Abstract
Scleroderma is a systemic autoimmune disease of unknown etiology whose hallmark features include endothelial cell dysfunction, fibroblast proliferation and immune dysregulation. Although virtually any organ can be pathologically involved in scleroderma, lung complications including interstitial lung disease (ILD) and pulmonary arterial hypertension (PAH) are the leading cause of death in patients with this condition. Currently, the molecular mechanisms leading to development of scleroderma-related lung disease are poorly understood; however, the systemic nature of this condition has led many to implicate circulating factors in the pathogenesis of some of its organ impairment. In this article, we focus on a new class of circulating factors derived from adipose-tissue called adipokines, which are known to be altered in scleroderma. Recently, the adipokines adiponectin and leptin have been found to regulate biological activities in endothelial, fibroblast and immune cell types in lung and in many other tissues. The pleiotropic nature of these circulating factors and their functional activity on many cell types implicated in the pathogenesis of ILD and PAH suggest these hormones may play a mechanistic role in the onset and/or progression of scleroderma-related lung diseases.
Keywords: Scleroderma, Adipokines, Adiponectin, Leptin, Interstitial lung disease, Pulmonary fibrosis, Pulmonary hypertension
Introduction
Scleroderma is a progressive, systemic disease characterized by vasculopathy and excessive collagen deposition in the skin and internal organs. Scleroderma can affect almost any organ in the body but lung manifestations, including PAH and ILD, are its most serious complications[1]. It is estimated that 60% of scleroderma-related deaths are attributable to lung involvement, and currently there are few effective treatments for these conditions[2].
The systemic nature of scleroderma as well as its involvement of tissues from diverse vascular beds has led many to implicate serum-derived factors in its pathogenesis. In the 1990's, the observation that an adipose tissue-derived hormone called leptin regulates appetite in the brain led to the immediate recognition of adipose tissue as an important endocrine organ[3]. Since that time, many other adipose-derived signaling factors have been identified and these hormones are now collectively referred to as adipokines. Adipokines act on virtually all tissues and regulate biological processes important in metabolism, immune regulation, vascular homeostasis and cell proliferation[4–8]. Although much of what we know about the functional role of adipokines is linked to obesity it is now increasingly apparent that endocrine function of adipose tissue is also altered in many other chronic conditions including connective tissue diseases[9–11]. This observation has led to emerging interest in understanding how adipose tissue dysfunction contributes to disease pathogenesis in non-obese individuals.
In this review, we will focus on the potential role of the adipokines adiponectin and leptin in ILD and PAH pathogenesis. We have elected to limit our discussion to adiponectin and leptin, the two most abundant hormones produced by adipose tissue, because each has well-documented activities in lung homeostasis[12–14]. The primary goal of this review is to stimulate further discussion on the possible role for adipokines in ILD and PAH pathogenesis and to promote further research in this new and exciting area of lung biology.
Interstitial Lung Disease and Pulmonary Arterial Hypertension
ILD and PAH are highly complex diseases and a full discussion of these conditions is beyond the scope of this review. For a more complete understanding of either disease, we refer the reader to one of several recent review articles[15–18].
Importantly, ILD is a non-specific term that refers to any chronic inflammatory disease of the lung interstitium. However, in patients with scleroderma, the term ILD often connotes a more serious condition that is associated with progressive scarring of the lung and portends a poor prognosis. The precise incidence of ILD in patients with scleroderma varies depending on how it is defined; more sensitive measurements such as high resolution CT scanning of the lung suggest that interstitial lung abnormalities are present in most patients with this disease[2]. Fortunately, life-threatening ILD occurs in only one-fifth of individuals with scleroderma [19]. Although immunosuppressive agents have been shown to slow the progression of ILD in some patients with scleroderma the overall efficacy of these treatments is quite limited[20].
In contrast to ILD, PAH is a disease that is confined to the pulmonary vasculature and is diagnosed based on sustained elevations in pulmonary artery pressures. In patients with scleroderma, PAH has a well-documented increased morbidity and mortality. The prevalence of PAH in scleroderma varies depending on whether patients have limited or diffuse disease but overall it is estimated that one-quarter of patients develop this condition[21]. Recent studies suggest that new treatments have improved mortality for PAH over the last decade [22]. However, despite the availability of these new pharmacological therapies, response to treatment is often transient and three-year mortality for scleroderma-associated PAH remains very high (25%) [22–24].
Scleroderma lung diseases: Are ILD and PAH connected?
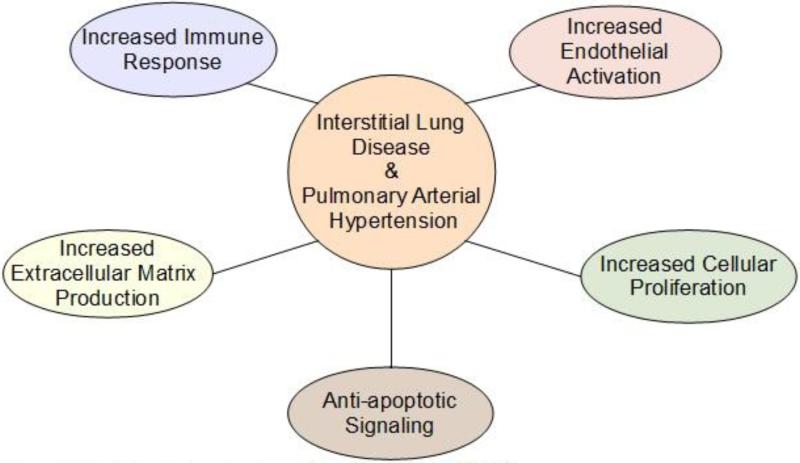
As highlighted above, ILD and PAH are ostensibly very different conditions and for this reason are usually discussed in separate contexts. However, the fact that they often co-exist in scleroderma, and in other diseases, strongly suggests a pathogenic link between these conditions. Relevant to this, when direct comparisons are made between these conditions (Figure 1) several striking similarities emerge. First, ILD and PAH are both diseases that involve predominantly cells of mesenchymal origin (e.g. fibroblast, myofibroblast, endothelial and smooth muscle cell types)[15,17]. Second, chronic inflammation plays an important role in both conditions and similar types of immune cells and pro-inflammatory cytokines are observed in pathological specimens from patients with these diseases[25]. Moreover, ILD and PAH share many pathogenic mechanisms including altered endothelial cell function, enhanced cell proliferation, increased apoptotic cell death and impaired tissue remodeling further suggesting a commonality between these two conditions[15,17]. Although the precise molecular signals linking these two diseases are unknown, the possibility that adipokines adiponectin and leptin modulate processes central to the pathogenesis of ILD and PAH will be discussed below.
Figure 1.
Pathological mechanisms shared by ILD and PAH.
Adiponectin
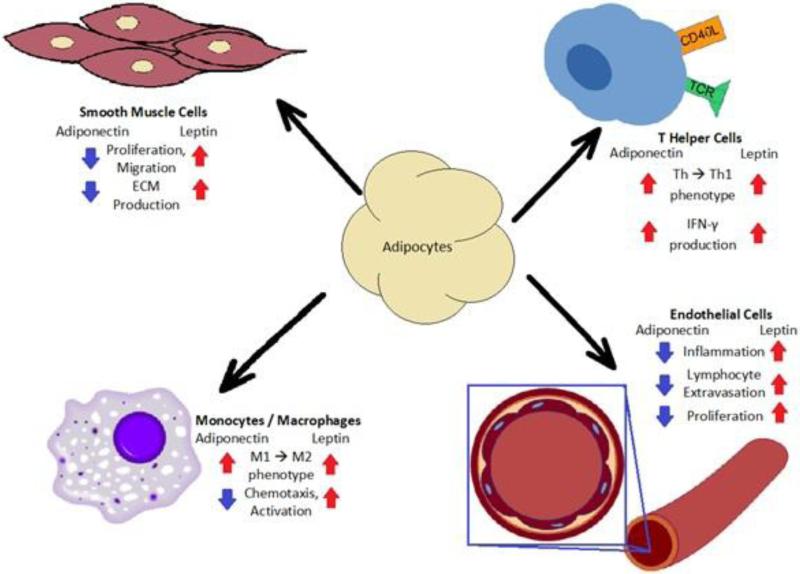
Adiponectin is perhaps the most important adipokine secreted from adipose tissue because of its well-documented role in regulating metabolism, inflammation, vascular homeostasis and tissue remodeling[8,26–29]. The ability of adiponectin to regulate diverse biological processes in many different cells (Figure 2) is attributable in part to its unique collagen-like domain that enables protein monomers to form small, medium and large complexes that possess distinct functional capabilities[30].
Figure 2.
Schematic depicting the pleiotropic effects of adiponectin and leptin.
Interestingly, and for reasons that are unclear, the concentration of adiponectin in the plasma far exceeds that of most other circulating hormones[31]. Moreover, plasma levels of adiponectin in healthy individuals are remarkably stable over time suggesting that maintenance of high serum concentrations is somehow important for organ homeostasis[32]. Consistent with this hypothesis, adiponectin production is down-regulated in response to pro-inflammatory cytokines and oxidative stress in obese subjects, and this reduction has been shown in mice to contribute, at least in part, to the pathogenesis of many obesity-related diseases including type II diabetes, systemic hypertension and peripheral vascular disease[33,34]. Relevant to this review, adiponectin production is also impaired in scleroderma and other connective tissue diseases; however, the impact of these changes on disease progression is currently not understood [35–37].
In addition to having well-described anti-diabetic properties, one of the most recognized functions of adiponectin is its ability to suppress vascular inflammation [8,27,29]. Although adiponectin has been shown to inhibit inflammatory responses in virtually every cell type the importance of adiponectin in controlling vascular homeostasis is highlighted by studies in adiponectin-deficient mice. Targeted deletion of the adiponectin gene leads to the development of a spontaneous lung phenotype characterized by activated lung endothelium, age-dependent increases in perivascular immune cell infiltration and increased pulmonary artery pressures[12]. In addition, adiponectin deficient mice also display an exaggerated eosinophilic inflammatory vascular response to allergic lung challenge that results in increased muscularization of the pulmonary arteries and worsening pulmonary hypertension[28]. Importantly, blocking immune cell infiltration in this model attenuates the development of pulmonary hypertension suggesting that anti-inflammatory actions of adiponectin are important for limiting the development of PAH. To our knowledge, no human studies have yet to explore the relationship between adiponectin and PAH in scleroderma.
As discussed above, adiponectin's anti-inflammatory activities are also mediated outside the vascular compartment. This is likely attributable to the fact that adiponectin has been shown to readily accumulate within most tissues[38,39]. Interestingly, high concentrations of adiponectin are reported in the airway lining fluid from the human and murine lung, and its accumulation has been shown to be dependent on the expression of T-cadherin in the lung endothelium[38,40]. Studies in mice demonstrate that deficiency of lung adiponectin leads to spontaneous activation of alveolar macrophage and to architectural distortion of distal airspaces of the lung[41]. Although structural changes are presumably related to increased pro-inflammatory cytokine production from alveolar macrophages it is equally plausible that changes result from direct loss of adiponectin actions on the lung's epithelium. Indeed, adiponectin receptor 1 has been shown to be expressed on lung epithelium suggesting that adiponectin may be important for regulating epithelial cell homeostasis[40]. This ability of adiponectin to modulate cellular processes in both the intra and extravascular compartments of the lung provide, at very least, a plausible explanation for how adiponectin could serve as a molecular link for two anatomically distinct lung diseases.
In addition to its role in controlling inflammation, there is now strong evidence that adiponectin is also an important regulator of tissue remodeling and cell proliferation in the lung[28,29,42,43]. For example, recent studies have demonstrated that deficiency in adiponectin promotes pulmonary artery smooth muscle proliferation in the setting of vascular inflammation and chronic hypoxia[42]. The molecular signals mediating these effects are poorly understood but several different mechanisms have been proposed including 1) direct activation of pathways that inhibit growth factor-mediated activation, 2) inhibition of the differentiation of smooth muscle cells into a proliferative phenotype, and 3) binding growth factors and hindering their bioavailability at a receptor level[5,29,44,45].
Importantly, adiponectin is also likely to influence tissue remodeling in the lung by its effects on non-smooth muscle cell types. This is supported by numerous studies demonstrating that adiponectin effectively inhibits tissue remodelling in many other tissues. For example, adiponectin has been shown to inhibit hepatic stellate cell proliferation and attenuate liver fibrosis, and to prevent myocardial fibrosis associated with pressure overload and ischemia[37,46]. Relevent to scleroderma, tissue levels of adiponectin have been shown to inversely correlate with skin scores in patients with diffuse disease[47]. Moreover, adiponectin has been demonstrated to suppress the expression of type I collagen in both normal and scleroderma fibroblasts, and to attenuate the stimulation of pro-fibrotic responses elicited by TGF-β through activation of adenosine monophosphate-activated protein kinase[48]. These findings suggest hypoadiponectinmia might be an important stimulus for pro-fibrotic responses (e.g. increased collagen production) in scleroderma.
Leptin
Leptin is also a multi-functional hormone involved in metabolism, immune regulation and tissue remodeling [5]. Leptin's metabolic actions are important for down-regulating feeding impulses in the hypothalamus of the brain. In lean individuals, leptin levels are reduced in the blood during fasting and production rapidly increases after eating[49]. Somewhat paradoxically, circulating levels of leptin are markedly increased in obese subjects and this is ascribed to receptor resistance and the loss of leptin's actions in the brain[50]. Relevant to this review, independent of body mass index, plasma levels of leptin are also impaired in patients with scleroderma suggesting that synthesis and/or activity is influenced by this disease[9].
The structural homology of leptin to the IL-6 family of cytokines led to early speculation about its immune-regulatory activities [51]. Since that time, it is now widely recognized that leptin functions as an important immune modulating protein. Overall, the predominant actions of leptin are in enhancing pro-inflammatory responses. Leptin has been shown to activate monocytes, dendritic cells and macrophages and to stimulate their production of pro-inflammatory cytokines[4,7,52]. In addition, leptin has been found to promote phagocytosis and augment the chemotaxis of antigen presenting cells into tissue [4,5]. Interestingly, although leptin is considered mostly a mediator of the innate immune system, studies in mice have found that leptin augments the development of chronic autoimmune diseases through its ability to modulate T-regulatory cell function[53].
The pleiotropic actions of leptin in immune regulation have led investigators to implicate leptin in the pathogenesis of many chronic inflammatory conditions[14,54] (Figure 2). To date, there are surprisingly very few studies that have investigated the role of leptin in the pathogenesis of scleroderma lung diseases. However, the limited data that is available suggests that leptin levels are increased in patients’ scleroderma and these findings correlated with other measures of inflammation (e.g. tumor necrosis factor alpha)[9]. While these findings provide only an association between leptin and scleroderma lung disease it is tempting to speculate that higher circulating levels may somehow act to exacerbate the pro-inflammatory state in these conditions.
Leptin receptors are ubiquitous in the lung and are found on many parenchymal cell types including endothelium, fibroblasts and the proximal and distal lung epithelium[55,56]. The functional role for these receptors appears to have less to do with promoting pro-inflammatory responses and more to do with enhancing cellular proliferation and tissue remodeling[7,57,58]. Leptin has been shown to augment cell growth in endothelial, fibroblast and epithelial cell populations but signaling pathways mediating these effects have not been well-characterized[58–60].
In addition to controlling proliferation, leptin also appears to play a role in enhancing tissue remodeling. This was exemplified in a recent study using mice with defective leptin receptor signaling (db/db). These mice were found to be resistant to the development of bleomycin-induced lung fibrosis[58]. Consistent with these findings, treatment of human lung fibroblasts with leptin in culture was found to enhance TGF-β–mediated expression of many profibrotic genes. Interestingly, leptin-induced expression of pro-fibrotic genes was not observed in fibroblast deficient in PPAR-γ activity suggesting that leptin's effects are dependent on PPAR-γ signaling. Since adiponectin expression is induced by PPAR-γ, these findings suggest there is a reciprocal relationship between leptin and adiponectin on signaling in fibrotic diseases [26].
Conclusions and Implications
Scleroderma related lung disease is a major cause of morbidity and mortality, but despite decades of research the mechanisms leading to the development of ILD and PAH remain poorly understood. The shared pathogenic mechanisms between these conditions have prompted investigators to search for new factors that might provide a link between these diseases. In this review, we discuss the possible role for the adipokines adiponectin and leptin in ILD and PAH pathogenesis. While this area of investigation is arguably in its infancy the pleiotropic nature of adiponectin and leptin suggest that future research in this area will provide valuable insight into the pathogenesis of these conditions.
Acknowledgment
This work was supported by the National Institutes of Health (NIH) grant no. R01HL105490.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Shannon Haley, Dilip Shah, Freddy Romero, and Ross Summer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Annals of the Rheumatic Diseases. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wells AU, Steen V, Valentini G. Pulmonary complications: one of the most challenging complications of systemic sclerosis. Rheumatology (Oxford, England) 2009;48(Suppl 3):iii40–4. doi: 10.1093/rheumatology/kep109. [DOI] [PubMed] [Google Scholar]
- 3.Leibel RL, Bahary N, Friedman JM. Genetic variation and nutrition in obesity: approaches to the molecular genetics of obesity. World Review of Nutrition and Dietetics. 1990;63:90–101. doi: 10.1159/000418501. [DOI] [PubMed] [Google Scholar]
- 4.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochemical and Biophysical Research Communications. 2009;384:311–5. doi: 10.1016/j.bbrc.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 5.Frühbeck G. Intracellular signalling pathways activated by leptin. The Biochemical Journal. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim GS, Hong JS, Kim SW, Koh J-M, An CS, Choi J-Y, et al. Leptin induces apoptosis via ERK/cPLA2/cytochrome c pathway in human bone marrow stromal cells. The Journal of Biological Chemistry. 2003;278:21920–9. doi: 10.1074/jbc.M204598200. [DOI] [PubMed] [Google Scholar]
- 7.Dreyer MG, Juge-Aubry CE, Gabay C, Lang U, Rohner-Jeanrenaud F, Dayer J-M, et al. Leptin activates the promoter of the interleukin-1 receptor antagonist through p42/44 mitogen-activated protein kinase and a composite nuclear factor kappa B/PU.1 binding site. The Biochemical Journal. 2003;370:591–9. doi: 10.1042/BJ20021270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomizawa A, Hattori Y, Kasai K, Nakano Y. Adiponectin induces NF-kappaB activation that leads to suppression of cytokine-induced NF-kappaB activation in vascular endothelial cells: globular adiponectin vs. high molecular weight adiponectin. Diabetes & Vascular Disease Research: Official Journal of the International Society of Diabetes and Vascular Disease. 2008;5:123–7. doi: 10.3132/dvdr.2008.020. [DOI] [PubMed] [Google Scholar]
- 9**.Pehlivan Y, Onat AM, Ceylan N, Turkbeyler IH, Buyukhatipoglu H, Comez G, et al. Serum leptin, resistin and TNF-α levels in patients with systemic sclerosis: the role of adipokines in scleroderma. International Journal of Rheumatic Diseases. 2012;15:374–9. doi: 10.1111/j.1756-185X.2012.01755.x. [This study is one of the first to investigate the relationship of circulating adipokines in scleroderma.] [DOI] [PubMed] [Google Scholar]
- 10.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflammatory Bowel Diseases. 2006;12:100–5. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 11.Kotulska A, Kucharz EJ, Brzezińska-Wcisło L, Wadas U. A decreased serum leptin level in patients with systemic sclerosis. Clinical Rheumatology. 2001;20:300–2. doi: 10.1007/s100670170053. [DOI] [PubMed] [Google Scholar]
- 12.Summer R, Fiack CA, Ikeda Y, Sato K, Dwyer D, Ouchi N, et al. Adiponectin deficiency: a model of pulmonary hypertension associated with pulmonary vascular disease. American Journal of Physiology Lung Cellular and Molecular Physiology. 2009;297:L432–8. doi: 10.1152/ajplung.90599.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Zhang J-P, Huang H, Wang Z-H, Cheng R, Cai W-B. Leptin promotes fetal lung maturity and upregulates SP-A expression in pulmonary alveoli type-II epithelial cells involving TTF-1 activation. PloS One. 2013;8:e69297. doi: 10.1371/journal.pone.0069297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vernooy JHJ, Ubags NDJ, Brusselle GG, Tavernier J, Suratt BT, Joos GF, et al. Leptin as regulator of pulmonary immune responses: involvement in respiratory diseases. Pulmonary Pharmacology & Therapeutics. 2013;26:464–72. doi: 10.1016/j.pupt.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. The American Journal of Pathology. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Hassoun PM. Lung involvement in systemic sclerosis. Presse Médicale (Paris, France: 1983) 2011;40:e3–e17. doi: 10.1016/j.lpm.2010.08.006. [This review provides the reader with a comprehensive overview of scleroderma-associated ILD and PAH.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, et al. Cellular and molecular basis of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2009;54:S20–31. doi: 10.1016/j.jacc.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18*.Mathai SC, Hassoun PM. Pulmonary arterial hypertension in connective tissue diseases. Heart Failure Clinics. 2012;8:413–25. doi: 10.1016/j.hfc.2012.04.001. [This article provides a nice review on pulmonary arterial hypertension in connective tissue disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benan M, Hande I, Gul O. The natural course of progressive systemic sclerosis patients with interstitial lung involvement. Clinical Rheumatology. 2007;26:349–54. doi: 10.1007/s10067-006-0302-6. [DOI] [PubMed] [Google Scholar]
- 20.Cappelli S, Guiducci S, Bellando Randone S, Matucci Cerinic M. Immunosuppression for interstitial lung disease in systemic sclerosis. European Respiratory Review. 2013;22:236–43. doi: 10.1183/09059180.00001813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coghlan JG, Mukerjee D. The heart and pulmonary vasculature in scleroderma: clinical features and pathobiology. Current Opinion in Rheumatology. 2001;13:495–9. doi: 10.1097/00002281-200111000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Chung L, Domsic RT, Lingala B, Alkassab F, Bolster M, Csuka ME, et al. Survival and predictors of mortality in systemic sclerosis associated pulmonary arterial hypertension: Outcomes from the PHAROS registry. Arthritis Care & Research. 2013 doi: 10.1002/acr.22121. [DOI] [PubMed] [Google Scholar]
- 23.Le Pavec J, Humbert M, Mouthon L, Hassoun PM. Systemic Sclerosis-associated Pulmonary Arterial Hypertension. American Journal of Respiratory and Critical Care Medicine. 2010;181:1285–93. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefèvre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: A systematic review and meta-analysis. Arthritis and Rheumatism. 2013 doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 25.Rubin R, Strayer DS. Interstitial Lung Disease; Pulmonary Hypertension. In: Rubin E, editor. Rubin's Pathology Clinicopathologic Foundations of Medicine. 5th Ed. Lippincott Williams & Wilkins; 2008. pp. 524–534.pp. 536–538. [Google Scholar]
- 26.Combs TP, Wagner JA, Berger J, Doebber T, Wang W-J, Zhang BB, et al. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143:998–1007. doi: 10.1210/endo.143.3.8662. [DOI] [PubMed] [Google Scholar]
- 27.Addabbo F, Nacci C, De Benedictis L, Leo V, Tarquinio M, Quon MJ, et al. Globular adiponectin counteracts VCAM-1-mediated monocyte adhesion via AdipoR1/NF-κB/COX-2 signaling in human aortic endothelial cells. American Journal of Physiology Endocrinology and Metabolism. 2011;301:E1143–54. doi: 10.1152/ajpendo.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, et al. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. American Journal of Respiratory Cell and Molecular Biology. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Weng M, Raher MJ, Leyton P, Combs TP, Scherer PE, Bloch KD, et al. Adiponectin decreases pulmonary arterial remodeling in murine models of pulmonary hypertension. American Journal of Respiratory Cell and Molecular Biology. 2011;45:340–7. doi: 10.1165/rcmb.2010-0316OC. [This study is the second study to illustrate the importance of adiponectin on lung vascular remodeling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schraw T, Wang Z V, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149:2270–82. doi: 10.1210/en.2007-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 32.Pischon T. Adiponectin: Stability in Plasma over 36 Hours and Within-Person Variation over 1 Year. Clinical Chemistry. 2003;49:650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]
- 33.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, et al. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. American Journal of Physiology Endocrinology and Metabolism. 2003;285:E527–33. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda M, Shimomura I. Roles of adiponectin and oxidative stress in obesity-associated metabolic and cardiovascular diseases. Reviews in Endocrine & Metabolic Disorders. 2013 doi: 10.1007/s11154-013-9271-7. [DOI] [PubMed] [Google Scholar]
- 35.Masui Y, Asano Y, Takahashi T, Shibata S, Akamata K, Aozasa N, et al. Clinical significance of monitoring serum adiponectin levels during intravenous pulse cyclophosphamide therapy in interstitial lung disease associated with systemic sclerosis. Modern Rheumatology / the Japan Rheumatism Association. 2013;23:323–9. doi: 10.1007/s10165-012-0660-7. [DOI] [PubMed] [Google Scholar]
- 36.Fantuzzi G. Adiponectin and inflammation: consensus and controversy. J Allergy Clin Immunol. 2008;121:326–30. doi: 10.1016/j.jaci.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Sam F, Walsh K. What can adiponectin say about left ventricular function? Heart (British Cardiac Society) 2010;96:331–2. doi: 10.1136/hrt.2009.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38*.Williams AS, Kasahara DI, Verbout NG, Fedulov A V, Zhu M, Si H, et al. Role of the adiponectin binding protein, T-cadherin (Cdh13), in allergic airways responses in mice. PloS One. 2012;7:e41088. doi: 10.1371/journal.pone.0041088. [This study illustrates the importance of T-cadherin in facilitating the mobilization of adiponectin into the lung.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denzel MS, Scimia M-C, Zumstein PM, Walsh K, Ruiz-Lozano P, Ranscht B. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. The Journal of Clinical Investigation. 2010;120:4342–52. doi: 10.1172/JCI43464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller M, Cho JY, Pham A, Ramsdell J, Broide DH. Adiponectin and functional adiponectin receptor 1 are expressed by airway epithelial cells in chronic obstructive pulmonary disease. Journal of Immunology (Baltimore, Md: 1950) 2009;182:684–91. doi: 10.4049/jimmunol.182.1.684. [DOI] [PubMed] [Google Scholar]
- 41.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, et al. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. American Journal of Physiology Lung Cellular and Molecular Physiology. 2008;294:L1035–42. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arita Y, Kihara S, Ouchi N, Maeda K, Kuriyama H, Okamoto Y, et al. Adipocyte-derived plasma protein adiponectin acts as a platelet-derived growth factor-BB-binding protein and regulates growth factor-induced common postreceptor signal in vascular smooth muscle cell. Circulation. 2002;105:2893–8. doi: 10.1161/01.cir.0000018622.84402.ff. [DOI] [PubMed] [Google Scholar]
- 43.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. The Journal of Biological Chemistry. 2002;277:37487–91. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 44.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiological Reviews. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 45.Ding M, Xie Y, Wagner RJ, Jin Y, Carrao AC, Liu LS, et al. Adiponectin induces vascular smooth muscle cell differentiation via repression of mammalian target of rapamycin complex 1 and FoxO4. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31:1403–10. doi: 10.1161/ATVBAHA.110.216804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Zhao C, Wang Y, He X, Shen C, Cao W, et al. [Adiponectin inhibits the activation of hepatic stellate cells induced by TGFb1 via up-regulating the expression of eNOS]. Zhonghua Gan Zang Bing Za Zhi = Zhonghua Ganzangbing Zazhi = Chinese Journal of Hepatology. 2011;19:917–22. doi: 10.3760/cma.j.issn.1007-3418.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Masui Y, Asano Y, Shibata S, Noda S, Aozasa N, Akamata K, et al. Serum adiponectin levels inversely correlate with the activity of progressive skin sclerosis in patients with diffuse cutaneous systemic sclerosis. Journal of the European Academy of Dermatology and Venereology: JEADV. 2012;26:354–60. doi: 10.1111/j.1468-3083.2011.04077.x. [DOI] [PubMed] [Google Scholar]
- 48**.Fang F, Liu L, Yang Y, Tamaki Z, Wei J, Marangoni RG, et al. The adipokine adiponectin has potent anti-fibrotic effects mediated via adenosine monophosphate-activated protein kinase: novel target for fibrosis therapy. Arthritis Research & Therapy. 2012;14:R229. doi: 10.1186/ar4070. [This publication is the first to demonstrate the anti-fibrotic effects of adiponectin in normal and scleroderma fibroblasts, elucidating signaling pathways important for this effect.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kolaczynski JW, Considine R V, Ohannesian J, Marco C, Opentanova I, Nyce MR, et al. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes. 1996;45:1511–5. doi: 10.2337/diab.45.11.1511. [DOI] [PubMed] [Google Scholar]
- 50.Prolo P, Wong ML, Licinio J. Leptin. The International Journal of Biochemistry & Cell Biology. 1998;30:1285–90. doi: 10.1016/s1357-2725(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 51.Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Letters. 1995;373:13–8. doi: 10.1016/0014-5793(95)00977-h. [DOI] [PubMed] [Google Scholar]
- 52.Loffreda S, Yang SQ, Lin HZ, Karp CL, Brengman ML, Wang DJ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12:57–65. [PubMed] [Google Scholar]
- 53**.Huertas A, Tu L, Gambaryan N, Girerd B, Perros F, Montani D, et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. The European Respiratory Journal. 2012;40:895–904. doi: 10.1183/09031936.00159911. [This study is one of the first to show that serum levels of leptin are increased in patients with scleroderma-associated PAH when compared to controls.] [DOI] [PubMed] [Google Scholar]
- 54.Biesiada G, Czepiel J, Ptak-Belowska A, Targosz A, Krzysiek-Maczka G, Strzalka M, et al. Expression and release of leptin and proinflammatory cytokines in patients with ulcerative colitis and infectious diarrhea. Journal of Physiology and Pharmacology: an Official Journal of the Polish Physiological Society. 2012;63:471–81. [PubMed] [Google Scholar]
- 55.Muoio DM, Lynis Dohm G. Peripheral metabolic actions of leptin. Best Practice & Research Clinical Endocrinology & Metabolism. 2002;16:653–66. doi: 10.1053/beem.2002.0223. [DOI] [PubMed] [Google Scholar]
- 56.Bjørbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Progress in Hormone Research. 2004;59:305–31. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 57.Hegyi K, Fülöp K, Kovács K, Tóth S, Falus A. Leptin-induced signal transduction pathways. Cell Biology International. 2004;28:159–69. doi: 10.1016/j.cellbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 58**.Jain M, Budinger GRS, Lo A, Urich D, Rivera SE, Ghosh AK, et al. Leptin promotes fibroproliferative acute respiratory distress syndrome by inhibiting peroxisome proliferator-activated receptor-γ. American Journal of Respiratory and Critical Care Medicine. 2011;183:1490–8. doi: 10.1164/rccm.201009-1409OC. [This publication is the first to demonstrate that leptin promotes the development of bleomycin- induced lung fibrosis in mice through augmenting TGF-β signaling.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y, Jia X, Qin J, Lu C, Zhu H, Li X, et al. Leptin inhibits PPARgamma gene expression in hepatic stellate cells in the mouse model of liver damage. Molecular and Cellular Endocrinology. 2010;323:193–200. doi: 10.1016/j.mce.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, Barrett KE. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. The British Journal of Surgery. 2007;94:346–54. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]