Abstract
We have developed a method of injecting Bacterial Artificial Chromosome (BAC) DNA into Xenopus embryos that is simple and efficient, and results in consistent and tissue-specific expression of transgenes cloned into BAC vectors. Working with large pieces of DNA, as can be accommodated by BACs, is necessary when studying large or complex genes and conducive to studying the function of long-range regulatory elements that act to control developmentally restricted gene expression. We recombineered fluorescent reporters into three Xenopus tropicalis BAC clones targeting three different genes and report that up to 60% of injected embryos express the reporter in a manner consistent with endogenous expression. The behavior of these BACs, which are replicated after injection, contrasts with that of smaller plasmids, which degrade relatively quickly when injected as circular molecules and generally fail to recapitulate endogenous expression when not integrated into the Xenopus genome.
Keywords: Eye Development, Microinjection, Transgene, Pax6, Rax, Xath5
INTRODUCTION
Xenopus has long been a widely used model for studying gene regulation during development. While much work has utilized the species Xenopus laevis, the development of Xenopus tropicalis as a model with a diploid genome (X. laevis is an allotetraploid) and faster generation time has increased the genetic tools available to Xenopus researchers. One advantage of using Xenopus embryos for studying gene function is that we can easily express a gene of interest by simple microinjection of RNA or DNA. RNA injections are suitable for over-expression in early embryonic stages because RNA is immediately translated, but with little temporal or spatial control. Injected RNAs eventually lose activity due to degradation and/or dilution as embryos age, making it unsuitable for examining effects of over-expression at later stages. In theory, injecting plasmid DNA could ameliorate some of these problems, as tissue- or temporal-specific promoters can be used to control gene expression while prolonging activity. In some cases, transient expression of plasmids has been of use in identifying key promoter and cis-regulatory element sequences in Xenopus (e.g., Damjanovski et al., 1998; Watabe et al., 1995), though in most cases these plasmids are expressed mosaically, that is, while the transgene may be expressed in the appropriate domains, it is only active in a small subset of cells that normally express the gene. Moreover, expression of injected DNA plasmids into embryos is often not only mosaic, but the specificity of promoters is often lost for unknown reasons (Etkin and Pearman, 1987; Fu et al., 1998). To overcome these problems, a number of methods for inserting transgenes have been developed, including methods depending on genomic integration of the transgene (Hamlet et al., 2006; Kroll and Amaya, 1996; Ogino et al., 2006; Pan et al., 2006; Sinzelle et al., 2006) or transient expression (Fu et al., 1998). These methods generally achieve 5-30% efficiency of correct expression in injected embryos (F0 generation).
Here we report a method using Bacterial Artificial Chromosome (BAC) clones that can achieve 50-60% efficiency in the F0 population. The BAC is injected into embryos as a circular molecule with no mechanism to mediate genomic integration, so most expression is likely transient. However, expression is tissue-specific and highly consistent, and persists until late tadpole stages. This fast, simple method for expressing a transgene in a tissue-specific manner provides an additional tool for Xenopus researchers with many promising applications.
RESULTS
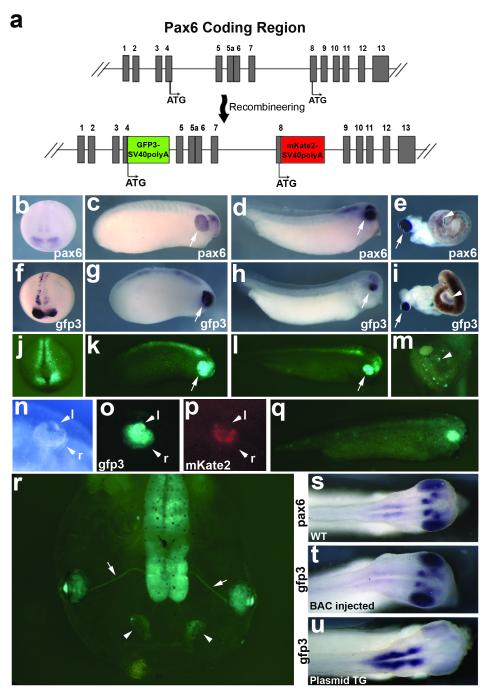
pax6 is a well studied developmental gene encoding a transcription factor that is the master regulator of eye development, and is also known to have many long-range cis-regulatory enhancers regulating its transcription (Kammandel et al., 1999; Kleinjan et al., 2006). We choose BAC clone CH216-109E08 to use as a pax6 gene expression reporter. This clone contains approximately 175kb of Xenopus tropicalis genomic sequence surrounding pax6. Importantly, this clone includes upstream and far-downstream cis-regulatory enhancer sequences shown to be important for correct expression (Kammandel et al., 1999; Kleinjan et al., 2006). We initially performed BAC recombineering to flank the genomic DNA with I-SceI sites for future meganuclease-mediated transgenesis experiments (Ogino et al., 2006; Pan et al., 2006; Wang et al., 2009). To create a pax6 reporter BAC, we needed to consider that pax6 is a complex gene with multiple promoters and two translational start sites (Haubst et al., 2004; Kim and Lauderdale, 2006). Using two fluorescent protein genes, gfp3 and mkate2, we created a dual reporter for multiple pax6 isoforms (Fig. 1a; Kim and Lauderdale, 2008; Shcherbo et al., 2007). We originally injected the pax6 reporter BAC by itself only as a control for I-SceI mediated transgenesis experiments (Ogino et al., 2006; Pan et al., 2006). Surprisingly, we found that the BAC alone was sufficient to recapitulate endogenous pax6 expression in a high percentage of embryos without any mediator of transgenesis (Table 1, Figs. 1b-m, Fig. 4e), as opposed to the mosaic expression commonly seen with plasmid injections (Fig. 4f; Etkin and Pearman, 1987; Ogino et al., 2006). pax6 is expressed in the developing eye, brain, pancreas, and intestine (Hirsch and Harris, 1997; Kelly and Melton, 2000). Our transient BAC reporter recapitulates endogenous pax6 expression at all stages examined until late tadpole stages (Figs.1b-m). Furthermore, we find evidence of the shortened pax6 isoform expressed in a subset of retinal tissues at late tadpole stages (Figs. 1n-p), consistent with reports of this isoform’s expression in mouse and zebrafish (Kim and Lauderdale, 2008; Lakowski et al., 2007). This BAC reporter also functions in Xenopus laevis embryos, implying that this method will be useful in this species as well (Fig. 1q). We compared embryos injected with our BAC reporter to an integrated 3.6kb Pax6-promoter-GFP line (Hirsch et al., 2002). Interestingly, the BAC reporter more accurately recapitulates endogenous expression patterns in the brain compared to the integrated line, which uses only the 3.6kb upstream promoter to drive gfp expression (Figs. 1s-u). We observe loss of obvious transgene expression around stage 50 in most injected embryos.
FIG. 1.
Expression of the pax6 dual reporter BAC in Xenopus embryos. (a) Detail of the pax6 coding region contained in BAC 109E08, showing construction of the pax6 BAC dual reporter injected into Xenopus tropicalis embryos shown in f-p, r and t, and into the Xenopus laevis embryo shown in q. Exons are numbered and shown as grey boxes. Alternate translational start sites indicated with “ATG,” with the most common isoforms using the exon 4 start site, and the shorter, retinal specific isoform using exon 8 start site. (b-e) In situ hybridization of uninjected siblings using a probe against pax6 mRNA to show endogenous expression (b, stage 18, anterior view; c, stage 23; d, stage 33; e stage 47 dissected gut). Expression is detected in the developing eye, brain, pancreas and intestine; developing eye indicated by arrows in c and d, pancreas indicated by arrow in e, intestinal expression indicated by arrowhead in e. (f-i) In situ hybridization with probe detecting gfp3 mRNA in BAC-injected embryos (f, stage 18, anterior view; g, stage 23; h, stage 33; I stage 47 dissected gut). Expression of the transgene is detected in the same tissues as endogenous pax6 expression: developing eye, brain, intestine, and pancreas; pancreas indicated by arrow in I, intestinal staining indicated by arrowhead in i. (The dark coloration of intestines is due to pigment remaining in tissues.) (j-l) Fluorescent images of gfp3 expression at same stages as in situ hybridized embryos above. Developing eye indicated by arrows in k and l. (m) Detail of stage 47 embryo gut. gfp3 speckling is seen in the intestine, indicated with arrowhead. Although pancreatic expression of BAC gfp3 mRNA is confirmed via in situ hybridization for gfp3, gfp3 protein is not observed in the pancreas. (n-p) Detail of retina and lens of stage 47 embryo injected with dual reporter. The more common isoforms of pax6 reported by the gfp3 transgene are seen widely expressed in both the lens and retina, while the less expressed, shorter isoform reported with the mkate2 transgene is excluded from the lens, and detected in only a subset of retinal cells. (q) gfp3 expression seen in a stage 33 X. laevis embryo also recapitulates endogenous expression. (r) gfp3 expression in a stage 47 embryo injected with the pax6 reporter BAC. Expression is still detected in the retina and lens, olfactory bulbs (arrows) and optic nerves (arrowheads). (s-u) Comparison of stage 33 embryos, dorsal view of head: pax6 endogenous expression (s), gfp3 transient expression from pax6 BAC (t) and gfp3 expression seen in an F1 embryo from an integrated line in which gfp3 expression is driven by the upstream 3.6kb pax6 promoter region (u). (j-r) Embryos were PTU-treated to visualize retinal tissue. Abbreviations: l = lens, r = retina.
Table 1.
Survival and observed transgene expression efficiencies from injected DNA
| Construct Injected | Amount (pg) |
Injected Embryos |
Scoreda | Survivalb (%) |
Correct Expressors |
Partialc | Ectopib | GFP- | Correct Expressors (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pax6 BAC | 20 | 216 | 197 | 91% | 127 | 37 | 9 | 24 | 64 |
| Pax6 BAC | 40 | 151 | 87 | 58% | 49 | 5 | 2 | 31 | 56 |
| Pax6 BAC | 80 | 114 | 46 | 40% | 18 | 6 | 3 | 19 | 39 |
| Xath5 BAC | 20 | 91 | 76 | 84% | 43 | 25 | 1 | 7 | 57 |
| Xath5 BAC | 40 | 129 | 33 | 26% | 14 | 5 | 0 | 14 | 42 |
| Xath5 BAC | 80 | 151 | 22 | 15% | 8 | 3 | 1 | 10 | 36 |
| RaxGFPfusion BAC | 20 | 113 | 55 | 49% | 18 | 2 | 7 | 28 | 33 |
| RaxGFPfusion BAC | 40 | 149 | 79 | 53% | 31 | 11 | 5 | 32 | 39 |
| RaxGFPfusion BAC | 80 | 154 | 17 | 11% | 3 | 0 | 2 | 12 | 18 |
| IS Pax6GFP | 20 | 138 | 62 | 45% | 0 | 0 | 54 | 8 | 0 |
| IS Pax6GFP | 40 | 100 | 19 | 19% | 0 | 0 | 18 | 1 | 0 |
| Pax6-GFP pTARBAC2.1 | 40 | 142 | 73 | 51% | 2 | 6 | 14 | 51 | 3 |
| Pax6-GFP pTARBAC2.1 | 80 | 150 | 39 | 26% | 0 | 1 | 9 | 29 | 0 |
Only embryos with normal appearance were scored.
Indicates the percentage of normal embryos scored from the injected count.
Indicates embryos expressing in a subset of correct tissues for a given reporter. IS Pax6GFP-injected and Pax6-GFP pTARBAC2.1-injected embryos were scored as correct expressors if GFP expression recapitulated either endogenous Pax6 expression or previously reported integrated transgene expression (Hirsch et al., 2002; Ogino et al., 2006).
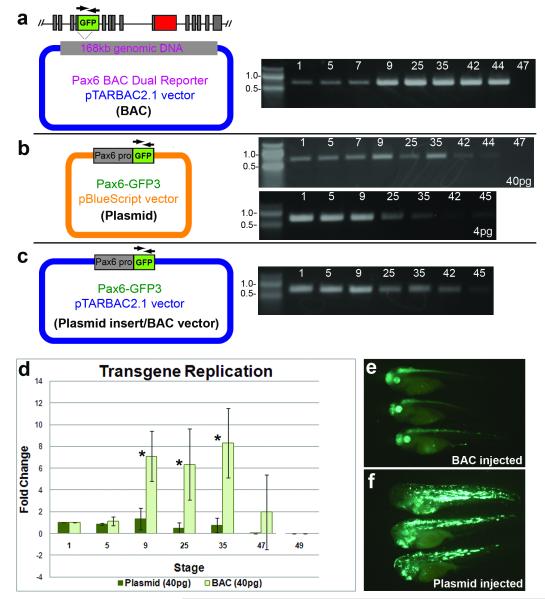
FIG. 4.
BAC constructs replicate more efficiently in developing embryos than smaller plasmids. (a) Schematic showing pax6 dual reporter BAC injected into embryos (40pg). DNA gel image shows results from PCR assay amplifying gfp3 at the indicated stages. (b) Schematic showing plasmid reporter injected into embryos for PCR replication assay. DNA gel images show PCR results from 2 different DNA amounts injected (40pg and 4pg, as indicated). (c) pax6 promoter-GFP in BAC vector construct injected into embryos for PCR assay. Gel shows PCR results from embryos collected at indicated stages. (d) Graph shows results of quantification of gel bands from 3 independent experiments performed for each construct, and though these experiments were done independently using slightly different PCR conditions, they are shown together in Fig. 4d to illustrate the difference in patterns in the two experiments. Error bars show standard deviations, and asterisks indicate p values < 0.05 for transgene amplification as compared to 1 hour-post-fertilization at stages 9 (p=0.016), 25 (p=0.037) and 35 (p=0.016). (e) Consistent expression of transgene is observed in pax6 BAC injected embryos. (f) Ectopic, mosaic and inconsistent expression observed in plasmid injected embryos.
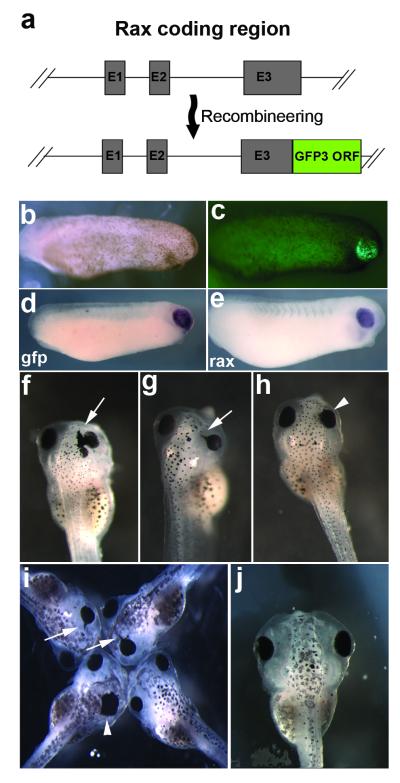
To test the universality of this method we choose two additional BAC clones to test: ISB1-349A23, a 78.5kb, rax gene-containing BAC, and CH216-38N10, a 166.5kb, xath5 (aka atoh7) gene-containing BAC. rax and xath5 encode transcription factors expressed in the developing retina (Kanekar et al., 1997; Mathers et al., 1997). For the rax BAC we opted to recombineer the gfp3 cassette after the rax protein C-terminus to create a rax-GFP fusion protein (Fig. 2a). This BAC extends only 2kb upstream of rax, so it may not include all necessary regulatory elements, although a proximal upstream regulatory region that appears to be the main embryonic enhancer controlling onset of expression is present (Hui Wang, RMG, unpublished observations). This BAC was successful at recapitulating endogenous rax expression in around 30% of scored embryos (Table 1, Figs. 2b-e). The rax-GFP fusion caused rax over-expression phenotypes such as medially-shifted eyes and ectopic retinal pigmented epithelium (RPE) in some embryos (Figs. 2f-h, j), similar to those observed when rax is over-expressed via mRNA injection (Fig. 2i, Mathers et al., 1997).
FIG. 2.
Expression of the rax-gfp3 fusion BAC. (a) Detail of rax coding region contained in BAC 349A23, showing construction of the rax GFP3 fusion construct. (b) Bright field view of stage 24 embryo injected with rax-gfp fusion BAC. (c) GFP fluorescent view of same embryo, showing expression of transgene in developing retina. (d) In situ hybridization against gfp3 mRNA in BAC-injected, stage 24 embryo. (e) In situ hybridization of WT sibling embryo showing endogenous rax mRNA expression. (f-h) rax overexpression phenotypes seen in stage 42-43 tadpoles injected with rax-gfp fusion BAC. Arrows indicate formation of ectopic retinal pigmented epithelium. Arrowheads indicate eyes shifted medially. (i) rax overexpression phenotypes observed in rax mRNA injected embryos. Arrows and arrowheads indicate the same structures as in f-h. (j) Normal, wildtype stage 42 embryo for comparison.
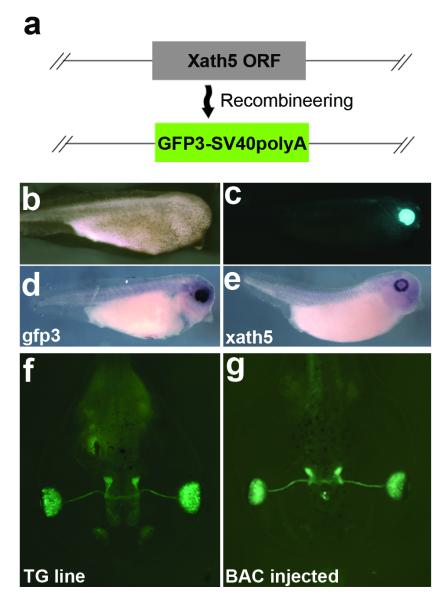
The xath5 BAC extends 65kb upstream and 101kb downstream of the xath5 coding region. We created a xath5 BAC reporter construct by replacing the singular xath5 exon with gfp3 (Fig. 3a). xath5 is expressed in the developing retina, olfactory placode, and pineal gland (Kanekar et al., 1997). Over 50% of xath5 BAC-injected embryos showed correct transgene expression at all stages through late tadpole (Table 1, Figs. 3b-e). We compared our xath5 reporter BAC-injected embryos to a 3.4kb xath5-promoter-gfp integrated line (Hirsch et al., 2002), and found that F0 expression of the BAC gfp3 was comparable to integrated F2 offspring extending into late tadpole stages (Figs. 3f,g).
FIG. 3.
Expression of the xath5 GFP reporter BAC. (a) Detail of xath5 coding region contained in BAC 38N10, showing replacement of coding region with gfp3 open reading frame (ORF). (b) Bright field image of stage 35 embryo injected with xath5 reporter BAC. (c) gfp fluorescence image of same embryo, showing gfp3 expression in developing retina. (d) In situ hybridization against gfp3 mRNA in BAC-injected, stage 35 embryo. (e) In situ hybridization of WT sibling embryo showing endogenous xath5 mRNA expression. (f) Stage 46 F2 tadpole from integrated line, using xath5 promoter to drive GFP expression. (g) Stage 46 xath5 BAC-injected tadpole GFP expression is comparable to integrated line expression. Embryos were PTU treated to visualize retinal tissue (b-g).
The ability of BACs to efficiently recapitulate endogenous expression patterns contrasts with difficulties faced by experimenters attempting to achieve consistent, non-mosaic expression using smaller plasmids in transient assays. To illustrate this we compared our BAC-injected embryos to embryos injected with a 7kb plasmid that uses the 3.6kb pax6 promoter driving gfp3, IS-Pax6GFP (Ogino et al., 2006). Injections of this plasmid under the same conditions as our pax6 BAC reporter yielded no embryos with correct expression (Table 1 and Fig. 4e,f, (Ogino et al., 2006).
We wondered if some component of the BAC vector was affecting transgene behavior, so we subcloned the Pax6-promoter-GFP cassette into this vector, pTARBAC2.1, creating the plasmid Pax6-GFP3-pTARBAC2.1 (Fig. 4c). When injected, this construct yielded only a small number of correctly expressing embryos, with very weak gfp expression (Table 1).
It may be the case that the BAC constructs are localized differently than the plasmids, or perhaps there is a difference in the way the DNA is packaged into chromatin. It is understood that nuclear structure is important for regulating proper DNA replication in eukaryotic cells (Margueron and Reinberg, 2010; Wu, 1999). To further investigate the disparity in expression, we performed a PCR-based experiment to assay for replication of the transgene in the embryo, since differences in replication efficiency may reflect differences in sub-cellular localization or chromatin structure. Embryos were injected with either plasmid or BAC and collected at indicated stages, ranging from 1 hour-post-fertilization to stage 47. Semi-quantitative PCR amplification of the gfp3 transgene was used to assay the amount of exogenous DNA in the embryos (Figs. 4a, b, d). Band intensities from each time point were compared with a baseline level from embryos collected 1 hour-post-fertilization (1hpf) to estimate fold-change in exogenous DNA amounts. Our results show an increase in the BAC transgene as the embryo develops, consistent with BAC replication (Fig. 4d). By contrast, this assay shows no significant increase of the plasmid transgene. To test whether the higher copy number of injected plasmid might be overwhelming the replication machinery, we performed the assay with less DNA, and detected no significant change in pattern of transgene levels during development (Fig. 4b). This assay was also performed with the Pax6-GFP-pTARBAC2.1 vector, which similarly showed no increase in the transgene (Fig. 4c). It has previously been reported that only a small percentage of injected circular plasmid molecules are replicated (Marini and Benbow, 1991), while the majority are degraded. This assay does not address what percentage of BAC molecules replicate in the embryo; it may be only a minority of the total injected molecules. Nevertheless, at least some subset of injected BAC molecules appears to be differentially replicated compared with plasmid constructs. All constructs seem to be degraded to a large extent by late tadpole stages, which could explain the loss of obvious BAC transgene expression around stage 50.
DISCUSSION
Here we have reported a new, simple and highly efficient method of driving tissue-specific transgene expression in Xenopus embryos. Since this method uses BAC clones as vectors, it is ideal for studying large or complex genes that would otherwise be difficult to manipulate using traditional subcloning methods. One important consideration for this method is choosing the best BAC for the purpose, as our studies indicate that BACs with optimized flanking sequence on each side of the gene-of-interest provide the highest expression efficiencies.
The mechanism by which this method leads to correct transgene expression is unclear. Past studies have shown that the behavior of DNA injected into Xenopus oocytes and eggs can vary greatly depending on its size and structure (Bendig, 1981; Etkin and Pearman, 1987; Harland and Laskey, 1980; Shiokawa et al., 1986). Examination of exogenous DNA injections into oocytes demonstrated that circular DNA is transcribed more efficiently than linearized DNA (Harland et al., 1983). However, DNA microinjection experiments using embryos concluded that circular DNA is very poorly replicated. Small linear DNAs are also inefficiently replicated when compared to the concatamers of these linear DNAs (Marini and Benbow, 1991; Marini et al., 1988). However, because the replication efficiency of linearized plasmids is improved by the formation of high molecular weight concatemers, it may be that the higher molecular weight of the large BAC molecule likewise improves its ability to replicate. It is possible that larger DNAs are packaged into chromatin-like structures that enhance their ability to be replicated. While differences in replication could account for differences in expression persistence, what is striking about this method is the improvement of expression specificity. This could be caused by differences in chromatin state or the intracellular localization of injected DNA. The differences we observe in DNA levels may be a reflection of a deeper change precipitated by specific sequences contained within the genomic DNA of the BAC.
Since this method results in eventual loss of observable transgene expression and furthermore does not utilize any known mediator of genomic insertion (e.g. co-injection with I-SceI or Tol2 mRNA), it is unlikely that genomic integration of the BAC is occurring. Although this transient expression is highly efficient and consistent in the F0 population, there will certainly be future applications for which the creation of BAC transgenic lines would be beneficial and likely necessary, and so a remaining question for future investigation is the possibility of BAC DNA genomic integration. BAC DNA has been successfully integrated in mouse and zebrafish using the Tol2 transposase mediated method (Suster et al., 2009), and in zebrafish using the I-SceI meganuclease-mediated method (Kimura et al., 2006). In these examples, integrated BAC transgenes can mitigate or eliminate problematic positional effects often observed with plasmid integration. Since BACs can also include the numerous regulatory elements necessary for correct gene expression, we predict that integration of BAC constructs will result in more accurate expression the desired transgene compared to other methods.
This method lends itself to numerous useful applications. One we have begun to explore is the use of BAC clones to rescue mutant lines; preliminary experiments indicate this method can successfully rescue mutant embryos when the proper dose of BAC was injected (TN, MBF, RMG, unpublished observations). Importantly, in theory this method could rescue lethal mutations to obtain mature, homozygous mutant adults without germline transmission of the rescuing transgene.
It will be valuable for future rescue experiments to accurately quantify the expression levels of injected transgenes. Although we can theoretically estimate the transgene copy number per cell as the embryos ages, it is not known what percentage of these are actively transcribed. Gene dosage sensitivities vary depending on the gene, and the onset of expression of a given gene will also be a factor, as presumably the number of BAC molecules per nuclei is reduced as the embryo ages (after the initial, early increase in copy number). In the case of our Rax-GFP fusion injections into wildtype embryos we observe a range of overexpression phenotypes from minor to severe (Fig. 2), and sometimes observe GFP+ embryos without any obvious overexpression phenotype, suggesting that although overall transcriptional levels of transgene are higher than endogenous expression (as evidenced by the overexpression phenotype), they may be close to the biologically-relevant range. Careful examination of transgene expression levels will need to be performed to ensure appropriate gene dosage for future experimentation.
BAC clones are also ideal for studying the activity of long-range regulatory enhancers, as their large size permits one to maintain endogenous genetic distance as well as interactions of multiple enhancers. We should mention that we often observe a difference in transgene intensity between the left and right sides of our embryos (e.g., Figs.1f, j), perhaps due to an uneven distribution of BAC molecules for the first cell division. We also sometimes observe embryos expressing the transgene in just one half of the embryo, which is useful as the other half can serve as control, or can allow one to follow cell migration pathways crossing the midline.
METHODS
Plasmid constructs and BAC recombineering
BAC clones were purchased from CHORI BACPAC. RpsL/kana cassttes were amplified with appropriate 50bp homology arms for recombineering using pSK+ KanaRpsL (Addgene plasmid 20871) as a template. The pax6 (CH216-109E08), rax (ISB1-349A23) and xath5 (CH216-38N10) BACs, rpsL/kana, gfp3 and mkate2 (Evrogen Cat. # FP182, Shcherbo et al., 2007) expression cassettes were transformed into SW102 cells (received from NCI Frederick Biological Resources Branch) and recombineered as described previously (Wang et al., 2009; Warming et al., 2005). Correct recombinants were identified and confirmed via PCR amplicon length analysis, restriction digests, amplicon sequencing and end-sequencing of BAC clones. Construction of the IS-Pax6-GFP3 plasmid was previously described (Ogino et al., 2006). To construct the Pax6-GFP3 pTARBAC2.1 vector, I-SceI sites were inserted into the pTARBAC2.1 vector arms flanking the genomic DNA in CH216-109E08 using recombineering as described above. This vector was cut with I-SceI enzyme to remove the genomic insert and gel-purified. Similarly, the pax6 promoter-gfp insert was removed with I-SceI enzyme and ligated into pTARBAC2.1 vector. Confirmation of vector construction was done with restriction digest analysis and DNA sequencing. A more detailed recombineering protocol is available upon request.
DNA and mRNA injections
Xenopus tropicalis eggs were fertilized and dejellied as described previously (Ogino et al., 2006). BAC DNA and the Pax6-GFP-pTARBAC2.1 construct were purified using the Nucleobond BAC100 kit, Clontech Cat. # 740579, and resuspended in microinjection buffer (10 mM Tris-HCl, pH 7.5, 0.1 mM EDTA, 30 μM spermine, 70 μM spermidine, 100 mM NaCl) and stored at 4°C (Schedl et al., 1993). Plasmid DNA was purified using the Qiagen Maxiprep Kit (Qiagen, USA). DNA concentrations were determined using a NanoDrop spectrophotometer. On the day of injections, DNA was diluted to 100ng/μl in sterile water, and further diluted in 1X I-SceI buffer to the appropriate concentration for injections. Xenopus tropicalis embryos were injected with 2nl DNA injection solution at the 1-cell stage, with injections occurring between 20 and 40 minutes post-fertilization. Xenopus laevis embryos were injected with 20pg BAC DNA in 4nl. Embryos were cultured in 0.002% Phenylthiourea (PTU) to prevent pigment formation and enable observation of fluorescent transgene expression in retinal tissues (Sims, 1962). rax capped mRNA was synthesized from pCS2-Xrx1 (a kind gift from Massimiliano Andreazzoli; Andreazzoli et al., 2003) using mMESSAGE mMACHINE® SP6 Kit (Invitrogen) and 20 pg mRNA was injected into one dorsal blastomere at early 4-cell stage of embryos (X. tropicalis) to observe overexpression phenotype.
Fluorescence Microscopy and in situ hybridization
Fluorescence in embryos was observed using a Zeiss Discovery V12 microscope with fluorescent illumination and 470nm GFP filter set to visualize gfp3, or 560nm Texas Red filter set to visualize mkate2. Photos were taken with a Zeiss AxioCam Mrc5 CCD camera assembled on the microscope, using Axiovision 4.8 software.
Whole-mount and dissected gut in situ hybridization was performed as described previously (Sive et al., 2000) using digoxygenin-labeled probes. The stained embryos/tissues/organs were bleached for better visualization of staining as described previously (Sive et al., 2000).
PCR replication assay
Embryos were injected with either 40pg or 4pg DNA as described above, and collected at indicated stages in groups of 10. Embryos were lysed overnight at 56°C in Lysis Buffer (50mM Tris, 1mM EDTA, 0.5% Tween 20, 200ug/ml proteinase K). The next day the samples were incubated at 95°C for 10 minutes to inactivate proteinase, and spun in a microcentrifuge at 4°C for 15 minutes at 15,000 RPM. 10 μl of the supernatant was diluted in 490μl sterile water, and 5μl of this solution was used as a template for the gfp3 PCR amplification reaction, using gfp3 primers described in (Offield et al., 2000), resulting in a 710bp amplicon. For each experimental condition, increasing PCR cycle amounts were assayed to determine an optimal cycle number within the exponential phase of the reaction. 40pg BAC, 40pg Pax6-GFP-pTARBAC2.1 and 4pg IS-Pax6GFP plasmid injected samples were PCR amplified using 35 cycles. 40pg IS-Pax6GFP injected samples were amplified using 25 cycles. PCR products were run on a 1.5% agarose gel and visualized using EtBr and UV light. Image J was used to quantify bands as previously described (Abramoff et al., 2004). Band intensities at each stage were divided by the baseline 1 hour-post-fertilization (hpf) intensity to estimate fold-change in amplifiable DNA. This experiment was performed independently three times for BAC and 40pg plasmid injections. Microsoft Excel was used to calculate data means, standard deviations, and p-values using the student’s t-test with two-tailed distribution.
ACKNOWLEDGMENTS
We wish to thank the NCI Frederick Biological Resources Branch for providing recombineering reagents, and Drs. J. Zhu and M. Andreazzoli for the sharing of plasmids. We would also like to thank Drs. V. van Heyningen and D. Kleinjan for advice regarding BAC experiments and recombineering, Dr. P. Skoglund and Ms. K. Pfister for assistance with X. laevis experiments, and members of the Grainger lab, especially Mr. R. Reeder for experimental assistance, and Dr. M. Fisher for experimental advice and comments on the manuscript.
Work funded by NIH grants EY019000 and EY017400
LITERATURE CITED
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Andreazzoli M, Gestri G, Cremisi F, Casarosa S, Dawid IB, Barsacchi G. Xrx1 controls proliferation and neurogenesis in Xenopus anterior neural plate. Development. 2003;130:5143–5154. doi: 10.1242/dev.00665. [DOI] [PubMed] [Google Scholar]
- Bendig MM. Persistence and expression of histone genes injected into Xenopus eggs in early development. Nature. 1981;292:65–67. doi: 10.1038/292065a0. [DOI] [PubMed] [Google Scholar]
- Damjanovski S, Huynh MH, Motamed K, Sage EH, Ringuette M. Regulation of SPARC expression during early Xenopus development: evolutionary divergence and conservation of DNA regulatory elements between amphibians and mammals. Dev Genes Evol. 1998;207:453–461. doi: 10.1007/s004270050136. [DOI] [PubMed] [Google Scholar]
- Etkin LD, Pearman B. Distribution, expression and germ line transmission of exogenous DNA sequences following microinjection into Xenopus laevis eggs. Development. 1987;99:15–23. doi: 10.1242/dev.99.1.15. [DOI] [PubMed] [Google Scholar]
- Etkin LD, Pearman B, Ansah-Yiadom R. Replication of injected DNA templates in Xenopus embryos. Exp Cell Res. 1987;169:468–477. doi: 10.1016/0014-4827(87)90207-2. [DOI] [PubMed] [Google Scholar]
- Etkin LD, Pearman B, Roberts M, Bektesh SL. Replication, integration and expression of exogenous DNA injected into fertilized eggs of Xenopus laevis. Differentiation. 1984;26:194–202. doi: 10.1111/j.1432-0436.1984.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Fu Y, Wang Y, Evans SM. Viral sequences enable efficient and tissue-specific expression of transgenes in Xenopus. Nat Biotechnol. 1998;16:253–257. doi: 10.1038/nbt0398-253. [DOI] [PubMed] [Google Scholar]
- Hamlet MRJ, Yergeau DA, Kuliyev E, Takeda M, Taira M, Kawakami K, Mead PE. Tol2 transposon-mediated transgenesis in Xenopus tropicalis. Genesis. 2006;44:438–445. doi: 10.1002/dvg.20234. [DOI] [PubMed] [Google Scholar]
- Harland RM, Weintraub H, McKnight SL. Transcription of DNA injected into Xenopus oocytes is influenced by template topology. Nature. 1983;302:38–43. doi: 10.1038/302038a0. [DOI] [PubMed] [Google Scholar]
- Harland RM, Laskey RA. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980;21:761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Haubst N, Berger J, Radjendirane V, Graw J, Favor J, Saunders GF, Stoykova A, Götz M. Molecular dissection of Pax6 function: the specific roles of the paired domain and homeodomain in brain development. Development. 2004;131:6131–6140. doi: 10.1242/dev.01524. [DOI] [PubMed] [Google Scholar]
- Hirsch N, Harris WA. Xenopus Pax-6 and retinal development. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- Hirsch N, Zimmerman LB, Gray J, Chae J, Curran KL, Fisher M, Ogino H, Grainger RM. Xenopus tropicalis transgenic lines and their use in the study of embryonic induction. Developmental Dynamics. 2002;225:522–535. doi: 10.1002/dvdy.10188. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinctcis-Essential Modules Direct the Time-Space Pattern of thePax6Gene Activity. Developmental Biology. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/s0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Kelly OG, Melton DA. Development of the pancreas in Xenopus laevis. Dev Dyn. 2000;218:615–627. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1027>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Analysis of Pax6 expression using a BAC transgene reveals the presence of a paired-less isoform of Pax6 in the eye and olfactory bulb. Dev Biol. 2006;292:486–505. doi: 10.1016/j.ydbio.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Kim J, Lauderdale JD. Overexpression of pairedless Pax6 in the retina disrupts corneal development and affects lens cell survival. Dev Biol. 2008;313:434–454. doi: 10.1016/j.ydbio.2007.10.043. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Okamura Y, Higashijima S-ichi. alx, a Zebrafish Homolog of Chx10, Marks Ipsilateral Descending Excitatory Interneurons That Participate in the Regulation of Spinal Locomotor Circuits. The Journal of Neuroscience. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinjan DA, Seawright A, Mella S, Carr CB, Tyas DA, Simpson TI, Mason JO, Price DJ, van Heyningen V. Long-range downstream enhancers are essential for Pax6 expression. Dev Biol. 2006;299:563–581. doi: 10.1016/j.ydbio.2006.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122:3173–3183. doi: 10.1242/dev.122.10.3173. [DOI] [PubMed] [Google Scholar]
- Lakowski J, Majumder A, Lauderdale JD. Mechanisms controlling Pax6 isoform expression in the retina have been conserved between teleosts and mammals. Developmental Biology. 2007;307:498–520. doi: 10.1016/j.ydbio.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Margueron R, Reinberg D. Chromatin structure and the inheritance of epigenetic information. Nat Rev Genet. 2010;11:285–296. doi: 10.1038/nrg2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini NJ, Benbow RM. Differential compartmentalization of plasmid DNA microinjected into Xenopus laevis embryos relates to replication efficiency. Mol Cell Biol. 1991;11:299–308. doi: 10.1128/mcb.11.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini NJ, Etkin LD, Benbow RM. Persistence and replication of plasmid DNA microinjected into early embryos of Xenopus laevis. Dev Biol. 1988;127:421–434. doi: 10.1016/0012-1606(88)90328-4. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Offield MF, Hirsch N, Grainger RM. The development of Xenopus tropicalis transgenic lines and their use in studying lens developmental timing in living embryos. Development. 2000;127:1789–1797. doi: 10.1242/dev.127.9.1789. [DOI] [PubMed] [Google Scholar]
- Ogino H, McConnell WB, Grainger RM. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech Dev. 2006;123:103–113. doi: 10.1016/j.mod.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Pan FC, Chen Y, Loeber J, Henningfeld K, Pieler T. I-SceI meganuclease-mediated transgenesis in Xenopus. Dev Dyn. 2006;235:247–252. doi: 10.1002/dvdy.20608. [DOI] [PubMed] [Google Scholar]
- Schedl A, Larin Z, Montoliu L, Thies E, Kelsey G, Lehrach H, Schütz G. A method for the generation of YAC transgenic mice by pronuclear microinjection. Nucleic Acids Res. 1993;21:4783–4787. doi: 10.1093/nar/21.20.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shcherbo D, Merzlyak EM, Chepurnykh TV, Fradkov AF, Ermakova GV, Solovieva EA, Lukyanov KA, Bogdanova EA, Zaraisky AG, Lukyanov S, Chudakov DM. Bright far-red fluorescent protein for whole-body imaging. Nat Meth. 2007;4:741–746. doi: 10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- Shiokawa K, Sameshima M, Tashiro K, Miura T, Nakakura N, Yamana K. Formation of nucleus-like structure in the cytoplasm of [lambda]-DNA-injected fertilized eggs and its partition into blastomeres during early embryogenesis in Xenopus laevis. Developmental Biology. 1986;116:539–542. doi: 10.1016/0012-1606(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Sims RT. The Action of Phenyl-Thiourea on Melanogenesis in Xenopus Laevis. Quarterly Journal of Microscopal Science. 1962;3:439–446. [Google Scholar]
- Sinzelle L, Vallin J, Coen L, Chesneau A, Du Pasquier D, Pollet N, Demeneix B, Mazabraud A. Generation of trangenic Xenopus laevis using the Sleeping Beauty transposon system. Transgenic Res. 2006;15:751–760. doi: 10.1007/s11248-006-9014-6. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger R, Harland R. Early Development of Xenopus Laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- Suster ML, Sumiyama K, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genomics. 2009;10:477. doi: 10.1186/1471-2164-10-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU JR. Regulation of eukaryotic DNA replication and nuclear structure. Cell Res. 1999;9:163–170. doi: 10.1038/sj.cr.7290014. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhao Y, Leiby M, Zhu J. A New Positive/Negative Selection Scheme for Precise BAC Recombineering. Mol Biotechnol. 2009;42:110–116. doi: 10.1007/s12033-009-9142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Research. 2005;33:e36. doi: 10.1093/nar/gni035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe T, Kim S, Candia A, Rothbächer U, Hashimoto C, Inoue K, Cho KW. Molecular mechanisms of Spemann’s organizer formation: conserved growth factor synergy between Xenopus and mouse. Genes Dev. 1995;9:3038–3050. doi: 10.1101/gad.9.24.3038. [DOI] [PubMed] [Google Scholar]
- Wu JR. Regulation of eukaryotic DNA replication and nuclear structure. Cell Res. 1999;9:163–170. doi: 10.1038/sj.cr.7290014. [DOI] [PubMed] [Google Scholar]