Abstract
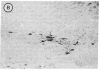
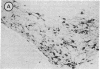
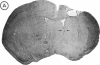
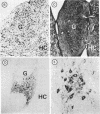
Attempts to reconstruct the damaged nigrostriatal pathway in experimental models of Parkinson disease have thus far been carried out in animals with neurotoxically induced dopamine deficiency. The present study establishes the weaver (wv/wv) mutant mouse as a genetic model of chronic striatal dopamine denervation by demonstrating a marked decrease of tyrosine hydroxylase-immunoreactive neurons in the substantia nigra pars compacta. Moreover, grafts of embryonic ventral mesencephalon taken from genetically normal mice and transplanted into the lateral ventricle of adult weaver mutants can survive and grow in the mutant host environment, express tyrosine hydroxylase immunoreactivity, and reinnervate the target regions of the recipient. These results provide evidence of integration of graft and host tissue and suggest that transplantation of dopamine neurons may be effectively applied to overcome nigrostriatal degeneration of genetic etiology.
Full text
PDF

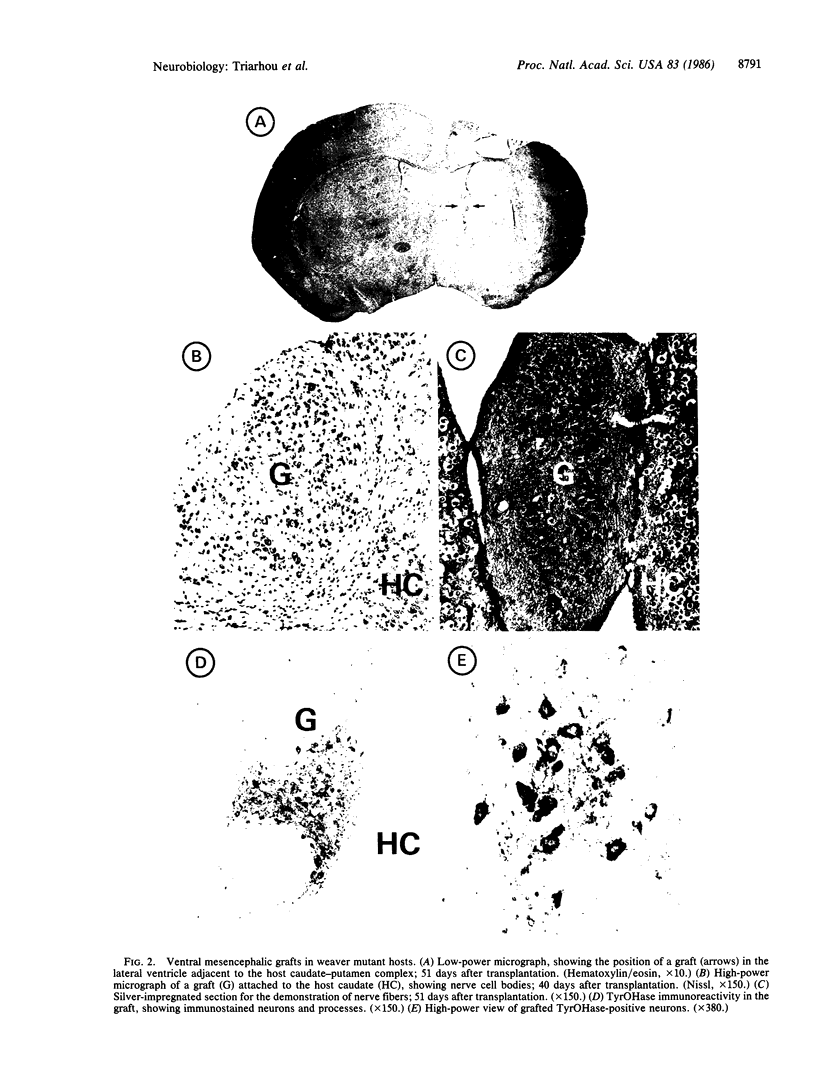

Images in this article
Selected References
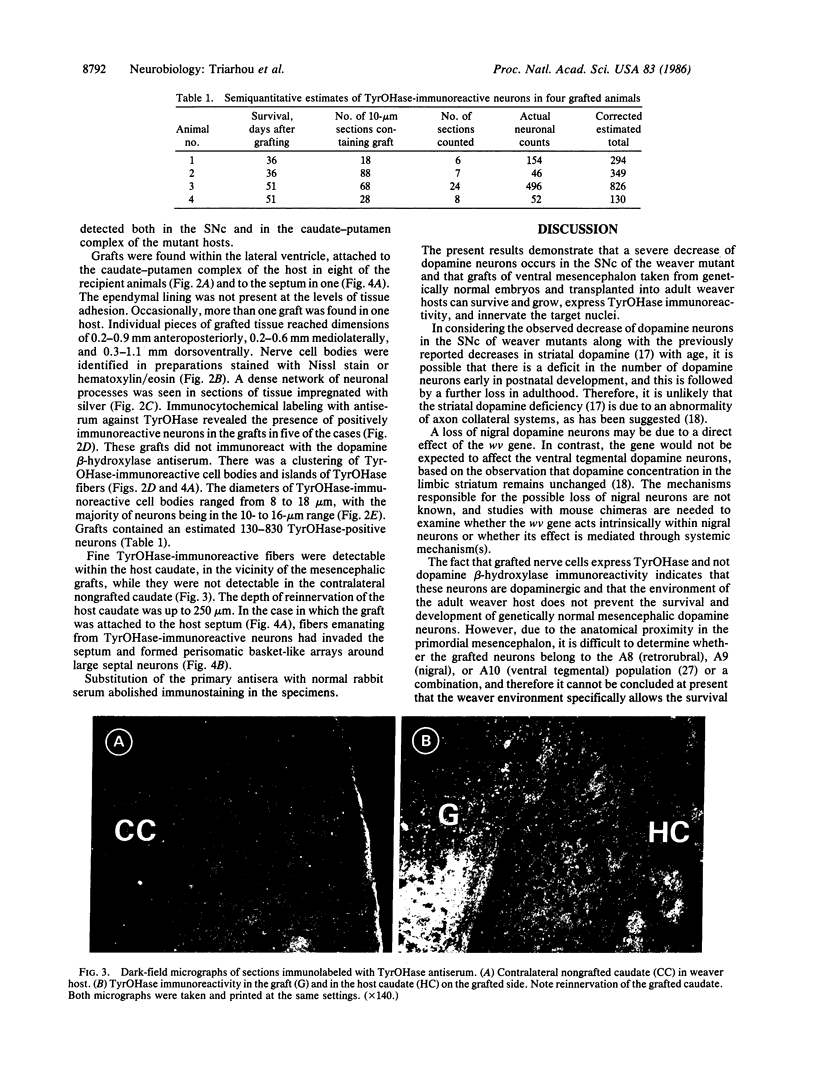
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo A. J., Björklund A., Stenevi U., Carlstedt T. Fetal mesencephalic neurons survive and extend long axons across peripheral nervous system grafts inserted into the adult rat striatum. Neurosci Lett. 1984 Mar 9;45(1):53–58. doi: 10.1016/0304-3940(84)90328-8. [DOI] [PubMed] [Google Scholar]
- Backlund E. O., Granberg P. O., Hamberger B., Knutsson E., Mårtensson A., Sedvall G., Seiger A., Olson L. Transplantation of adrenal medullary tissue to striatum in parkinsonism. First clinical trials. J Neurosurg. 1985 Feb;62(2):169–173. doi: 10.3171/jns.1985.62.2.0169. [DOI] [PubMed] [Google Scholar]
- Baker H., Joh T. H., Reis D. J. Genetic control of number of midbrain dopaminergic neurons in inbred strains of mice: relationship to size and neuronal density of the striatum. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4369–4373. doi: 10.1073/pnas.77.7.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björklund A., Dunnett S. B., Stenevi U., Lewis M. E., Iversen S. D. Reinnervation of the denervated striatum by substantia nigra transplants: functional consequences as revealed by pharmacological and sensorimotor testing. Brain Res. 1980 Oct 20;199(2):307–333. doi: 10.1016/0006-8993(80)90692-7. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979 Nov 30;177(3):555–560. doi: 10.1016/0006-8993(79)90472-4. [DOI] [PubMed] [Google Scholar]
- Burns R. S., Chiueh C. C., Markey S. P., Ebert M. H., Jacobowitz D. M., Kopin I. J. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne D. B., Duvoisin R. C., McGeer E. Speculations on the etiology of Parkinson's disease. Adv Neurol. 1984;40:353–360. [PubMed] [Google Scholar]
- Cotzias G. C., Papavasiliou P. S., Gellene R. Modification of Parkinsonism--chronic treatment with L-dopa. N Engl J Med. 1969 Feb 13;280(7):337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- DEMYER W. Impregnation of axons and terminal buttons in routine, paraffin, or frozen sections of central and peripheral nervous tissue; adaptation of Hortega's silver carbonate method for neurofibrils. Am J Clin Pathol. 1958 May;29(5):449–454. doi: 10.1093/ajcp/29.5.449. [DOI] [PubMed] [Google Scholar]
- EHRINGER H., HORNYKIEWICZ O. [Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system]. Klin Wochenschr. 1960 Dec 15;38:1236–1239. doi: 10.1007/BF01485901. [DOI] [PubMed] [Google Scholar]
- Einarson L. A Method for Progressive Selective Staining of Nissl and Nuclear Substance in Nerve Cells. Am J Pathol. 1932 May;8(3):295–308.5. [PMC free article] [PubMed] [Google Scholar]
- Fallon J. H. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J Neurosci. 1981 Dec;1(12):1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed W. J., Morihisa J. M., Spoor E., Hoffer B. J., Olson L., Seiger A., Wyatt R. J. Transplanted adrenal chromaffin cells in rat brain reduce lesion-induced rotational behaviour. Nature. 1981 Jul 23;292(5821):351–352. doi: 10.1038/292351a0. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Bolam J. P., Björklund A., Stenevi U., Dunnett S. B., Powell J. F., Smith A. D. Efferent synaptic connections of grafted dopaminergic neurons reinnervating the host neostriatum: a tyrosine hydroxylase immunocytochemical study. J Neurosci. 1985 Mar;5(3):603–616. doi: 10.1523/JNEUROSCI.05-03-00603.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden G. S. Embryologic demonstration of a nigro-striatal projection in the mouse. Brain Res. 1972 Sep 15;44(1):278–282. doi: 10.1016/0006-8993(72)90384-8. [DOI] [PubMed] [Google Scholar]
- Jaeger C. B. Cytoarchitectonics of substantia nigra grafts: a light and electron microscopic study of immunocytochemically identified dopaminergic neurons and fibrous astrocytes. J Comp Neurol. 1985 Jan 1;231(1):121–135. doi: 10.1002/cne.902310110. [DOI] [PubMed] [Google Scholar]
- Lindvall O. Mesencephalic dopaminergic afferents to the lateral septal nucleus of the rat. Brain Res. 1975 Apr 4;87(1):89–95. doi: 10.1016/0006-8993(75)90785-4. [DOI] [PubMed] [Google Scholar]
- Mahalik T. J., Finger T. E., Stromberg I., Olson L. Substantia nigra transplants into denervated striatum of the rat: ultrastructure of graft and host interconnections. J Comp Neurol. 1985 Oct 1;240(1):60–70. doi: 10.1002/cne.902400105. [DOI] [PubMed] [Google Scholar]
- Perlow M. J., Freed W. J., Hoffer B. J., Seiger A., Olson L., Wyatt R. J. Brain grafts reduce motor abnormalities produced by destruction of nigrostriatal dopamine system. Science. 1979 May 11;204(4393):643–647. doi: 10.1126/science.571147. [DOI] [PubMed] [Google Scholar]
- Pierce E. T. Time of origin of neurons in the brain stem of the mouse. Prog Brain Res. 1973;40(0):53–65. doi: 10.1016/S0079-6123(08)60679-2. [DOI] [PubMed] [Google Scholar]
- Rakic P., Sidman R. L. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973 Nov 15;152(2):103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Roffler-Tarlov S., Graybiel A. M. Weaver mutation has differential effects on the dopamine-containing innervation of the limbic and nonlimbic striatum. Nature. 1984 Jan 5;307(5946):62–66. doi: 10.1038/307062a0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. J., Sawyer B. D., Perry K. W., Fuller R. W., Foreman M. M., Ghetti B. Dopamine deficiency in the weaver mutant mouse. J Neurosci. 1982 Mar;2(3):376–380. doi: 10.1523/JNEUROSCI.02-03-00376.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Strömberg I., Johnson S., Hoffer B., Olson L. Reinnervation of dopamine-denervated striatum by substantia nigra transplants: immunohistochemical and electrophysiological correlates. Neuroscience. 1985 Apr;14(4):981–990. doi: 10.1016/0306-4522(85)90270-2. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968 Dec;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Yokochi M., Narabayashi H., Iizuka R., Nagatsu T. Juvenile parkinsonism--some clinical, pharmacological, and neuropathological aspects. Adv Neurol. 1984;40:407–413. [PubMed] [Google Scholar]