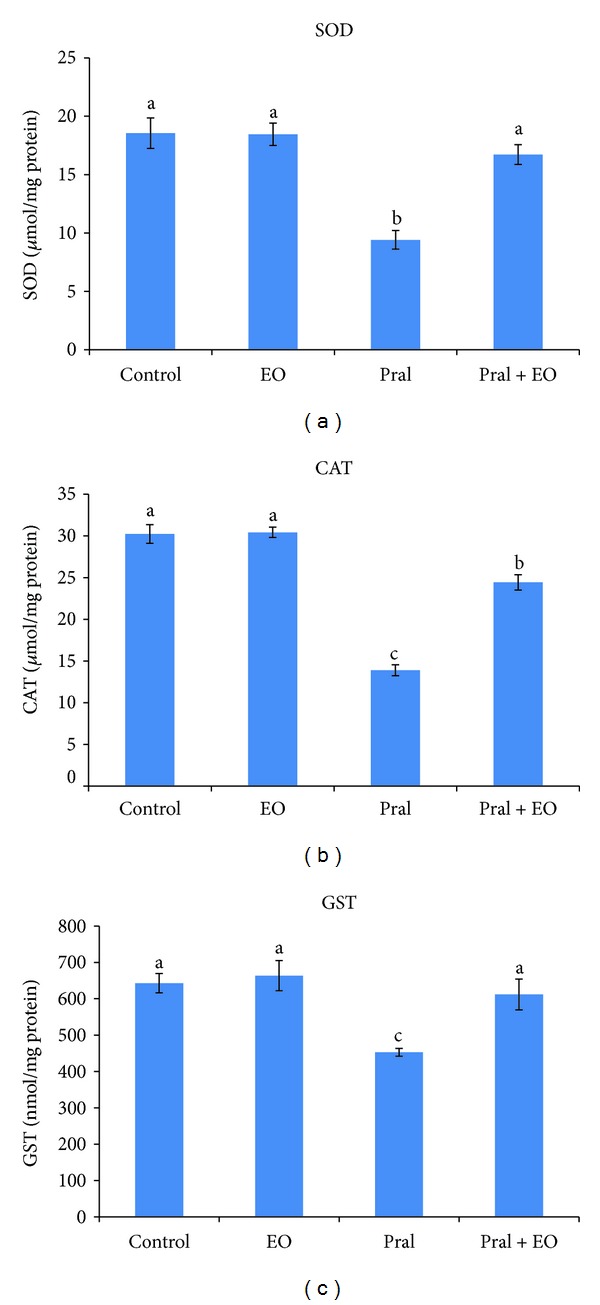
Effect of Origanum majorana essential oil (EO) on prallethrin (Pral)-induced alterations in SOD, CAT, and GST activities in liver tissue of control and treated rats. Each value is a mean of 7 rats ± SE; a,b,c values are not sharing superscripts letters (a, b, c) differ significantly at P ≤ 0.05.
3.6. Histological Changes
The histopathological changes were graded and summarized in (Table 1). As shown in Figure 5, liver sections stained with H&E showed normal histological structure of the central vein and surrounding hepatocytes in control groups (Figure 5(a)). In prallethrin-treatment group, dilatation and congestion of the portal vein, oedema, infiltration of inflammatory cells, and necrosis were recorded (Figures 5(b1)–5(b3)). Sections of liver from rats, treated with EO alone, showed to be similar to control (Figure 5(c)). Coadministration of EO to prallethrin-treated rats showed dilation, congestion, oedema, few inflammatory cells infiltration, and diffuse kupffer cells and did not reveal any necrosis area (Figures 5(d1) and 5(d2)). However, quantitative assessment of histopathological injury, based on scoring severity of injury in the liver, showed mild to moderate injury after EO coadministration to prallethrin-treated rats (Table 1).
Table 1.
Histopathological changes in the liver of male rats exposed to prallethrin (Pral) and the protective effect of Origanum majorana essential oil (EO), based on scoring severity of injury.
| Observation | Control | EO | Pral | Pral + EO |
|---|---|---|---|---|
| Inflammatory cells in the portal area | − | − | +++ | + |
| Focal necrosis in the hepatic parenchyma | − | − | ++ | − |
| Diffuse kupffer proliferation | − | − | − | ++ |
Normal (−), minimal (+), mild (++), moderate (+++).
Figure 5.
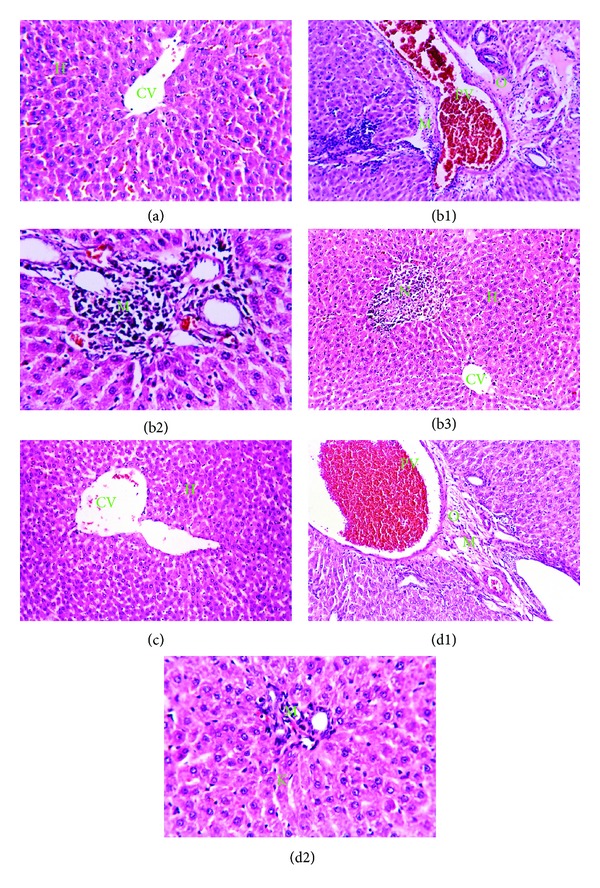
Paraffin sections of liver stained by haematoxylin and eosin (H&E) for histopathological changes. Control group (a) showing the normal histological structure of the central vein (CV) and surrounding hepatocytes (H) (x64). Prallethrin group showing (b1) severe dilatation and congestion of the portal vein (PV) with oedema (O) in portal area (x40), (b2) massive number of inflammatory cells infiltration (M) in the portal area (x80), and (b3) focal necrosis (N) in the hepatic parenchyma (x40). O. majorana (EO) group (c) showing intact normal histopathological structure of the central vein (CV) and surrounding hepatocytes (x40). Prallethrin-O. majorana (EO) group showing (d1) dilation and congestion in the portal vein (PV), oedema (O), and few inflammatory cells infiltration (M) with dilated bile duct (bd) in portal area (x40) and (d2) diffuse kupffer cells proliferation (K) in between the hepatocytes (x80).
4. Discussion
In toxicological studies, body, organ weights, and biochemical parameters are measured to evaluate a broad range of physiological and biochemical functions, affected target organ identification, and tissue injury assessment. In the present study, rats treated with prallethrin at 64.0 mg/kg b.wt. (1/10 LD50) daily for 28 days showed no mortality or signs of toxicity throughout the experimental period. Also, food and water consumption were not significantly affected (untabulated data). Our results revealed that treatment of prallethrin caused significant reduction in rat body weight gain while increased relative liver weight compared to control group. In addition, coadministration of EO prevented the toxicity of prallethrin. Increase in liver weight in prallethrin intoxication rat may be due to the increment of biotransformation enzymes [38–40]. Long-term feeding studies with laboratory animals have shown adverse effects of pyrethroid (e.g., cypermethrin); it caused reduced growth rate and increased liver weight in rats [38, 41]. Findings of present study are consistent with previous studies with different pesticides on mammals [42–44].
Liver is a target organ and plays a major role in detoxification and excretion of many endogenous and exogenous compounds. It plays important role in metabolism [45] and biotransformation of toxic compound [46]. Therefore, any type of injury or impairment of its function produces hepatotoxicity and causes health complications. Liver biomarker enzymes, for example, AST, ALT, and ALP, have been commonly associated with liver dysfunction/damage. Hayes et al. [47] reported that one of the indicators for liver damage and function is increase in the activities of transaminases (AST and ALT) in the serum. They play a role in amino acids catabolism and biosynthesis. ALP mainly reaches the liver from bone, excreted into the bile; therefore its elevation in serum can be associated with hepatobiliary disease [48]. The present study revealed that prallethrin-induction in rats remarkably increased the level of ALT, AST, and ALP. This increase may be indicative of initial cell injury occurring in advance of gross hepatic pathology. It causes hepatocyte injuries and altered membrane integrity and as a result enzymes in hepatocytes leak out [49]. The activities of transaminases and ALP were increased in rat after exposure to prallethrin [50], permethrin [51], fluvalinate [52], cypermethrin and deltamethrin [53], and fenvalerate in buffalo calves [54]. However, coadministration of EO to prallethrin intoxicated rats decrease ALT, AST, and ALP activity to within normal levels. These results indicated the ability of EO to protect against prallethrin-induced hepatocyte injury, which is in agreement with a previous study [55] that reported the protective consequence of polyphenolic compounds against xenobiotic-induced liver injury.
Reactive oxygen species (ROS) are causally related to oxidative stress. Many studies have demonstrated that overproduction of ROS can further aggravate oxidative stress and have implicated ROS in a number of disease processes, including heart disease [56], diabetes [57], liver injury [19, 20, 43, 58, 59], cancer [60], and aging [61]. Maintaining the balance between ROS and antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT), and glutathione-s-transferase (GST), is, therefore, crucial and could be an important mechanism for preventing damage by oxidative stress. This balance has been suggested to have an important role in preventing pesticides toxicity [19, 20, 43, 58, 59].
In fact, liver was the major site of pyrethroid metabolism which accumulated a great concentration of its metabolites [62, 63]. Their toxic effects occurred probably through generation of reactive oxygen species causing damage to various membranous components of the cell. Our results revealed that prallethrin caused a statistically significant decrease in the activity of SOD, CAT, and GST in liver of rats. SOD catalyses the dismutation of superoxide anion (O2 ∙−) to H2O2 and O2. Because H2O2 is still harmful to cells, CAT catalyses the decomposition of H2O2 to water. GST is a detoxifying enzyme that catalyzes the conjugation of a variety of electrophilic substrates to the thiol group of GSH, producing less toxic forms [64]. Thus, the coordinate actions of various cellular antioxidants in mammalian cells are critical for effectively detoxifying free radicals. Therefore, any impairment in this pathway will affect the activities of other enzymes in the cascade [65, 66]. However, reduction in the activity of SOD will result in an increased level of O2 ∙−, while a decrease in the activity of CAT will lead to accumulation of H2O2 in the cell, which leads to peroxidation of membrane lipids via Fenton-type reaction. TBARS, the final metabolites of peroxidized polyunsaturated fatty acids, are considered as a late biomarker of oxidative stress [67] and are a good indicator of the degree of lipid peroxidation [16]. In the present study, we observed significant increase (P ≤ 0.05) in the levels of TBARS in liver of prallethrin-treated rat. Our results revealed that prallethrin exposure induces oxidative stress in the liver of rat as indicated by decreased SOD, CAT, and GST activity and elevated TBARS concentrations, which would further induce lipid peroxidation, initiate free radicals damage to hepatocellular membrane, and lead to liver injury. The possible explanation for this effect could be that the increase in the formation of liver lipid peroxidation in prallethrin-intoxicated animals acted as a signal to maintain lower levels of antioxidant enzymes (SOD, CAT, and GST) in order to enhance the triggering of the detoxification process for the pyrethroid. So, the decrease in the activity of SOD, CAT, and GST in prallethrin-intoxicated animals indicates insufficient detoxification of prallethrin in rats. The decline of antioxidant enzymes activity, in our study, supported earlier findings [68, 69] which demonstrated that exposure of rats to pyrethroids decreased antioxidant enzymes activity. The increment in lipid peroxidation, as assessed by the elevated levels of TBARS following insecticides administration, has been well documented [16, 19, 29, 59, 70, 71]. Cypermethrin exposure to rats resulted in free radical-mediated tissue damage as indicated by elevated cerebral and hepatic lipid peroxidation [62]. Cypermethrin and fenvalerate increased the oxidative stress and LPO in liver, kidneys, and heart tissues of rats [72]. Our results corroborated previous reports [73–75]who have demonstrated that pyrethroids exposure like fenvalerate and deltamethrin altered antioxidant defense mechanisms and enhanced lipid peroxidation in rat liver. Therefore, oxidative stress and LPO has been implicated in the toxicology of pyrethroids [70, 71]. In fact, LPO alters the physiological functions of cell membranes and plays an important role in cellular membrane damage. It has been shown to perturb the bilayer structure and modify membrane properties such as membrane fluidity, permeability to different substances, and bilayer thickness.
The elevation of AST, ALT, and ALP enzymes activity in this study suggests probable liver tissue damage due to prallethrin as evidenced by the histopathological lesions like dilatation, congestion, oedema, inflammatory cells, and necrosis. Regardless of the causing agent of the hepatic lesion, the liver will apparently react in five ways: (1) necrosis, (2) degeneration, (3) inflammation, (4) regeneration, and (5) fibrosis. Necrosis may follow practically any lesion whose changes are significant, taking a toll on hepatocytes. However, before it becomes characteristically necrotic, hepatocytes may become swollen and edematous, with irregularly compact cytoplasm and great clear spaces. The decrease of CAT, SOD, and GST activities and increased TBARS level suggest that prallethrin causes hepatic damage and pathogenesis may be through the generation of free radicals and oxidative damage which certainly play a vital role in the pathogenesis of liver injury. The present study has demonstrated that the EO exerts a hepatoprotective effect against prallethrin-induced hepatotoxicity in rat. Increased levels of antioxidant enzymes and a reduction in the amount of lipid peroxides are likely to be the major mechanisms by which EO prevents development of the liver damage induced by prallethrin. Supporting this hypothesis, we observed significant increase in SOD, CAT, and GST activity and decrease in the levels of TBARS in liver of prallethrin-treated rat by the administration of EO. This might be due to hydroxyl radicals scavenging activities of EO. The EO of O. majorana shows potent antioxidant activity and many antioxidant components are found in EO [76–78]. The high potential of phenolics components to scavenger radicals might be explained by their ability to donate a hydrogen atom from their phenolic hydroxyl groups [27]. It contains phenolic terpenoids (thymol, carvacrol), flavonoids (diosmetin, luteolin, and apigenin), tannins, hydroquinone, phenolic glycosides (arbutin, methyl arbutin, vitexin, and orientinthymonin), triacontane, sitosterol, oleanolic acid and cis-sabinene hydrate [79–82]. It has been reported that the antioxidant activity of EOs could not be attributed to the major compounds, and minor compounds might play a significant role in the antioxidant activity, and synergistic effects were reported [77]. In the present study, the most prominent components of O. majorana L. EO were 4-terpineol (29.97%), γ-terpinene (15.40%), trans-sabinene hydrate (10.93), α-terpinene (6.86%), and 3-cycolohexene-1-1 methanal,a,a4-trimethyl-,(S)-(CAS) (6.54%) [27]. Therefore, the possible mechanisms of O. majorana EO hepatoprotective could arise from the free radical scavenging effect, preventing lipid peroxidation and improvement of the antioxidant/detoxification system in liver. Furthermore, the free radical scavenger effect of O. majorana EO has been reported by many authors [77, 83–86]. Several studies have indicated that treatment with antioxidants can ameliorate the toxicity of pyrethroids [71, 87].
5. Conclusion
In view of the data of the present study, it can deduce that prallethrin caused oxidative damage and liver injury in male rats. These results could be useful for increasing information on the potential toxicity of this pyrethroid. The coadministration of O. majorana EO attenuated the toxic effect of prallethrin. These results demonstrate that administration of EO appeared to be a promising agent for protection against prallethrin-induced oxidative damage and hepatotoxicity. Therefore, administration of O. majorana may be useful, easy, and economical to protect humans exposed to pyrethroids against their toxic effects.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
The authors thank the Academy of Scientific Research & Technology (ASRT, Egypt) and IMRCP UMR 5623, Faculty of Pharmacy, University Paul Sabatier (France), for supporting this study within the IMHOTEP program (Project no. 109/EGY/FR 8-02). Also, the authors are grateful to Professor Dr. Adel Mohamed Bakeer Kholoussy, Professor of Pathology, Faculty of Veterinary Medicine, Cairo University, for reading the histopathological sections.
References
- 1.Pauluhn J. Hazard identification and risk assessment of pyrethroids in the indoor environment. Toxicology Letters. 1999;107(1–3):193–199. doi: 10.1016/s0378-4274(99)00047-8. [DOI] [PubMed] [Google Scholar]
- 2.Aprea C, Stridori A, Sciarra G. Analytical method for the determination of urinary 3-phenoxybenzoic acid in subjects occupationally exposed to pyrethroid insecticides. Journal of Chromatography B. 1997;695(2):227–236. doi: 10.1016/s0378-4347(97)00190-4. [DOI] [PubMed] [Google Scholar]
- 3.Anand SS, Kim K-B, Padilla S, et al. Ontogeny of hepatic and plasma metabolism of deltamethrin in vitro: role in age-dependent acute neurotoxicity. Drug Metabolism and Disposition. 2006;34(3):389–397. doi: 10.1124/dmd.105.007807. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge WN. An assessment of the toxicological properties of pyrethroids and their neurotoxicity. Critical Reviews in Toxicology. 1990;21(2):89–104. doi: 10.3109/10408449009089874. [DOI] [PubMed] [Google Scholar]
- 5.Vijverberg HPM, van der Zalm JM, van Den Bercken J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature. 1982;295(5850):601–603. doi: 10.1038/295601a0. [DOI] [PubMed] [Google Scholar]
- 6.Bradbury SP, Coats JR. Comparative toxicology of the pyrethroid insecticides. Reviews of Environmental Contamination and Toxicology. 1989;108:133–177. doi: 10.1007/978-1-4613-8850-0_4. [DOI] [PubMed] [Google Scholar]
- 7.Mandhane SN, Chopde CT. Neurobehavioral effects of low level fenvalerate exposure in mice. Indian Journal of Experimental Biology. 1997;35(6):623–627. [PubMed] [Google Scholar]
- 8.López O, Hernández AF, Rodrigo L, et al. Changes in antioxidant enzymes in humans with long-term exposure to pesticides. Toxicology Letters. 2007;171(3):146–153. doi: 10.1016/j.toxlet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Glass R. Chronic and long-term effects of pesticides use in agriculture: current knowledge and limits. Toxicology Letters. 2008;180:p. S21. [Google Scholar]
- 10.Michelangeli F, Robson MJ, East JM, Lee AG. The conformation of pyrethroids bound to lipid bilayers. Biochimica et Biophysica Acta. 1990;1028(1):49–57. doi: 10.1016/0005-2736(90)90264-o. [DOI] [PubMed] [Google Scholar]
- 11.El-Demerdash FM. Lambda-cyhalothrin-induced changes in oxidative stress biomarkers in rabbit erythrocytes and alleviation effect of some antioxidants. Toxicology in Vitro. 2007;21(3):392–397. doi: 10.1016/j.tiv.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Fetoui H, Garoui EM, Makni-ayadi F, Zeghal N. Oxidative stress induced by lambda-cyhalothrin (LTC) in rat erythrocytes and brain: attenuation by vitamin C. Environmental Toxicology and Pharmacology. 2008;26(2):225–231. doi: 10.1016/j.etap.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine Pollution Bulletin. 2001;42(8):656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- 14.Sarkar SN, Balasubramanian SV, Sikdar SK. Effect of fenvalerate, a pyrethroid insecticide on membrane fluidity. Biochimica et Biophysica Acta. 1993;1147(1):137–142. doi: 10.1016/0005-2736(93)90324-s. [DOI] [PubMed] [Google Scholar]
- 15.Antunes-Madeira MC, Videira RA, Madeira VMC. Effects of parathion on membrane organization and its implications for the mechanisms of toxicity. Biochimica et Biophysica Acta. 1994;1190(1):149–154. doi: 10.1016/0005-2736(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 16.Mansour SA, Mossa A-TH. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. Pesticide Biochemistry and Physiology. 2009;93(1):34–39. [Google Scholar]
- 17.Perez-Albarsanz MA, Lopez-Aparicio P, Senar S, Recio MN. Effects of lindane on fluidity and lipid composition in rat renal cortex membranes. Biochimica et Biophysica Acta. 1991;1066(2):124–130. doi: 10.1016/0005-2736(91)90178-b. [DOI] [PubMed] [Google Scholar]
- 18.Jones OT, Lee AG. Effects of pyrethroids on the activity of a purified (Ca2+-Mg2+)-ATPase. Pesticide Biochemistry and Physiology. 1986;25(3):420–430. [Google Scholar]
- 19.Mansour SA, Mossa A-TH. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pesticide Biochemistry and Physiology. 2010;96(1):14–23. [Google Scholar]
- 20.Mansour SA, Mossa AH. Adverse effects of lactational exposure to chlorpyrifos in suckling rats. Human and Experimental Toxicology. 2010;29(2):77–92. doi: 10.1177/0960327109357276. [DOI] [PubMed] [Google Scholar]
- 21.Matsunaga MM, Higo A, Nishibe I, Dohara KA, Shinjo J. Studies on prallethrin, a new synthetic pyrethroid for indoor applications. The insecticidal activities of prallethrin. Japanese Journal of Sanitary Zoology. 1987;38:219–223. [Google Scholar]
- 22.Akhtar A, Deshmukh AA, Raut CG, Somkuwar AP, Bhagat SS. Prallethrin induced serum biochemical changes in Wistar rats. Pesticide Biochemistry and Physiology. 2012;102(2):160–168. [Google Scholar]
- 23.van Den Broucke CO, Lemli JA. Antispasmodic activity of Origanum compactum. Planta Medica. 1980;38(4):317–331. doi: 10.1055/s-2008-1074884. [DOI] [PubMed] [Google Scholar]
- 24.Leung AY. Encyclopedia of Common Natural Ingredients Used in Food Drugs and Cosmetics. New York, NY, USA: John Wiley & Sons; 1980. [Google Scholar]
- 25.Assaf MH, Ali AA, Makboul MA. Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Medica. 1987;53(4):343–345. doi: 10.1055/s-2006-962734. [DOI] [PubMed] [Google Scholar]
- 26.Simon JE, Chadwick AF, Craker LE. An indexed Bibliography 1971–1980. The scientific literature on selected herbs and aromatic and medicinal plants of temperate zone. In: Hamden CT, editor. Archon Books. 1984. p. p. 770. [Google Scholar]
- 27.Mossa ATH, Nawwar GAM. Free radical scavenging and antiacetylcholinesterase activities of Origanum majorana L. essential oil. Human and Experimental Toxicology. 2011;30(10):1501–1513. doi: 10.1177/0960327110391686. [DOI] [PubMed] [Google Scholar]
- 28.Tomlin CDS. The e-Pesticide Manual. 13th edition. Farnham, UK: The British Crop Protection Council; 2004. [Google Scholar]
- 29.El-Ashmawy IM, El-Nahas AF, Salama OM. Protective effect of volatile oil, alcoholic and aqueous extracts of Origanum majorana on lead acetate toxicity in mice. Basic and Clinical Pharmacology and Toxicology. 2005;97(4):238–243. doi: 10.1111/j.1742-7843.2005.pto_136.x. [DOI] [PubMed] [Google Scholar]
- 30.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Young DS, Pestaner LC, Gibberman V. Effects of drugs on clinical laboratory tests. Clinical Chemistry. 1975;21(5):D431–D432. [PubMed] [Google Scholar]
- 32.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clinica Chimica Acta. 1978;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 33.Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 34.Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 35.Habig WH, Pabst MJ, Jakoby WB. Glutathione S transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 36.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. The Journal of Biological Chemistry. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 37.Michael JD. The Toxicologist's Pocket Handbook. 2nd edition. Informa Healthcare USA: New York, NY, USA; 2008. [Google Scholar]
- 38.Shakoori AR, Ali SS, Saleem MA. Effects of six months’ feeding of cypermethrin on the blood and liver of albino rats. Journal of Biochemical Toxicology. 1988;3:59–71. doi: 10.1002/jbt.2570030107. [DOI] [PubMed] [Google Scholar]
- 39.Gupta PK, Kumar S. Cumulative toxicity of deltamethrin in mice. Journal of Environmental Biology. 1991;12(1):45–50. [Google Scholar]
- 40.Singh VK, Dixit P, Saxena PN. Cybil induced hepatobiochemical changes in wistar rats. Journal of Environmental Biology. 2005;26(4):725–727. [PubMed] [Google Scholar]
- 41.Extoxnet (Extension Toxicology Network) Cypermethrin, Cooperative Extension offices of Cornell University, Michigan State University, Oregon State University, and University of California, Davis, 1993.
- 42.Grue CE. Response of common grackles to dietary concentrations of four organophosphate pesticides. Archives of Environmental Contamination and Toxicology. 1982;11(5):617–626. doi: 10.1007/BF01056371. [DOI] [PubMed] [Google Scholar]
- 43.Mossa AH. Genotoxicity of pesticides [Ph.D. thesis] Pesticide Chemistry and Toxicology, Faculty of Agriculture, Damanhour, Alexandria University; 2004. [Google Scholar]
- 44.Mossa AH, Refaie AA, Ramadan A. Effect of exposure to mixture of four organophosphate insecticides at no observed adverse effect level dose on rat liver: the protective role of vitamin C. Research Journal of Environmental Toxicology. 2011;5(6):323–335. [Google Scholar]
- 45.Guyton AC, Hall JE. Text Book of Medical Physiology. 9th edition. Bangalore, India: Prism Book; 1996. [Google Scholar]
- 46.Hodgson EA. Textbook of Modern Toxicology. 3rd edition. Hoboken, NJ, USA: John Wiley & Sons; 2004. [Google Scholar]
- 47.Hayes RB, Sheffet A, Spirtas R. Cancer mortality among a cohort of chromium pigment workers. American Journal of Industrial Medicine. 1989;16(2):127–133. doi: 10.1002/ajim.4700160204. [DOI] [PubMed] [Google Scholar]
- 48.Burtes CA, Ashwood ER. Text Book of Clinical Chemistry. Philadelphia, Pa, USA: W. B. Sunders; 1986. [Google Scholar]
- 49.Cheng HL, Hu YY, Wang RP, Liu C, Liu P, Zhu DY. Protective actions of salvianolic acid A on hepatocyte injured by peroxidation in vitro. World Journal of Gastroenterology. 2000;6(3):402–404. doi: 10.3748/wjg.v6.i3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akhtar A, Deshmukh AA, Raut CG, Somkuwar AP, Bhagat SS. Prallethrin induced serum biochemical changes in Wistar rats. Pesticide Biochemistry and Physiology. 2012;102(2):160–168. [Google Scholar]
- 51.Shah MA, Gupta PK. Subacute toxicity studies on permethrin. Indian Journal of Toxicology. 2001;8(1):61–67. [Google Scholar]
- 52.Garg UK, Pal AK, Jha GJ, Jadhao SB. Haemato-biochemical and immuno-pathophysiological effects of chronic toxicity with synthetic pyrethroid, organophosphate and chlorinated pesticides in broiler chicks. International Immunopharmacology. 2004;4(13):1709–1722. doi: 10.1016/j.intimp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Abbassy M, Mossa AH. Haemato-biochemical effects of formulated and technical cypermethrin and deltamethrin insecticides in male rats. Journal of Pharmacology and Toxicology. 2012;7:312–321. [Google Scholar]
- 54.Singh G, Sharma LD, Ahmad AH, Singh SP. Fenvalerate induced dermal toxicity in buffalo calves. Journal of Applied Animal Research. 1999;16(2):205–210. [Google Scholar]
- 55.Xiao-hui H, Liang-qi C, Xi-ling C, Kai S, Yun-jian L, Long-juan Z. Polyphenol epigallocatechin-3-gallate inhibits oxidative damage and preventive effects on carbon tetrachloride-induced hepatic fibrosis. The Journal of Nutritional Biochemistry. 2007;3:511–515. doi: 10.1016/j.jnutbio.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 56.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. Journal of Clinical Investigation. 2005;115(3):500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicology and Applied Pharmacology. 2006;212(2):167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury. Journal of Gastroenterology and Hepatology. 2000;15(7):718–724. doi: 10.1046/j.1440-1746.2000.02207.x. [DOI] [PubMed] [Google Scholar]
- 59.Mansour SA, Mossa A-TH. Adverse effects of exposure to low doses of chlorpyrifos in lactating rats. Toxicology and Industrial Health. 2011;27(3):213–224. doi: 10.1177/0748233710384054. [DOI] [PubMed] [Google Scholar]
- 60.Klaunig JE, Kamendulis LM. The Role of oxidative stress in carcinogenesis. Annual Review of Pharmacology and Toxicology. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- 61.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mechanisms of Ageing and Development. 2004;125(10-11):811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 62.Giray B, Gürbay A, Hincal F. Cypermethrin-induced oxidative stress in rat brain and liver is prevented by Vitamin E or allopurinol. Toxicology Letters. 2001;118(3):139–146. doi: 10.1016/s0378-4274(00)00277-0. [DOI] [PubMed] [Google Scholar]
- 63.Rickard J, Brodie ME. Correlation of blood and brain levels of the neurotoxic pyrethroid deltamethrin with the onset of symptoms in rats. Pesticide Biochemistry and Physiology. 1985;23(2):143–156. [Google Scholar]
- 64.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Critical Reviews in Biochemistry and Molecular Biology. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 65.Sinet PM, Garber P. Inactivation of the human CuZn superoxide dismutase during exposure to O 2 − and H2O2 . Archives of Biochemistry and Biophysics. 1981;212(2):411–416. doi: 10.1016/0003-9861(81)90382-9. [DOI] [PubMed] [Google Scholar]
- 66.Kono Y, Fridovich I. Superoxide radical inhibits catalase. The Journal of Biological Chemistry. 1982;257(10):5751–5754. [PubMed] [Google Scholar]
- 67.Cheeseman KH. Mechanisms and effects of lipid peroxidation. Molecular Aspects of Medicine. 1993;14(3):191–197. doi: 10.1016/0098-2997(93)90005-x. [DOI] [PubMed] [Google Scholar]
- 68.Sinha C, Seth K, Islam F, et al. Behavioral and neurochemical effects induced by pyrethroid-based mosquito repellent exposure in rat offsprings during prenatal and early postnatal period. Neurotoxicology and Teratology. 2006;28(4):472–481. doi: 10.1016/j.ntt.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Kanbur M, Liman BC, Eraslan G, Altinordulu S. Effects of cypermethrin, propetamphos, and combination involving cypermethrin and propetamphos on lipid peroxidation in mice. Environmental Toxicology. 2008;23(4):473–479. doi: 10.1002/tox.20360. [DOI] [PubMed] [Google Scholar]
- 70.Banerjee BD, Seth V, Bhattacharya A, Pasha ST, Chakraborty AK. Biochemical effects of some pesticides on lipid peroxidation and free-radical scavengers. Toxicology Letters. 1999;107(1–3):33–47. doi: 10.1016/s0378-4274(99)00029-6. [DOI] [PubMed] [Google Scholar]
- 71.Abdollahi M, Ranjbar A, Shadnia S, Nikfar S, Rezaie A. Pesticides and oxidative stress: a review. Medical Science Monitor. 2004;10(6):RA141–RA147. [PubMed] [Google Scholar]
- 72.Kale M, Rathore N, John S, Bhatnagar D. Lipid peroxidative damage on pyrethroid exposure and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicology Letters. 1999;105(3):197–205. doi: 10.1016/s0378-4274(98)00399-3. [DOI] [PubMed] [Google Scholar]
- 73.Prasanthi K, Muralidhara M, Rajini PS. Fenvalerate-induced oxidative damage in rat tissues and its attenuation by dietary sesame oil. Food and Chemical Toxicology. 2005;43(2):299–306. doi: 10.1016/j.fct.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Tuzmen N, Candan N, Kaya E, Demiryas N. Biochemical effects of chlorpyrifos and deltamethrin on altered antioxidative defense mechanisms and lipid peroxidation in rat liver. Cell Biochemistry and Function. 2008;26(1):119–124. doi: 10.1002/cbf.1411. [DOI] [PubMed] [Google Scholar]
- 75.Yousef MI, Awad TI, Mohamed EH. Deltamethrin-induced oxidative damage and biochemical alterations in rat and its attenuation by Vitamin E. Toxicology. 2006;227(3):240–247. doi: 10.1016/j.tox.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 76.Ruberto G, Baratta MT. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chemistry. 2000;69(2):167–174. [Google Scholar]
- 77.Singh G, Marimuthu P, de Heluani CS, Catalan C. Antimicrobial and antioxidant potentials of essential oil and acetone extract of Myristica fragrans Houtt. (aril part) Journal of Food Science. 2005;70(2):M141–M148. [Google Scholar]
- 78.Kim H-J, Chen F, Wu C, Wang X, Chung HY, Jin Z. Evaluation of antioxidant activity of australian tea tree (Melaleuca alternifolia) oil and its components. Journal of Agricultural and Food Chemistry. 2004;52(10):2849–2854. doi: 10.1021/jf035377d. [DOI] [PubMed] [Google Scholar]
- 79.Sawa T, Nakao M, Akaike T, Ono K, Maeda H. Alkylperoxyl radical-scavenging activity of various flavonoids and other phenolic compounds: implications for the anti-tumor-promoter effect of vegetables. Journal of Agricultural and Food Chemistry. 1999;47(2):397–402. doi: 10.1021/jf980765e. [DOI] [PubMed] [Google Scholar]
- 80.Leung YA. Encyclopedia of Common Natural Ingredients Used in Food Drug and Cosmetics. New York, NY, USA: John Wiley & Sons; 1980. [Google Scholar]
- 81.Assaf MH, Ali AA, Makboul MA. Preliminary study of phenolic glycosides from Origanum majorana; quantitative estimation of arbutin; cytotoxic activity of hydroquinone. Planta Medica. 1987;53(4):343–345. doi: 10.1055/s-2006-962734. [DOI] [PubMed] [Google Scholar]
- 82.Novak J, Langbehn J, Pank F, Franz CM. Essential oil compounds in a historical sample of marjoram (Origanum majorana L., Lamiaceae) Flavour and Fragrance Journal. 2002;17(3):175–180. [Google Scholar]
- 83.Roth LS. Mosby's Handbook of herbs and Natural Supplements. Mosby, A Harcort Health Sciences; 2001. [Google Scholar]
- 84.Lamaison JL, Petitjean-Freytet C, Carnat A. Medicinal lamiaceae with antioxidative activities, potential sources of rosmarinic acid. Pharmaceutica Acta Helvetiae. 1991;66(7):185–188. [PubMed] [Google Scholar]
- 85.Pieroni A, Janiak V, Dürr CM, Lüdeke S, Trachsel E, Heinrich M. In vitro antioxidant activity of non-cultivated vegetables of ethnic Albanians in southern Italy. Phytotherapy Research. 2002;16(5):467–473. doi: 10.1002/ptr.1243. [DOI] [PubMed] [Google Scholar]
- 86.Dorman HJD, Bachmayer O, Kosar M, Hiltunen R. Antioxidant properties of aqueous extracts from selected lamiaceae species grown in Turkey. Journal of Agricultural and Food Chemistry. 2004;52(4):762–770. doi: 10.1021/jf034908v. [DOI] [PubMed] [Google Scholar]
- 87.Aldana L, Tsutsumi V, Craignill A, Silveria MI, Eijia JG. Alpha Tocopherol Modulates Liver Toxicity of Pyrethroid Cypermethrin, Toxicology Letters. 2001;25 doi: 10.1016/s0378-4274(01)00427-1. [DOI] [PubMed] [Google Scholar]


