Abstract
Amphetamines are a class of psychostimulant drugs that are widely abused for their stimulant, euphoric, empathogenic and hallucinogenic properties. Many of these effects result from acute increases in dopamine and serotonin neurotransmission. Subsequent to these acute effects, methamphetamine and 3,4 methylenedioxymethamphetamine (MDMA) produce persistent damage to dopamine and serotonin nerve terminals. This review summarizes the numerous interdependent mechanisms including excitotoxicity, mitochondrial damage and oxidative stress that have been demonstrated to contribute to this damage. Emerging non-neuronal mechanisms by which the drugs may contribute to monoaminergic terminal damage, as well as the neuropsychiatric consequences of this terminal damage are also presented. Methamphetamine and 3,4-methylenedioxymethamphetamine (MDMA) have similar chemical structures and pharmacologic properties compared to other abused substances including cathinone (khat), as well as a relatively new class of novel synthetic amphetamines known as ‘bath salts’ that have gained popularity amongst drug abusers.
Keywords: Methamphetamine, 3-4-methylenedioxymethamphetamine, neurotoxicity, excitotoxicity, oxidative stress, psychosis
Introduction
Amphetamines represent a class of widely abused psychostimulant drugs which include methamphetamine (Meth) and its derivative, 3,4-methylenedioxymethamphetamine (MDMA). Amphetamine abuse is a growing concern around the world due primarily to its ability to produce significant short term feelings of euphoria. An intense research focus has been directed towards understanding these acute effects as they promote the abuse liability of the amphetamines; however, the long term consequences of their abuse are rapidly emerging and include evidence of brain injury and neurotoxicity. This review will highlight the underlying mechanisms associated with the neurotoxicity of amphetamines and discuss the neuropsychological consequences associated with the neuronal damage produced by the amphetamines. In addition, the review will introduce relatively new potential modulators that may contribute to the long-term effects of Meth and MDMA.
Neurotoxicity of Methamphetamine and MDMA
Acute Effects on Neurotransmitter Release
Methamphetamine treatment causes acute increases in both dopamine (DA) and serotonin (5HT) release. These increases result from the direct and indirect action of the drug on the DA transporter (DAT) and 5HT transporter (SERT). Meth is known to be a substrate for both transporters and is transported into the axon terminal (Rothman and Baumann, 2003, Fleckenstein et al., 2007). Following intracellular transport or diffusion, amphetamines can disrupt the vesicle proton gradient to cause an increase in cytoplasmic DA and 5HT from vesicular compartments (Sulzer and Rayport, 1990). Meth also affects cytoplasmic monoamine concentrations and DA release via altering the function of the vesicular monoamine transporter (VMAT-2) (Brown et al., 2002, Hansen et al., 2002, Riddle et al., 2002). Subsequent to increases in cytoplasmic DA and 5HT, reversal of the directionality of the DA and 5HT transporters causes significant, action potential-independent neurotransmitter efflux (Sulzer et al., 1995). Short-term decreases in neurotransmitter reuptake also contribute to increases in extracellular DA (Fleckenstein et al., 1997, Haughey et al., 2000). Secondary to increases in extracellular DA, Meth also causes acute increases in striatal glutamate as a result of D1 DA receptor-mediated disinhibition of corticostriatal glutamate release.(Mark et al., 2004). Unlike Meth, MDMA does not produce significant acute increases in striatal glutamate (Nash and Yamamoto, 1992a) but does appear to increase the extracellular concentration of glutamate in the hippocampus that may be mediated in part, through non-neuronal mechanisms (Nash and Yamamoto, 1992b, Anneken and Gudelsky, 2012). Both Meth and MDMA also increase 5HT release through similar transporter mediated mechanisms, though MDMA has a preferential affinity for SERT over DAT and consequently more pronounced effects on 5HT efflux (Rudnick and Wall, 1992, Rothman and Baumann, 2003).
Persistent DA and 5HT Terminal Damage
Subsequent to the acute effects of exposure, Meth produces long-term damage to dopaminergic and serotonergic axon terminals in the striatum, hippocampus, and prefrontal cortex (Ricaurte et al., 1980, Wagner et al., 1980, Seiden et al., 1988). In contrast, MDMA produces damage to serotonergic, but not dopaminergic axon terminals in the striatum, hippocampus, and prefrontal cortex (Battaglia et al., 1987, O’Hearn et al., 1988). The damage associated with Meth and MDMA has been shown to persist for at least 2 years in rodents, non-human primates and humans (Seiden et al., 1988, Woolverton et al., 1989, McCann et al., 1998, Volkow et al., 2001a, McCann et al., 2005) Neurochemical markers of this toxicity include decreases in the expression of tyrosine and tryptophan hydroxylase, the rate limiting enzymes for DA and 5HT, respectively as well as decreases in DA and 5HT tissue content, and decreases in DAT and SERT expression coupled with decreases in neurotransmitter uptake Vmax without changes in Km (Hotchkiss and Gibb, 1980, Wagner et al., 1980, Ricaurte et al., 1982, Ricaurte et al., 1985, Commins et al., 1987). Beyond changes in tissue content and neurotransmitter specific proteins, morphological changes indicative of axon terminal damage have been reported including the presence of swollen, distorted nerve terminals and positive Fink-Heimer staining, as well as edematous and degenerative changes seen using electron microscopy (Lorez, 1981, Ricaurte et al., 1982, Sharma and Kiyatkin, 2009). Further confirmation of the damage to terminals produced by substituted amphetamine exposure, recent Meth and MDMA self-administration studies with longer access/exposure paradigms show decreases in DAT and SERT and tyrosine hydroxylase, as well as decreases in tissue content, all indicative of drug-induced monoaminergic terminal damage (Krasnova et al., 2010, Do and Schenk, 2011, McFadden et al., 2012a, McFadden et al., 2012b).
Neuronal Damage
Though not as extensively studied, there is also supporting evidence that Meth may produce cell death, in addition to damaging DA and 5HT terminals. Increases in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), a marker for apoptotic cell death, has been reported after exposure to Meth in the prefrontal cortex and striatum (Kadota and Kadota, 2004, Cadet et al., 2005, Zhu et al., 2006b). This cell death has been identified in different subpopulations of GABA-interneurons. Within the hippocampus, cell death of calbindin-containing GABA interneurons has been demonstrated after Meth and Meth-contributes to the death of parvalbumin containing striatal GABA interneurons (Zhu et al., 2006a, Kuczenski et al., 2007). Mitochondrial damage and endoplasmic reticulum stress have been associated with this Meth-induced apoptosis (Cadet et al., 2005). Specifically, Meth has been shown to produce apoptosis through increases in caspase-3 activity and the Fas/FasL cell death pathways (Jayanthi et al., 2005). Meth also produces DNA damage and alterations in the expression of Bcl-2 related genes, which may contribute to GABA interneuron cell death (Jayanthi et al., 2001, Jeng et al., 2006).
Fewer studies have shown cell death after MDMA but there is evidence that MDMA causes apoptosis in primary hippocampal cultures (Stumm et al., 1999). MDMA has also been reported to cause cell death in cultured cortical neurons in a 5-HT2A receptor-dependent manner. In these studies, drug exposure produces increases in reactive nitrogen species as well as increases in caspase-3, indicative of apoptotic cell death (Capela et al., 2007). In vivo studies have demonstrated that MDMA exposure increases protein levels of apoptosis associated proteins such as BAX, cytochrome c and caspase-3 levels in the hippocampus (Tamburini et al., 2006, Wang et al., 2009, Soleimani Asl et al., 2012, Anneken et al., 2013). Furthermore, Wang et al. 2009 showed an increase in TUNEL staining in the hippocampus of MDMA treated rats that may be associated with the loss of parvalbumin interneurons (Anneken et al., 2013).
Mechanisms of Neurotoxicity
Excitotoxicity describes a series of events initiated by excessive glutamate release, which promotes increases in intracellular calcium levels leading to the activation of calcium-dependent proteolytic enzymes, free radical and nitric oxide (NO) production, and activation of apoptotic pathways, ultimately culminating in cellular damage (Bruno et al., 1993, Nicholls, 2004). Excessive glutamate release has been proposed to play a role in the neurotoxicity caused by both Meth and MDMA. Binge doses of Meth, but not MDMA, cause increases in extracellular glutamate in the rat striatum through activation of the striatonigral pathway (Nash and Yamamoto, 1992a, Stephans and Yamamoto, 1994, Mark et al., 2004) and inhibition of these increases in glutamate is protective against Meth neurotoxicity to DA terminals within the region (Mark et al., 2004). The evidence of excitotoxic damage within the striatum is marked by increases in calpain-specific spectrin proteolysis, mediated by glutamate in an AMPA receptor-dependent manner (Staszewski and Yamamoto, 2006).
There is limited evidence for the role of excitotoxicity in nerve terminal damage caused by MDMA. Early studies suggesting that NMDA receptors were involved in 5HT terminal damage after MDMA exposure were confounded by the fact that an NMDA antagonist also prevented hyperthermia (Finnegan et al., 1989). When hyperthermia as maintained in the presence of NMDA antagonism, NMDA antagonists no longer prevented MDMA induced 5HT terminal damage (Colado et al., 1998).
Excitotoxicity has also been proposed as a mechanism leading to the decreases in cell bodies after Meth and MDMA. Increases in glutamate in the striatum and hippocampus are known to correlate with increases in glutamate levels during Meth exposure. Recently, it was shown that somatostatin, which is a known inhibitor of glutamate neurotransmission, has the ability to reduce Meth-induced cell death in the striatum (Afanador et al., 2013). The decrease in the number of parvalbumin in the hippocampus of MDMA treated rats has been linked to the increases in extracellular glutamate and cyclooxygenase activity observed during MDMA exposure (Anneken et al., 2013).
Oxidative Stress
Oxidative stress represents another mechanism by which both Meth and MDMA cause neuronal damage. Reactive oxygen species are formed subsequent to substituted amphetamine exposure through numerous mechanisms. Acute increases in cytoplasmic and extracellular DA contribute to oxidatively damaged axon terminals after both Meth and MDMA exposure (Schmidt et al., 1985, Stone et al., 1988, Sprague et al., 1998) Direct oxidation of DA leading to quinone formation, iron-catalyzed DA metabolism via the Fenton Reaction, and metabolism of DA by monoamine oxidase-A contribute to superoxide and hydrogen peroxide production (Graham, 1978, Yamamoto and Zhu, 1998, LaVoie and Hastings, 1999). In addition, Meth or MDMA treatment leads to oxidative stress via increases in reactive nitrogen species resulting from increased nitric oxide synthase activity (Imam et al., 2000, Darvesh et al., 2005), the latter most likely derived from increases in glutamate activated NMDA or AMPA receptors (Eyerman and Yamamoto, 2007). Oxidative stress is further enhanced by depletions in antioxidant enzymes after drug exposure (Jayanthi et al., 1998, Jayanthi et al., 1999). Subsequent to Meth exposure, oxidative damage is manifested as lipid peroxidation and protein carbonyl formation (Yamamoto and Zhu, 1998, Gluck et al., 2001). Specific nitration and nitrosylation of proteins important for monoamine synthesis and release including VMAT-2 and tyrosine and tryptophan hydroxylase have also been reported (Kuhn et al., 1999, Kuhn and Geddes, 1999, Eyerman and Yamamoto, 2007). The oxidative modification of these proteins is particularly significant as it restricts their activity and contributes to their degradation, thus playing a major role in the neurotoxicity profile of the amphetamines. Antioxidant treatments have also been shown to be neuroprotective against the damage produced by Meth or MDMA and substantiates the significant contribution of oxidative stress to the neurotoxicity of substituted amphetamines (Gudelsky, 1996, Sanchez et al., 2003, Fukami et al., 2004).
Altered Metabolism and Mitochondrial Damage
Meth and MDMA also contribute to monoaminergic terminal damage though altered energy metabolism, including mitochondrial damage. During exposure to Meth or MDMA, there is a high demand for energy and acute increases in energy metabolism in brain regions that exhibit increased activity produced by the drugs (Pontieri et al., 1990, Quate et al., 2004). Soon after the excessive increase in energy metabolism reported during drug exposure, there is evidence of compromised energy metabolism and depleted energy stores (Chan et al., 1994, Huang et al., 1999, Darvesh et al., 2002). These decreases in energy metabolism are further exacerbated by damage to mitochondria and specific complexes of the electron transport chain including complex II–III and IV (Burrows et al., 2000). More specifically, decreases in complex II after Meth and MDMA (Burrows et al., 2000b; Nixdorf et al., 2001) were shown to be mediated by glutamate and nitric oxide (Brown et al., 2005) and when substrates for complex II–III are supplied after drug exposure or when increases in glutamate were blocked, mitochondria damage as well as DA and 5HT terminal damage were prevented. Collectively, these results provide strong support for the role of glutamate in mediating compromised mitochondrial bioenergetics to produce axon terminal damage (Stephans et al., 1998, Darvesh and Gudelsky, 2005).
Emerging Mechanisms of Neurotoxicity
Numerous, complex interacting mechanisms have been identified as significant contributors to the neurotoxic effects of Meth and MDMA, yet many studies only consider how these mechanisms originate within neurons. Because both drugs are always administered systemically and may affect both brain and peripheral organ function, it is important to consider how the non-neuronal effects of substituted amphetamines contribute to the neuronal damage produced by the drugs. Identification and understanding of these non-neuronal contributors is important because they have the potential to significantly contribute to the excitotoxicity, oxidative stress and metabolic compromise that follows Meth and MDMA exposure. Furthermore, the elucidation of these non-neuronal mediators of toxicity could reveal novel targets for the treatment of the complex neurotoxicity produced by Meth and MDMA.
Peripheral Organ Damage
Meth and MDMA have been reported to cause significant peripheral organ damage (Smith and Fischer, 1970, Turillazzi et al., 2010). Clinical reports suggest that the cardiac, renal, muscular, and hepatic organ systems may be especially susceptible to damage by Meth and MDMA (Jones et al., 1994, Ellis et al., 1996, Milroy et al., 1996, Kamijo et al., 2002, Ben-Abraham et al., 2003, Wijetunga et al., 2003, Ago et al., 2006). Specifically, MDMA has been shown to oxidatively modify hepatocellular mitochondrial proteins (Moon et al., 2008). The mechanism by which amphetamines contribute to mitochondrial damage in peripheral organs is unknown, though it can be speculated that the drugs produce reactive oxygen species subsequent to the metabolism of the drugs in the liver by the cytochrome p450 system, or secondarily as a result of the hyperthermic or cardiac effects of the drugs (Skibba et al., 1989, Wang et al., 1990, Hong et al., 1991, Cherner et al., 2010, Pourahmad et al., 2010). The significance of drug-mediated peripheral organ damage is highlighted by the dependence of the brain on peripheral organ function and that alterations in peripheral physiology can contribute to neuronal pathology. For example, Meth has been shown to produce liver damage and concurrent increases in both peripheral and brain ammonia, a neurotoxic byproduct of protein and amino acid metabolism (Halpin and Yamamoto, 2012). Liver damage is associated with these increases in ammonia as the liver metabolizes ammonia to urea via the urea cycle so it can be efficiently excreted via the kidneys (Felipo and Butterworth, 2002b). When these increases in both plasma and brain ammonia were blocked, Meth induced damage to DA and 5HT terminals was also attenuated (Halpin and Yamamoto, 2012). In fact, ammonia per se has been shown to cause neurotoxicity via excitotoxicity, oxidative damage, and metabolic compromise similar to that produced by Meth and MDMA (Kosenko et al., 1995, Felipo and Butterworth, 2002a, Kosenko et al., 2003, Gorg et al., 2007). Accordingly, ammonia should now be considered as a peripherally derived mediator of Meth neurotoxicity that likely contributes synergistically to the neurotoxic mechanisms that were classically thought to result from the neuronal effects of the drug.
Inflammation
Meth and MDMA have been shown to trigger inflammatory responses in areas where DA and 5HT terminals are damaged. Given the potential damaging effects of inflammation within the CNS, these processes have been suggested to play a role in the damage to monoamine nerve terminals. Of particular interest has been microglial activation, which occurs during MDMA and Meth exposure. Meth causes activation of microglia in the striatum, cortex and hippocampus (Escubedo et al., 1998, Guilarte et al., 2003, Pubill et al., 2003, Thomas et al., 2004). The mechanism through which this activation occurs is not known; however dopaquinones are known to be strong activators of microglia (Kuhn et al., 2006). Thus, the increased cytosolic DA and oxidative stress during Meth can promote the production of dopaquinones and potentially promote microglial activation.
Glutamate may contribute to inflammation and microglial activation during Meth. For instance, glutamate receptor activation is known to stimulate microglial activation whereas antagonism suppresses the appearance of microglial activation (Neumann, 2001, Thomas and Kuhn, 2005). Furthermore, microglial activation correlates regionally with increases in extracellular glutamate observed during MDMA and Meth. During Meth exposure, microglial activation and increases in glutamate are seen in the striatum, prefrontal cortex and hippocampus; however the activation of microglia and increases in glutamate are not observed within the striatum and prefrontal cortex during MDMA exposure (Nash and Yamamoto, 1992a, Stephans and Yamamoto, 1994, Pubill et al., 2003, Mark et al., 2004).
The ability of activated microglia to promote neuronal damage during Meth and MDMA exposure has not been fully elucidated. Nevertheless, cytokines produced during microglial activation are known to enhance glutamate neurotransmission, and thus promote excitotoxicity (Zou and Crews, 2005). Increases in FasL, a member of the TNFα family of cytokines, can be seen in the rat striatum as early as 2–4 hour after a high Meth dose (Jayanthi et al., 2005). Similarly, other cytokines including IL5, TNF-α and IL-1α have been reported to be increased in the mouse striatum following Meth (Sriram et al., 2006, Goncalves et al., 2008). Whether these cytokines contribute to Meth toxicity is unknown, but Sriram et al. 2006 showed that attenuation of microglial activation by minocycline did not reduce DA terminal damage. A caveat however, is that TNFα was still increased despite a reduction in microglial activation. The role of cytokines has also been implicated in mediating MDMA toxicity as several studies have shown that MDMA can increase IL1β in the rat frontal cortex (Orio et al., 2004, O’Shea et al., 2005, Kuhn et al., 2006). It is not known if IL1β mediates the MDMA-induced damage to 5HT neurons but when IL-1β alone was administered intracerebroventricularly, toxicity to 5HT terminals was observed (Kuhn et al., 2006).
Cannabinoids
Cannabinoids are a class of molecules which are released during neuronal activation and have a role in modulating inflammation as well as neurotransmission. Two subtypes of receptors mediate most of the actions caused by cannabinoids with CB1 receptors being primarily located within the CNS and CB2 receptors in the periphery, mostly on immune related cells (Matsuda et al., 1990, Munro et al., 1993). The role of CB2 receptors within the CNS is not as clear due to the relatively sparse expression of CB2 receptors within the CNS. However, it has been shown more recently that microglia increase their expression of CB2 receptors during pathologic situations, mainly when inflammation occurs and when microglia become activated (Maresz et al., 2005, Yiangou et al., 2006) supporting the notion that CB2 receptors are known to have a regulatory role in inhibiting microglial activation and function (Puffenbarger et al., 2000, Ramirez et al., 2005). Interestingly, MDMA was shown to increase CB2 receptors on microglia (Torres et al., 2010). Given the suggested role of inflammatory processes and microglial activation with MDMA neurotoxicity, these changes in CB2 receptors may represent an attempt to suppress inflammatory processes and damage during MDMA exposure.
Within the CNS, CB1 receptors are localized predominantly on the terminals of neurons, where they function to regulate release of neurotransmitters (Ishac et al., 1996, Katona et al., 1999). Most notably, these receptors are present on glutamate and GABA terminals, where they regulate the release of their respective neurotransmitters. CB1 receptors have been shown to inhibit release of glutamate in hippocampal and striatal neurons (Shen et al., 1996, Gerdeman and Lovinger, 2001, Huang et al., 2001). Additionally, CB1 receptor activation leads to increases in extracellular DA release within the nucleus accumbens, striatum and prefrontal cortex (Ng Cheong Ton et al., 1988, Chen et al., 1990, Malone and Taylor, 1999). This is interesting in light of the fact that CB1 receptors are not found on DA terminals, but rather colocalize with glutamatergic and GABAergic terminals (Herkenham et al., 1991, Tsou et al., 1998). Thus, it has been proposed that within the striatum, cannabinoids cause DA release indirectly, through its effects on glutamate (Fernandez-Ruiz et al., 2010). Recently, it was shown that a CB1 antagonist attenuates Meth-induced deficits in evoked dopamine release in the nucleus accumbens (Loewinger et al., 2012). Furthermore, a CB1 antagonist were shown to prevent the increases in DA caused by (+)-amphetamine within the nucleus accumbens shell region (Kleijn et al., 2012). In light of this evidence, there exists the possibility that CB1 receptors are involved in DA release caused by substituted-amphetamines and thus neurotoxicity.
Consequences of Neurotoxicity
Neuropsychological Effects
Given the numerous reports of cellular and terminal damage caused by Meth and MDMA to the CNS, neuropsychological impairments are likely consequences. Exposure to amphetamines also is correlated with numerous long-term neuropsychological impairments which are associated with the persistent and significant DA and 5HT terminal damage seen in the striatum, prefrontal cortex, and hippocampus after drug exposure. Long term use of Meth is associated with impaired impulse control, working memory, decision making, attention, and motor coordination (Rogers et al., 1999, Volkow et al., 2001b, Simon et al., 2002, Clark et al., 2006, Johanson et al., 2006). Withdrawal from Meth is associated with disturbed sleep, anxiety, depressed mood, reduced energy and agitation (McGregor et al., 2005). It is often believed that these long term impairments contribute to the high relapse potential observed with past Meth use. Abstinent patients who were previously dependent on the drug also have a high potential for relapse, exhibit deficits in cortex-mediated decision making, and have decreases in cortical dopaminergic activity known to be predictive of relapse (Paulus et al., 2005, Wang et al., 2012). In addition to the neuropsychiatric consequences associated with drug use, Meth use has also been associated with an increased risk of developing Parkinson’s disease (Callaghan et al., 2010, Granado et al., 2013)
The use of MDMA has been associated with a number of neuropsychological impairments, consistent with the serotonergic damage in the prefrontal cortex, hippocampus and striatum produced by the drug. Several researchers have reported memory impairments in abstinent MDMA abusers (Krystal et al., 1992, Curran and Travill, 1997, Parrott et al., 1998). In addition to these memory impairments, there are significant reductions in executive functioning and attention, as well as enhanced impulsivity amongst MDMA abusers (Morgan, 1998, McCann et al., 1999). As with Meth these cognitive impairments have been suggested to underlie drug addiction processes observed with MDMA. While each of these impairments have been shown in numerous Meth- and MDMA-dependent subjects, it is also worth noting that the presence of these symptoms before substituted amphetamine use is unknown and may precede and contribute to their use and addiction.
Protracted Psychosis
The long-term damage produced by Meth or MDMA may also represent a vulnerability factor contributing to protracted psychosis and psychotic disorders, in addition to the acute psychotic effects of the drugs (Flaum and Schultz, 1996). Abuse of amphetamines is associated with a significant increase in the prevalence of psychosis and this association is especially strong in the context of Meth use and amphetamine abuse in the mid-late teen years (Harris and Batki, 2000, McKetin et al., 2006). Psychosis presenting subsequent to amphetamine-type stimulant use or in the context of schizophrenia share many common features and have been suggested to share a common neural substrate (Hermens et al., 2009). Of these, substituted-amphetamine induced alterations to DA, GABA and glutamate neurotransmitter systems are likely the most significant contributors to protracted psychosis and vulnerability to psychotic disorders.
Hyperactivity of mesolimbic DA pathways is strongly associated with the positive symptoms of schizophrenia and a target of many antipsychotic drugs. At first glance, the hyperactive mesolimbic DA activity postulated to contribute to psychosis appears to be paradoxically associated with the dopaminerrgic depletions produced by Meth; however mesolimbic dopaminergic projections are relatively spared from Meth-induced dopamine terminal damage (Granado et al., 2010). Resiliency of mesolimbic dopaminergic projections to the terminal damage produced by Meth may be the result of increased antioxidant capacity, neuropeptide production, or a differential expression of glutamate receptors and monoamine transporters, and blunted glutamate release (Hung and Lee, 1996, Chung et al., 2005, Fitzpatrick et al., 2005, Sava et al., 2006). Although beyond the scope of the discussion of amphetamine-induced psychosis, it is worth noting that the mesocortical DA depletions produced by Meth may contribute to the negative, cognitive and affective symptoms associated with schizophrenia. Beyond changes in DA function, the alteration of glutamate and GABA neurotransmission produced by Meth represent mechanisms by which the drug contributes to psychosis. Sensory gating is important for modulating the perceptual distortions contributing to psychosis and has been shown to be affected by Meth (Hadamitzky et al., 2011). Meth-induced decreases in sensorimotor gating have been attributed to alterations in both glutamate and downstream GABA neurotransmission in cortico-striatal-thalamic-cortical sensory filtering circuits (Arai et al., 2008, Mizoguchi et al., 2009). Prolonged psychosis has also been reported after MDMA use, and although this has not been studied as in-depth as Meth-associated psychosis, alterations in hippocampal activity could potentially contribute to the psychotomimetic effects of MDMA (Nifosi et al., 2009, Potash et al., 2009, Patel et al., 2011).
Conclusion
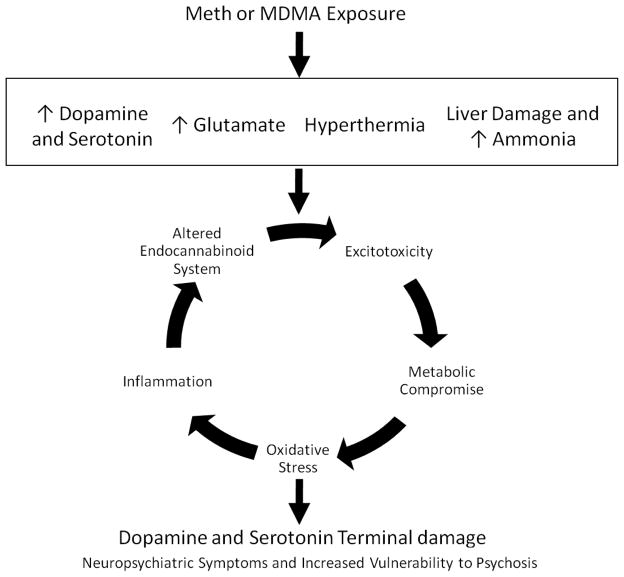
In conclusion, numerous interacting mechanisms have been established to contribute to the damage produced by Meth and MDMA. These mechanisms include excitotoxicity, oxidative stress and metabolic compromise. More recently, novel contributors to Meth and MDMA neurotoxicity have been identified and include inflammation, peripheral organ damage and the endocannabinoid system (Figure 1). Further understanding of the monoaminergic terminal damage produced by substituted amphetamines is important because this damage is associated with numerous neuropsychological effects as well as increased vulnerability to developing psychosis. Beyond these effects, understanding the neurotoxic consequences of Meth and MDMA is important when more broadly considering the effects of other synthetic amphetamines and cannabis-related compounds. For instance, Meth and MDMA have similar chemical structures and pharmacologic targets to other commonly abused substances including cathinone (khat), as well as a relatively new series of synthetic amphetamines which have gained popularity amongst drug abusers, known as ‘bath salts’. These compounds consist of cathinone derivitaves, including mephedrone, methylone, methedrone and buthylone. These ingredients of bath salts have been reported to have similar pharmacologic profiles as methamphetamine and MDMA (Hadlock et al., 2011, Baumann et al., 2012, Cameron et al., 2013). Reports also note that acute intoxication resulting from exposure to these novel compounds share characteristics with those seen after substituted amphetamine exposure (James et al., 2011, Kasick et al., 2012). Accordingly, understanding of the neuronal damage and neuropsychiatric consequences produced by the substituted amphetamines methamphetamine and MDMA may afford valuable insight to our understanding of the acute and long term consequences of exposure to cathinones and bath salts.
Figure 1. Mechanisms of Meth and MDMA Neurotoxicity.
Meth and MDMA produce persistent monoaminergic terminal damage that is associated with neuropsychiatric symptoms as well as increased vulnerability to psychosis. Acutely, drug exposure contributes to increases in DA and/or 5HT, and glutamate. These substituted amphetamines also produce acute hyperthermia and can contribute to liver damage. Downstream of these short-term effects, numerous interacting and feed-forward mechanisms have been identified as contributors to the neurotoxicity of these drugs of abuse. These mechanisms include excitotoxicity, metabolic compromise, oxidative stress, and more recently, inflammation and altered endocannbinoid system function.
Footnotes
Conflicts of Interest:
All authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afanador L, Mexhitaj I, Diaz C, Ordonez D, Baker L, Angulo JA. The role of the neuropeptide somatostatin on methamphetamine and glutamate-induced neurotoxicity in the striatum of mice. Brain research. 2013;1510:38–47. doi: 10.1016/j.brainres.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ago M, Ago K, Hara K, Kashimura S, Ogata M. Toxicological and histopathological analysis of a patient who died nine days after a single intravenous dose of methamphetamine: a case report. Leg Med (Tokyo) 2006;8:235–239. doi: 10.1016/j.legalmed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Anneken JH, Cunningham JI, Collins SA, Yamamoto BK, Gudelsky GA. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: role of cyclooxygenase. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:58–65. doi: 10.1007/s11481-012-9420-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anneken JH, Gudelsky GA. MDMA produces a delayed and sustained increase in the extracellular concentration of glutamate in the rat hippocampus. Neuropharmacology. 2012;63:1022–1027. doi: 10.1016/j.neuropharm.2012.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Takahashi K, Kamei H, Nabeshima T, Yamada K. Involvement of pallidotegmental neurons in methamphetamine- and MK-801-induced impairment of prepulse inhibition of the acoustic startle reflex in mice: reversal by GABAB receptor agonist baclofen. Neuropsychopharmacology. 2008;33:3164–3175. doi: 10.1038/npp.2008.41. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Yeh SY, O’Hearn E, Molliver ME, Kuhar MJ, De Souza EB. 3,4-Methylenedioxymethamphetamine and 3,4-methylenedioxyamphetamine destroy serotonin terminals in rat brain: quantification of neurodegeneration by measurement of [3H]paroxetine-labeled serotonin uptake sites. The Journal of pharmacology and experimental therapeutics. 1987;242:911–916. [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Abraham R, Szold O, Rudick V, Weinbroum AA. ‘Ecstasy’ intoxication: life-threatening manifestations and resuscitative measures in the intensive care setting. Eur J Emerg Med. 2003;10:309–313. doi: 10.1097/00063110-200312000-00013. [DOI] [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. The Journal of pharmacology and experimental therapeutics. 2002;302:497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Bruno V, Scapagnini U, Canonico PL. Excitatory amino acids and neurotoxicity. Functional neurology. 1993;8:279–292. [PubMed] [Google Scholar]
- Burrows KB, Gudelsky G, Yamamoto BK. Rapid and transient inhibition of mitochondrial function following methamphetamine or 3,4-methylenedioxymethamphetamine administration. European journal of pharmacology. 2000;398:11–18. doi: 10.1016/s0014-2999(00)00264-8. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Jayanthi S, Deng X. Methamphetamine-induced neuronal apoptosis involves the activation of multiple death pathways. Review. Neurotoxicity research. 2005;8:199–206. doi: 10.1007/BF03033973. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sajeev G, Kish SJ. Incidence of Parkinson’s disease among hospital patients with methamphetamine-use disorders. Mov Disord. 2010;25:2333–2339. doi: 10.1002/mds.23263. [DOI] [PubMed] [Google Scholar]
- Cameron KN, Kolanos R, Solis E, Jr, Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. British journal of pharmacology. 2013;168:1750–1757. doi: 10.1111/bph.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela JP, Fernandes E, Remiao F, Bastos ML, Meisel A, Carvalho F. Ecstasy induces apoptosis via 5-HT(2A)-receptor stimulation in cortical neurons. Neurotoxicology. 2007;28:868–875. doi: 10.1016/j.neuro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Chan P, Di Monte DA, Luo JJ, DeLanney LE, Irwin I, Langston JW. Rapid ATP loss caused by methamphetamine in the mouse striatum: relationship between energy impairment and dopaminergic neurotoxicity. Journal of neurochemistry. 1994;62:2484–2487. doi: 10.1046/j.1471-4159.1994.62062484.x. [DOI] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology. 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F, Atkinson JH, Heaton R, Grant I. Cytochrome P450–2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: preliminary findings. J Int Neuropsychol Soc. 2010;16:890–901. doi: 10.1017/S1355617710000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biological psychiatry. 2006;60:515–522. doi: 10.1016/j.biopsych.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Colado MI, Granados R, O’Shea E, Esteban B, Green AR. Role of hyperthermia in the protective action of clomethiazole against MDMA (‘ecstasy’)-induced neurodegeneration, comparison with the novel NMDA channel blocker AR-R15896AR. British journal of pharmacology. 1998;124:479–484. doi: 10.1038/sj.bjp.0701859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. The Journal of pharmacology and experimental therapeutics. 1987;241:338–345. [PubMed] [Google Scholar]
- Curran HV, Travill RA. Mood and cognitive effects of +/−3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’): week-end ‘high’ followed by midweek low. Addiction. 1997;92:821–831. [PubMed] [Google Scholar]
- Darvesh AS, Gudelsky GA. Evidence for a role of energy dysregulation in the MDMA-induced depletion of brain 5-HT. Brain research. 2005;1056:168–175. doi: 10.1016/j.brainres.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Shankaran M, Gudelsky GA. 3,4-Methylenedioxymethamphetamine produces glycogenolysis and increases the extracellular concentration of glucose in the rat brain. The Journal of pharmacology and experimental therapeutics. 2002;301:138–144. doi: 10.1124/jpet.301.1.138. [DOI] [PubMed] [Google Scholar]
- Darvesh AS, Yamamoto BK, Gudelsky GA. Evidence for the involvement of nitric oxide in 3,4-methylenedioxymethamphetamine-induced serotonin depletion in the rat brain. The Journal of pharmacology and experimental therapeutics. 2005;312:694–701. doi: 10.1124/jpet.104.074849. [DOI] [PubMed] [Google Scholar]
- Do J, Schenk S. Self-administered MDMA produces dose- and time-dependent serotonin deficits in the rat brain. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00370.x. [DOI] [PubMed] [Google Scholar]
- Ellis AJ, Wendon JA, Portmann B, Williams R. Acute liver damage and ecstasy ingestion. Gut. 1996;38:454–458. doi: 10.1136/gut.38.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escubedo E, Guitart L, Sureda FX, Jimenez A, Pubill D, Pallas M, Camins A, Camarasa J. Microgliosis and down-regulation of adenosine transporter induced by methamphetamine in rats. Brain research. 1998;814:120–126. doi: 10.1016/s0006-8993(98)01065-8. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J Neurochem. 2007;103:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- Felipo V, Butterworth RF. Mitochondrial dysfunction in acute hyperammonemia. Neurochem Int. 2002a;40:487–491. doi: 10.1016/s0197-0186(01)00119-x. [DOI] [PubMed] [Google Scholar]
- Felipo V, Butterworth RF. Neurobiology of ammonia. Prog Neurobiol. 2002b;67:259–279. doi: 10.1016/s0301-0082(02)00019-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Ruiz J, Hernandez M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS neuroscience & therapeutics. 2010;16:e72–91. doi: 10.1111/j.1755-5949.2010.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan KT, Skratt JJ, Irwin I, Langston JW. The N-methyl-D-aspartate (NMDA) receptor antagonist, dextrorphan, prevents the neurotoxic effects of 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neuroscience letters. 1989;105:300–306. doi: 10.1016/0304-3940(89)90637-x. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick E, Ashkan K, Wallace BA, Benabid AL, Mitrofanis J. Differential survival patterns among midbrain dopaminergic cells of MPTP-treated monkeys and 6OHDA-lesioned rats. Anat Embryol (Berl) 2005;210:101–123. doi: 10.1007/s00429-005-0003-y. [DOI] [PubMed] [Google Scholar]
- Flaum M, Schultz SK. When does amphetamine-induced psychosis become schizophrenia? The American journal of psychiatry. 1996;153:812–815. doi: 10.1176/ajp.153.6.812. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Gibb JW, Hanson GR. A rapid and reversible change in dopamine transporters induced by methamphetamine. Eur J Pharmacol. 1997;323:R9–10. doi: 10.1016/s0014-2999(97)00148-9. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fukami G, Hashimoto K, Koike K, Okamura N, Shimizu E, Iyo M. Effect of antioxidant N-acetyl-L-cysteine on behavioral changes and neurotoxicity in rats after administration of methamphetamine. Brain research. 2004;1016:90–95. doi: 10.1016/j.brainres.2004.04.072. [DOI] [PubMed] [Google Scholar]
- Gerdeman G, Lovinger DM. CB1 cannabinoid receptor inhibits synaptic release of glutamate in rat dorsolateral striatum. Journal of neurophysiology. 2001;85:468–471. doi: 10.1152/jn.2001.85.1.468. [DOI] [PubMed] [Google Scholar]
- Gluck MR, Moy LY, Jayatilleke E, Hogan KA, Manzino L, Sonsalla PK. Parallel increases in lipid and protein oxidative markers in several mouse brain regions after methamphetamine treatment. J Neurochem. 2001;79:152–160. doi: 10.1046/j.1471-4159.2001.00549.x. [DOI] [PubMed] [Google Scholar]
- Goncalves J, Martins T, Ferreira R, Milhazes N, Borges F, Ribeiro CF, Malva JO, Macedo TR, Silva AP. Methamphetamine-induced early increase of IL-6 and TNF-alpha mRNA expression in the mouse brain. Annals of the New York Academy of Sciences. 2008;1139:103–111. doi: 10.1196/annals.1432.043. [DOI] [PubMed] [Google Scholar]
- Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- Graham DG. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Molecular pharmacology. 1978;14:633–643. [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, Moratalla R. Methamphetamine and Parkinson’s disease. Parkinsons Dis. 2013;2013:308052. doi: 10.1155/2013/308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R. Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration : early loss of TH in striosomes after methamphetamine. Neurotoxicity research. 2010;18:48–58. doi: 10.1007/s12640-009-9106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA. Effect of ascorbate and cysteine on the 3,4-methylenedioxymethamphetamine-induced depletion of brain serotonin. J Neural Transm. 1996;103:1397–1404. doi: 10.1007/BF01271253. [DOI] [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behavioural brain research. 2011;217:386–390. doi: 10.1016/j.bbr.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. The Journal of pharmacology and experimental therapeutics. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen JP, Riddle EL, Sandoval V, Brown JM, Gibb JW, Hanson GR, Fleckenstein AE. Methylenedioxymethamphetamine decreases plasmalemmal and vesicular dopamine transport: mechanisms and implications for neurotoxicity. The Journal of pharmacology and experimental therapeutics. 2002;300:1093–1100. doi: 10.1124/jpet.300.3.1093. [DOI] [PubMed] [Google Scholar]
- Harris D, Batki SL. Stimulant psychosis: symptom profile and acute clinical course. The American journal on addictions / American Academy of Psychiatrists in Alcoholism and Addictions. 2000;9:28–37. doi: 10.1080/10550490050172209. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Brown JM, Wilkins DG, Hanson GR, Fleckenstein AE. Differential effects of methamphetamine on Na(+)/Cl(−)-dependent transporters. Brain research. 2000;863:59–65. doi: 10.1016/s0006-8993(00)02094-1. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermens DF, Lubman DI, Ward PB, Naismith SL, Hickie IB. Amphetamine psychosis: a model for studying the onset and course of psychosis. Med J Aust. 2009;190:S22–25. doi: 10.5694/j.1326-5377.2009.tb02370.x. [DOI] [PubMed] [Google Scholar]
- Hong R, Matsuyama E, Nur K. Cardiomyopathy associated with the smoking of crystal methamphetamine. JAMA. 1991;265:1152–1154. [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. The Journal of pharmacology and experimental therapeutics. 1980;214:257–262. [PubMed] [Google Scholar]
- Huang CC, Lo SW, Hsu KS. Presynaptic mechanisms underlying cannabinoid inhibition of excitatory synaptic transmission in rat striatal neurons. The Journal of physiology. 2001;532:731–748. doi: 10.1111/j.1469-7793.2001.0731e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Tsai SJ, Su TW, Sim CB. Effects of repeated high-dose methamphetamine on local cerebral glucose utilization in rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;21:427–434. doi: 10.1016/S0893-133X(99)00029-9. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EH. The mesolimbic dopaminergic pathway is more resistant than the nigrostriatal dopaminergic pathway to MPTP and MPP+ toxicity: role of BDNF gene expression. Brain Res Mol Brain Res. 1996;41:14–26. doi: 10.1016/0169-328x(96)00062-9. [DOI] [PubMed] [Google Scholar]
- Imam SZ, Islam F, Itzhak Y, Slikker W, Jr, Ali SF. Prevention of dopaminergic neurotoxicity by targeting nitric oxide and peroxynitrite: implications for the prevention of methamphetamine-induced neurotoxic damage. Annals of the New York Academy of Sciences. 2000;914:157–171. doi: 10.1111/j.1749-6632.2000.tb05193.x. [DOI] [PubMed] [Google Scholar]
- Ishac EJ, Jiang L, Lake KD, Varga K, Abood ME, Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. British journal of pharmacology. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James D, Adams RD, Spears R, Cooper G, Lupton DJ, Thompson JP, Thomas SH National Poisons Information S. Clinical characteristics of mephedrone toxicity reported to the U.K. National Poisons Information Service. Emergency medicine journal : EMJ. 2011;28:686–689. doi: 10.1136/emj.2010.096636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death Bcl-2 genes in the mouse neocortex. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Andrews AM, Cadet JL. Overexpression of human copper/zinc superoxide dismutase in transgenic mice attenuates oxidative stress caused by methylenedioxymethamphetamine (Ecstasy) Neuroscience. 1999;91:1379–1387. doi: 10.1016/s0306-4522(98)00698-8. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Ladenheim B, Cadet JL. Methamphetamine-induced changes in antioxidant enzymes and lipid peroxidation in copper/zinc-superoxide dismutase transgenic mice. Annals of the New York Academy of Sciences. 1998;844:92–102. [PubMed] [Google Scholar]
- Jeng W, Ramkissoon A, Parman T, Wells PG. Prostaglandin H synthase-catalyzed bioactivation of amphetamines to free radical intermediates that cause CNS regional DNA oxidation and nerve terminal degeneration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:638–650. doi: 10.1096/fj.05-5271com. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Jones AL, Jarvie DR, McDermid G, Proudfoot AT. Hepatocellular damage following amphetamine intoxication. J Toxicol Clin Toxicol. 1994;32:435–444. doi: 10.3109/15563659409011046. [DOI] [PubMed] [Google Scholar]
- Kadota T, Kadota K. Neurotoxic morphological changes induced in the medial prefrontal cortex of rats behaviorally sensitized to methamphetamine. Archives of histology and cytology. 2004;67:241–251. doi: 10.1679/aohc.67.241. [DOI] [PubMed] [Google Scholar]
- Kamijo Y, Soma K, Nishida M, Namera A, Ohwada T. Acute liver failure following intravenous methamphetamine. Vet Hum Toxicol. 2002;44:216–217. [PubMed] [Google Scholar]
- Kasick DP, McKnight CA, Klisovic E. “Bath salt” ingestion leading to severe intoxication delirium: two cases and a brief review of the emergence of mephedrone use. The American journal of drug and alcohol abuse. 2012;38:176–180. doi: 10.3109/00952990.2011.643999. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijn J, Wiskerke J, Cremers TI, Schoffelmeer AN, Westerink BH, Pattij T. Effects of amphetamine on dopamine release in the rat nucleus accumbens shell region depend on cannabinoid CB1 receptor activation. Neurochemistry international. 2012;60:791–798. doi: 10.1016/j.neuint.2012.03.002. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Kaminsky Y, Grau E, Minana MD, Grisolia S, Felipo V. Nitroarginine, an inhibitor of nitric oxide synthetase, attenuates ammonia toxicity and ammonia-induced alterations in brain metabolism. Neurochem Res. 1995;20:451–456. doi: 10.1007/BF00973101. [DOI] [PubMed] [Google Scholar]
- Kosenko E, Llansola M, Montoliu C, Monfort P, Rodrigo R, Hernandez-Viadel M, Erceg S, Sanchez-Perez AM, Felipo V. Glutamine synthetase activity and glutamine content in brain: modulation by NMDA receptors and nitric oxide. Neurochem Int. 2003;43:493–499. doi: 10.1016/s0197-0186(03)00039-1. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Price LH, Opsahl C, Ricaurte GA, Heninger GR. Chronic 3,4-methylenedioxymethamphetamine (MDMA) use: effects on mood and neuropsychological function? The American journal of drug and alcohol abuse. 1992;18:331–341. doi: 10.3109/00952999209026070. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Everall IP, Crews L, Adame A, Grant I, Masliah E. Escalating dose-multiple binge methamphetamine exposure results in degeneration of the neocortex and limbic system in the rat. Experimental neurology. 2007;207:42–51. doi: 10.1016/j.expneurol.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Aretha CW, Geddes TJ. Peroxynitrite inactivation of tyrosine hydroxylase: mediation by sulfhydryl oxidation, not tyrosine nitration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19:10289–10294. doi: 10.1523/JNEUROSCI.19-23-10289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Annals of the New York Academy of Sciences. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Geddes TJ. Peroxynitrite inactivates tryptophan hydroxylase via sulfhydryl oxidation. Coincident nitration of enzyme tyrosyl residues has minimal impact on catalytic activity. The Journal of biological chemistry. 1999;274:29726–29732. doi: 10.1074/jbc.274.42.29726. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J Neurosci. 1999;19:1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger GC, Beckert MV, Tejeda HA, Cheer JF. Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism. Neuropharmacology. 2012;62:2192–2201. doi: 10.1016/j.neuropharm.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorez H. Fluorescence histochemistry indicates damage of striatal dopamine nerve terminals in rats after multiple doses of methamphetamine. Life sciences. 1981;28:911–916. doi: 10.1016/0024-3205(81)90053-9. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. British journal of pharmacology. 1999;128:21–26. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. Journal of neurochemistry. 2005;95:437–445. doi: 10.1111/j.1471-4159.2005.03380.x. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McCann UD, Mertl M, Eligulashvili V, Ricaurte GA. Cognitive performance in (+/−) 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users: a controlled study. Psychopharmacology. 1999;143:417–425. doi: 10.1007/s002130050967. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Quantitative PET studies of the serotonin transporter in MDMA users and controls using [11C]McN5652 and [11C]DASB. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;30:1741–1750. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hadlock GC, Allen SC, Vieira-Brock PL, Stout KA, Ellis JD, Hoonakker AJ, Andrenyak DM, Nielsen SM, Wilkins DG, Hanson GR, Fleckenstein AE. Methamphetamine self-administration causes persistent striatal dopaminergic alterations and mitigates the deficits caused by a subsequent methamphetamine exposure. The Journal of pharmacology and experimental therapeutics. 2012a;340:295–303. doi: 10.1124/jpet.111.188433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden LM, Hunt MM, Vieira-Brock PL, Muehle J, Nielsen SM, Allen SC, Hanson GR, Fleckenstein AE. Prior methamphetamine self-administration attenuates serotonergic deficits induced by subsequent high-dose methamphetamine administrations. Drug and alcohol dependence. 2012b;126:87–94. doi: 10.1016/j.drugalcdep.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor C, Srisurapanont M, Jittiwutikarn J, Laobhripatr S, Wongtan T, White JM. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100:1320–1329. doi: 10.1111/j.1360-0443.2005.01160.x. [DOI] [PubMed] [Google Scholar]
- McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- Milroy CM, Clark JC, Forrest AR. Pathology of deaths associated with “ecstasy” and “eve” misuse. J Clin Pathol. 1996;49:149–153. doi: 10.1136/jcp.49.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi H, Arai S, Koike H, Ibi D, Kamei H, Nabeshima T, Kim HC, Takuma K, Yamada K. Therapeutic potential of nicotine for methamphetamine-induced impairment of sensorimotor gating: involvement of pallidotegmental neurons. Psychopharmacology (Berl) 2009;207:235–243. doi: 10.1007/s00213-009-1651-z. [DOI] [PubMed] [Google Scholar]
- Moon KH, Upreti VV, Yu LR, Lee IJ, Ye X, Eddington ND, Veenstra TD, Song BJ. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8:3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ. Recreational use of “ecstasy” (MDMA) is associated with elevated impulsivity. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1998;19:252–264. doi: 10.1016/S0893-133X(98)00012-8. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain research. 1992a;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxymethamphetamine. Brain research. 1992b;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Neumann H. Control of glial immune function by neurons. Glia. 2001;36:191–199. doi: 10.1002/glia.1108. [DOI] [PubMed] [Google Scholar]
- Ng Cheong Ton JM, Gerhardt GA, Friedemann M, Etgen AM, Rose GM, Sharpless NS, Gardner EL. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain research. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial dysfunction and glutamate excitotoxicity studied in primary neuronal cultures. Current molecular medicine. 2004;4:149–177. doi: 10.2174/1566524043479239. [DOI] [PubMed] [Google Scholar]
- Nifosi F, Martinuzzi A, Toffanin T, Costanzo R, Vestri A, Battaglia M, Bertagnoni GE, Lupi A, Amista P, Carollo C, Perini G. Hippocampal remodelling after MDMA neurotoxicity: a single case study. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2009;10:961–968. doi: 10.1080/15622970701870933. [DOI] [PubMed] [Google Scholar]
- O’Hearn E, Battaglia G, De Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea E, Sanchez V, Orio L, Escobedo I, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine increases pro-interleukin-1beta production and caspase-1 protease activity in frontal cortex, but not in hypothalamus, of Dark Agouti rats: role of interleukin-1beta in neurotoxicity. Neuroscience. 2005;135:1095–1105. doi: 10.1016/j.neuroscience.2005.06.084. [DOI] [PubMed] [Google Scholar]
- Orio L, O’Shea E, Sanchez V, Pradillo JM, Escobedo I, Camarero J, Moro MA, Green AR, Colado MI. 3,4-Methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: studies on the relationship with acute hyperthermia and 5-HT depletion. Journal of neurochemistry. 2004;89:1445–1453. doi: 10.1111/j.1471-4159.2004.02443.x. [DOI] [PubMed] [Google Scholar]
- Parrott AC, Lees A, Garnham NJ, Jones M, Wesnes K. Cognitive performance in recreational users of MDMA of ‘ecstasy’: evidence for memory deficits. Journal of psychopharmacology. 1998;12:79–83. doi: 10.1177/026988119801200110. [DOI] [PubMed] [Google Scholar]
- Patel A, Moreland T, Haq F, Siddiqui F, Mikul M, Qadir H, Raza S. Persistent Psychosis After a Single Ingestion of “Ecstasy” (MDMA) The primary care companion to CNS disorders. 2011:13. doi: 10.4088/PCC.11l01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of general psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Pontieri FE, Crane AM, Seiden LS, Kleven MS, Porrino LJ. Metabolic mapping of the effects of intravenous methamphetamine administration in freely moving rats. Psychopharmacology. 1990;102:175–182. doi: 10.1007/BF02245919. [DOI] [PubMed] [Google Scholar]
- Potash MN, Gordon KA, Conrad KL. Persistent Psychosis and Medical Complications After a Single Ingestion of MDMA “Ecstasy”: A Case Report and Review of the Literature. Psychiatry. 2009;6:40–44. [PMC free article] [PubMed] [Google Scholar]
- Pourahmad J, Eskandari MR, Nosrati M, Kobarfard F, Khajeamiri AR. Involvement of mitochondrial/lysosomal toxic cross-talk in ecstasy induced liver toxicity under hyperthermic condition. Eur J Pharmacol. 2010;643:162–169. doi: 10.1016/j.ejphar.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn-Schmiedeberg’s archives of pharmacology. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- Quate L, McBean DE, Ritchie IM, Olverman HJ, Kelly PA. Acute methylenedioxymethamphetamine administration: effects on local cerebral blood flow and glucose utilisation in the Dark Agouti rat. Psychopharmacology. 2004;173:287–295. doi: 10.1007/s00213-004-1784-z. [DOI] [PubMed] [Google Scholar]
- Ramirez BG, Blazquez C, Gomez del Pulgar T, Guzman M, de Ceballos ML. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte G, Bryan G, Strauss L, Seiden L, Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985;229:986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Guillery RW, Seiden LS, Schuster CR, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain research. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. European journal of pharmacology. 2002;449:71–74. doi: 10.1016/s0014-2999(02)01985-4. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, Baker NB, Hunter J, Carthy T, Booker E, London M, Deakin JF, Sahakian BJ, Robbins TW. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Camarero J, O’Shea E, Green AR, Colado MI. Differential effect of dietary selenium on the long-term neurotoxicity induced by MDMA in mice and rats. Neuropharmacology. 2003;44:449–461. doi: 10.1016/s0028-3908(02)00411-2. [DOI] [PubMed] [Google Scholar]
- Sava V, Reunova O, Velasquez A, Song S, Sanchez-Ramos J. Neuroanatomical mapping of DNA repair and antioxidative responses in mouse brain: Effects of a single dose of MPTP. Neurotoxicology. 2006;27:1080–1093. doi: 10.1016/j.neuro.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. The Journal of pharmacology and experimental therapeutics. 1985;233:539–544. [PubMed] [Google Scholar]
- Seiden LS, Commins DL, Vosmer G, Axt K, Marek G. Neurotoxicity in dopamine and 5-hydroxytryptamine terminal fields: a regional analysis in nigrostriatal and mesolimbic projections. Ann N Y Acad Sci. 1988;537:161–172. doi: 10.1111/j.1749-6632.1988.tb42104.x. [DOI] [PubMed] [Google Scholar]
- Sharma HS, Kiyatkin EA. Rapid morphological brain abnormalities during acute methamphetamine intoxication in the rat: an experimental study using light and electron microscopy. J Chem Neuroanat. 2009;37:18–32. doi: 10.1016/j.jchemneu.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. Journal of addictive diseases. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Skibba JL, Stadnicka A, Kalbfleisch JH. Hyperthermic liver toxicity: a role for oxidative stress. J Surg Oncol. 1989;42:103–112. doi: 10.1002/jso.2930420208. [DOI] [PubMed] [Google Scholar]
- Smith DE, Fischer CM. An analysis of 310 cases of acute high-dose methamphetamine toxicity in Haight-Ashbury. Clin Toxicol. 1970;3:117–124. doi: 10.3109/15563657008990106. [DOI] [PubMed] [Google Scholar]
- Soleimani Asl S, Farhadi MH, Moosavizadeh K, Samadi Kuchak Saraei A, Soleimani M, Jamei SB, Joghataei MT, Samzadeh-Kermani A, Hashemi-Nasl H, Mehdizadeh M. Evaluation of Bcl-2 Family Gene Expression in Hippocampus of 3, 4-methylenedioxymethamphetamine Treated Rats. Cell journal. 2012;13:275–280. [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Everman SL, Nichols DE. An integrated hypothesis for the serotonergic axonal loss induced by 3,4-methylenedioxymethamphetamine. Neurotoxicology. 1998;19:427–441. [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-alpha. Journal of neurochemistry. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Staszewski RD, Yamamoto BK. Methamphetamine-induced spectrin proteolysis in the rat striatum. Journal of neurochemistry. 2006;96:1267–1276. doi: 10.1111/j.1471-4159.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Whittingham TS, Douglas AJ, Lust WD, Yamamoto BK. Substrates of energy metabolism attenuate methamphetamine-induced neurotoxicity in striatum. Journal of neurochemistry. 1998;71:613–621. doi: 10.1046/j.1471-4159.1998.71020613.x. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Stone DM, Johnson M, Hanson GR, Gibb JW. Role of endogenous dopamine in the central serotonergic deficits induced by 3,4-methylenedioxymethamphetamine. The Journal of pharmacology and experimental therapeutics. 1988;247:79–87. [PubMed] [Google Scholar]
- Stumm G, Schlegel J, Schafer T, Wurz C, Mennel HD, Krieg JC, Vedder H. Amphetamines induce apoptosis and regulation of bcl-x splice variants in neocortical neurons. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1065–1072. doi: 10.1096/fasebj.13.9.1065. [DOI] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990;5:797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Tamburini I, Blandini F, Gesi M, Frenzilli G, Nigro M, Giusiani M, Paparelli A, Fornai F. MDMA induces caspase-3 activation in the limbic system but not in striatum. Annals of the New York Academy of Sciences. 2006;1074:377–381. doi: 10.1196/annals.1369.037. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. MK-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain research. 2005;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. The Journal of pharmacology and experimental therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Torres E, Gutierrez-Lopez MD, Borcel E, Peraile I, Mayado A, O’Shea E, Colado MI. Evidence that MDMA (‘ecstasy’) increases cannabinoid CB2 receptor expression in microglial cells: role in the neuroinflammatory response in rat brain. Journal of neurochemistry. 2010;113:67–78. doi: 10.1111/j.1471-4159.2010.06578.x. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Turillazzi E, Riezzo I, Neri M, Bello S, Fineschi V. MDMA toxicity and pathological consequences: a review about experimental data and autopsy findings. Curr Pharm Biotechnol. 2010;11:500–509. doi: 10.2174/138920110791591481. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. The American journal of psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wang AM, Suojanen JN, Colucci VM, Rumbaugh CL, Hollenberg NK. Cocaine- and methamphetamine-induced acute cerebral vasospasm: an angiographic study in rabbits. AJNR Am J Neuroradiol. 1990;11:1141–1146. [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhu SP, Kuang WH, Li J, Sun X, Huang MS, Sun XL. Neuron apoptosis induced by 3,4-methylenedioxy methamphetamine and expression of apoptosis-related factors in rat brain. Sichuan da xue xue bao Yi xue ban = Journal of Sichuan University Medical science edition. 2009;40:1000–1002. 1037. [PubMed] [Google Scholar]
- Wijetunga M, Seto T, Lindsay J, Schatz I. Crystal methamphetamine-associated cardiomyopathy: tip of the iceberg? J Toxicol Clin Toxicol. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain research. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Zhu W. The effects of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther. 1998;287:107–114. [PubMed] [Google Scholar]
- Yiangou Y, Facer P, Durrenberger P, Chessell IP, Naylor A, Bountra C, Banati RR, Anand P. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC neurology. 2006;6:12. doi: 10.1186/1471-2377-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006a;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo N, Angulo JA. Methamphetamine-induced striatal apoptosis in the mouse brain: comparison of a binge to an acute bolus drug administration. Neurotoxicology. 2006b;27:131–136. doi: 10.1016/j.neuro.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain research. 2005;1034:11–24. doi: 10.1016/j.brainres.2004.11.014. [DOI] [PubMed] [Google Scholar]