Abstract
Approximately 1% of all live births exhibit a minor or major congenital anomaly. Of these approximately one-third display craniofacial abnormalities which are a significant cause of infant mortality and dramatically affect national health care budgets. To date, more than 700 distinct craniofacial syndromes have been described and in this review, we discuss the etiology, pathogenesis and management of facial dysostoses with a particular emphasis on Treacher Collins, Nager and Miller syndromes. As we continue to develop and improve medical and surgical care for the management of individual conditions, it is essential at the same time to better characterize their etiology and pathogenesis. Here we describe recent advances in our understanding of the development of facial dysostosis with a view towards early in-utero identification and intervention which could minimize the manifestation of anomalies prior to birth. The ultimate management for any craniofacial anomaly however, would be prevention and we discuss this possibility in relation to facial dysostosis.
Keywords: Facial dysostosis, mandibulofacial dysostosis, acrofacial dysotosis, Treacher Collins syndrome, Nager syndrome, Miller syndrome, neural crest cells, ribosome biogenesis, spliceosome
INTRODUCTION
The craniofacial complex is anatomically the most intricate part of the body. Composed of a sophisticated assemblage of cranial specializations including the central and peripheral nervous systems and bone and cartilage components of the skull and jaw, the head must house and protect the brain as well as the majority of the body’s primary sense organs. Proper craniofacial development requires the coordinated integration of progenitor tissues and their derivatives in concert with the precise regulation of cell proliferation, migration and differentiation which is established during early embryogenesis. For example, the oral apparatus requires the coordinated development and proper orientation of the bony mandible and the muscles of mastication with their appropriate cranial nerve innervation. Collectively, these structures function to facilitate jaw opening and closing.
One-third of all congenital defects affect the head and face [Gorlin et al., 1990]. The majority of these defects are categorized based on the extent and location of alterations to the craniofacial skeleton which is derived primarily from a migratory stem and progenitor cell population called the neural crest (reviewed in [Noden and Trainor, 2005]. Therefore, most craniofacial anomalies are associated with defects in neural crest cell development. Depending on which phase of neural crest cell formation, proliferation, migration and/or differentiation is affected during embryogenesis, vastly distinct craniofacial anomalies may arise [Walker and Trainor, 2006]. For example, as described later in this chapter, mandibulofacial dysostosis in the form of Treacher Collins syndrome arises due to defects in neural crest cell formation [Dixon et al., 2006; Jones et al., 2008]. In contrast defects in neural crest cell migration and/or differentiation lead to conditions of craniosynostosis [Liu et al, 1999; Merrill et al., 2006]. In order to develop therapeutic avenues for minimizing or preventing craniofacial anomalies, it is essential to understand the precise etiology and pathogenesis of individual malformation syndromes. This requires a thorough understanding of the normal developmental events that induce neural crest cells to form, maintain their survival, guide their migration and influence their differentiation in the context of normal craniofacial development.
FACIAL DYSOSTOSIS
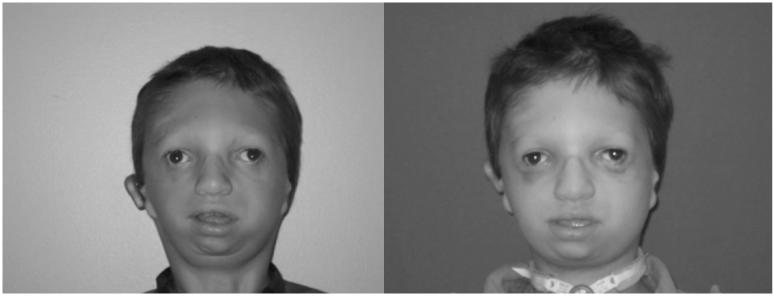
Facial dysostosis describes a set of clinically and etiologically heterogeneous congenital craniofacial anomalies. These disorders arise as a consequence of abnormal development of the first and second pharyngeal arches and their derivatives during embryogenesis. The pharyngeal arches together with other facial prominences such as the lateral and medial nasal prominences are reiterated outgrowths on the surface of the embryo that constitute the blueprint of the adult vertebrate face. Malformation of the pharyngeal arches typically manifests at birth as maxillary, malar and mandibular hypoplasia, cleft palate, and/or ear defects. Facial dysostoses can be subdivided into acrofacial dysostoses and mandibulofacial dysostoses. Acrofacial dysostoses present with craniofacial anomalies similar to those observed in mandibulofacial dysostosis but with the addition of limb defects. Several distinct mandibulofacial dysostosis syndromes have been described. Clinically, the most well understood is Treacher Collins syndrome (OMIM 154500), which is most commonly caused by mutations in at least three distinct genes involved in pre-rDNA transcription [Dauwerse et al., 2011; Dixon et al., 2006; Treacher Collins Syndrome Collaborative Group, 1996; Valdez et al., 2004]. Mandibulofacial dysostosis (OMIM610536) with microcephaly syndrome shares some overlapping features with Treacher Collins syndrome and has been associated with disruption of a spliceosomal GTPase [Lines et al., 2012; Luquetti et al., 2013]. Among several acrofacial dysostoses, causative mutations have recently been identified in an enzyme involved in pyrimidine biosynthesis in association with Miller syndrome (OMIM 263750) [Fang et al., 2012;], and in a component of the pre-mRNA spliceosomal complex, with respect to Nager syndrome (OMIM 154400) [Bernier et al., 2012]. Here we explore recent progress in understanding the etiology, pathogenesis and management of Treacher Collins, Nager and Miller syndromes and the potential for therapeutic prevention of facial dysostoses (Fig. 1).
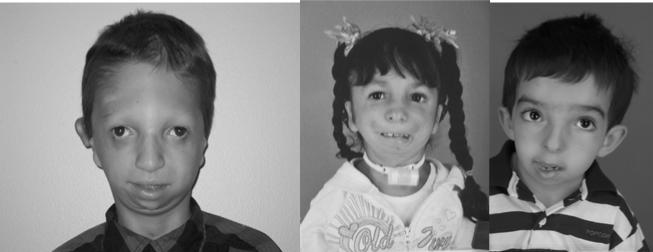
Fig 1. Facial Dysostoses Phenotypes.
Treacher Collins (left), Nager Syndrome (middle), Miller Syndrome (right). Photos courtesy of James P. Bradley, MD University of California-LA
TREACHER COLLINS SYNDROME
Treacher Collins syndrome is a congenital disorder of craniofacial development [Treacher Collins 1900] and is also known as mandibulofacial dysostosis and Franschetti-Zwahlen-Klein syndrome [Franceschetti and Klein, 1949]. The characteristic features of Treacher Collins syndrome include hypoplasia of the facial bones, particularly the maxilla, mandible and zygomatic complex together with inferolateral orbital cleft leading to downward slanting of the palpebral fissures with colobomas of the lower eyelids and absence of the medial third of the lower eyelashes (Fig. 1) [Poswillo, 1975]. Furthermore, in a large proportion of cases the palate is high, arched and frequently cleft. Hypoplasia of the jaw often results in dental malocclusion, with anterior open bite. Alterations in the size, shape and position of the external ears are common and usually associated with atresia of the external auditory canals and anomalies of the middle ear ossicles. Radiographic analyses of the middle ears of individuals with Treacher Collins syndrome has revealed irregular or absent auditory ossicles. Fusions between the rudimentary malleus and incus are common with partial absence of the stapes and oval window and complete absence of the middle ear and epitympanic space [Stovin et al., 1960]. As a result, bilateral conductive hearing loss is common, whereas mixed or sensorineural hearing loss is rare [Phelps et al., 1981]. Other clinical features of Treacher Collins syndrome may include defects in brain development such as microcephaly, intellectual disability and psychomotor delay [Cohen et al., 1995; Milligan et al., 1994; Sakai et al., 2012; Teber et al., 2004]; however these features are associated with fewer than 5% of affected individuals. It is important to note that there is a considerable degree of inter- and intramilial variability in severity associated with Treacher Collins syndrome, with some individuals so mildly affected that without genetic confirmation an unequivocal diagnosis may not be possible.
Treacher Collins syndrome occurs with an incidence of about 1 in 50,000 live births. Genetic, physical and transcript mapping techniques revealed that Treacher Collins syndrome is primarily associated with autosomal dominant mutations in the TCOF1 gene, which is located on chromosome 5 [Treacher Collins Syndrome Collaborative Group, 1996]. To date, over 200 largely family-specific mutations have been documented throughout the TCOF1 gene and these include deletions, insertions, splicing, mis-sense and nonsense mutations (http://genoma.ib.usp.br/TCOF1_database/). Deletions ranging in size from 1 to 40 nucleotides are the most common and within that group a reoccurring 5bp deletion in exon 24 accounts for 17% of TCS cases. Recently however, whole exome sequencing revealed causative mutations in POLR1C and POLR1D, which localize to chromosomes 6 and 13 respectively (Dauwerse and others 2011). POLR1D and POLR1C are subunits of RNA polymerase I and III, which similar to TCOF1’s role as an RNA polymerase 1 binding factor, implicates each of these genes in ribomosome biogenesis which is essential for cell growth and proliferation. At least 17 distinct mutations in POLR1D have been described and similar to TCOF1 they elicit their effect in an autosomal dominant manner. In contrast, the seven distinct mutations in POLR1C associated with Treacher Collins syndrome are all autosomal recessive [Dauwerse et al., 2011].
Penetrance of the genetic mutations underlying Treacher Collins syndrome is high, yet inter- and intra-familial variation in the severity of the phenotype is a striking feature of the condition [Dixon et al., 1994; Marres et al., 1995]. Severe cases of Treacher Collins syndrome have resulted in perinatal death [Edwards et al., 1996], however individuals can be so mildly affected that it prevents an unequivocal diagnosis. Furthermore, it is not uncommon for mildly affected individuals to be diagnosed with Treacher Collins syndrome retrospectively after the birth of a more severely affected child. Thus the disease spectrum includes subclinically affected individuals, and consequently the population prevalence is likely to be an underestimate. Furthermore, no genotype-phenotype correlation has been observed with respect to Treacher Collins syndrome and similarly there is no clear evidence of an association between disease severity and parental origin or type of pathogenic mutation, male or female, sporadic or familial [Edwards et al., 1997; Gladwin et al., 2000; Splendore et al., 2000; Teber et al., 2004]. Interestingly however, recent cephalometric analyses of the craniofacial skeleton in age- and sex- matched individuals with Treacher Collins syndrome has suggested that craniofacial deficiencies may be more significant in females [Chong et al., 2008]. Collectively, the variable severity indicates that genetic background, environmental factors and stochastic events may contribute to the clinical variation observed in patients with Treacher Collins syndrome [Dixon and Dixon, 2004].
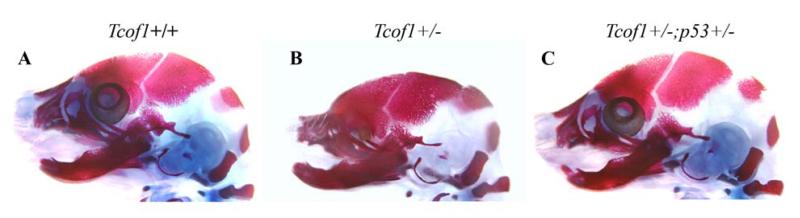
Animal models of Treacher Collins syndrome successfully mimic the characteristic features and variability observed in humans (Fig. 2) [Dixon and Dixon, 2004]. These models have been instrumental in deciphering the pathogenesis of this congenital craniofacial disorder. The majority of Tcof1+/− mice on a pure DBA background exhibit minor craniofacial anomalies including some subtle doming of the head and slight frontonasal hypoplasia. However, these mice are post-natal viable and fertile [Dixon and Dixon, 2004; Dixon et al., 2006]. In contrast mixed DBA;C57BL/6 background Tcof1+/− mice, where the mother was C57BL/6; exhibit severe craniofacial anomalies including frontonasal hypoplasia, particularly of the maxilla and mandible, together with high arched or cleft palate, and choanal atresia or agenesis of the nasal passages (Fig. 2A, B). The zygomatic arch, tympanic ring and middle ear ossicles are all hypoplastic and misshapen Dixon et al., 2006]. These mixed background mice mimic the severe form of Treacher Collins syndrome observed in humans and die within 24 hours of birth due to breathing difficulties and an inability to feed. Thus variability in the penetrance and severity of facial defects presents in mice just as it is in humans.
Fig 2. Prevention of Treacher Collins syndrome Craniofacial Anomalies.
Bone (alizarin red) and cartilage (alcian blue) stained E18.5 (A) Tcof1+/+, (B), Tcof1+/− and (C) Tcof1+/−; p53+/− mouse embryos demonstrating that suppressing p53 activity has the capacity in animal models to prevent the pathogenesis of craniofacial anomalies characteristic of Treacher Collins syndrome.
The majority of the cartilage and bone that makes up the craniofacial complex is derived from neural crest cells. Consequently, most craniofacial abnormalities are attributed to problems in neural crest cell development. Tcof1 is broadly expressed during mouse embryogenesis in both embryonic and adult tissues and interestingly, between E8.5-10.5, elevated levels of Tcof1 expression are observed in the neuroepithelium and neural crest cell derived facial mesenchyme [Dixon et al., 2006]. This is consistent with a potential role for Tcof1 in the formation and migration of neural crest cells. In support of this idea lineage tracing and gene expression analyses revealed a deficit in the number of migrating neural crest cells by as much as 25% in E8.5 Tcof1+/− mouse embryos compared to wild-type littermates [Dixon et al., 2006]. This deficiency in neural crest cell number was due to extensive neuroepithelial apoptosis in E8.0-10.5 Tcof1+/− embryos, which diminishes the neural stem cell pool from which neural crest cells are generated. Furthermore, the apoptosis is p53 dependent as nuclear activation and stabilization of p53 was observed in the neuroepithelium of Tcof1+/− embryos [Jones et al., 2008]. In addition, proliferation was reduced in the neuroepithelium of Tcof1+/− embryos and also compromised in migrating neural crest cells. Thus the initial deficiency in the number of neural crest cells formed in Tcof1+/− embryos is further compounded by their reduced proliferative capacity leading to a cumulative reduction in the number of migrating neural crest cells. Therefore the general cranioskeletal hypoplasia observed in individuals with Treacher Collins syndrome arises due to a deficiency in the number of migrating neural crest cells from which the majority of the cranial bone and cartilage is derived. Hence Tcof1 plays a critical role in neuroepithelial survival and is required for neural crest cell formation and proliferation [Dixon et al., 2006; Jones et al., 2008] TCOF1 encodes a 144 kDA low complexity, serine/alanine-rich, protein known as Treacle [Treacher Collins Syndrome Collaborative Group, 1996] and the majority of mutations in TCOF1 lead to truncations of its C-terminal end [Dixon et al., 2007]. Truncated proteins have not been detected in patient fibroblasts, indicating that mRNAs with premature termination codons are degraded by nonsense mediated mRNA decay. Treacle is a putative nucleolar phosphoprotein that functions in rDNA transcription and thus ribosome biogenesis, via direct binding of upstream binding factor (UBF) and RNA polymerase I [Valdez et al., 2004]. Approximately 95% of all transcription and metabolism is dedicated solely to the process of ribosome biogenesis and under normal cellular growth conditions Mdm2 polyubiquitlyates p53 targeting it for degradation. In contrast, under conditions of perturbed ribosome biogenesis, unincorporated ribosomal proteins bind to Mdm2 inhibiting its polyubiquitlyating capacity and this leads to activation and stabilization of p53 [Lindstrom et al., 2007; Rubbi and Milner, 2003]. Consistent with this, deficient mature ribosome biogenesis has been observed in Tcof1+/− embryos and this correlates with nucleolar stress activation of p53, p53 dependent neuroepithelial cell death, and decreased numbers of migrating neural crest cells which underlies the pathogenesis of craniofacial malformations in Treacher Collins syndrome [Dixon et al., 2006; Jones et al., 2008].
Interestingly, genetic and pharmacological inhibition of p53 in Tcof1+/− embryos can suppress neuroepithelial apoptosis ensuring the normal production of migrating neural crest cells. Remarkably, this can prevent the pathogenesis of craniofacial anomalies characteristic of Treacher Collins syndrome in animal models (Fig. 2B, C) [Jones et al., 2008]. In theory this indicates Treacher Collins syndrome may be clinically preventable. However, p53 inhibition carries considerable risk of tumor formation and metastasis, which outweighs any potential clinical benefit. Hence it will be important in the future to identify alternative (p53 independent) strategies for the potential therapeutic prevention of Treacher Collins syndrome. Interestingly, the rescue phenomenon occurred without restoration of ribosome biogenesis, which implies that Tcof1/Treacle may play other essential roles in neural progenitor cell and neural crest cell survival distinct from its previously recognized function in ribosome biogenesis. In agreement with this, Treacle has been shown in vivo to localize to the centrosome during metaphase and play a key role in regulating chromosome segregation and the plane of neural progenitor cell division during brain development [Sakai et al., 2012]. Furthermore, Treacle has also been linked in vitro to the regulation of oxidative stress [Duan et al., 2010]. Perturbation of one or more of these functions may also underpin the activation of p53 and provide additional avenues for the prevention of Treacher Collins syndrome. Thus, Tcof1+/− haploinsufficient mice have provided an important resource to decipher the in vivo cellular basis of Treacher Collins syndrome together with the biochemical function of Treacle. It will be interesting, in the future, to explore the function of POLR1C and POLR1D and determine whether they share similar or overlapping functions with TCOF1 during embryogenesis and in the pathogenesis of Treacher Collins syndrome.
MANDIBULOFACIAL DYSOSTOSIS WITH MICROCEPHALY
Mandibulofacial dysostosis with microcephaly is a rare sporadic syndrome comprising craniofacial malformations and microcephaly. Characteristic features include midface hypoplasia, micrognathia, choanal atresia, cleft palate and sensorineural hearing loss, each of which occurs in a significant proportion of affected individuals. The syndrome was first described in 1996 [Guion-Almeida et al., 2006] and recently a number of whole exome sequencing studies have revealed causative mutations in EFTUD2 [Czeschik et al., 2013; Lines et al., 2012; Luquetti et al., 2013]. Collectively, a wide variety of mutation types have been uncovered, including large deletions and frameshift, splice-site, nonsense, and missense mutations, which is consistent with haploinsufficiency. EFTUD2 encodes the 116kD U5 small nuclear ribonuclepoprotein, which is a highly conserved spliceosomal GTPase that plays a central regulatory role in catalytic splicing and post-splicing-complex disassembly. Mandibulofacial dyostosis with microcephaly was thus the first multiple-malformation syndrome attributed to a defect of the major spliceosome, which is critical for removing introns and ligating exon splicing during transcription. The defects observed in individuals with mandibulofacial dysostosis with microcephaly syndrome could be due to aberrant splicing of genes involved in craniofacial development. However, to date, nothing is known about the spatiotemporal activity of EFTUD2 during embryogenesis. Hence expression analyses as well as the generation of Eftud2 loss-of-function animal models are thus eagerly anticipated.
NAGER SYNDROME
Although the limbs are typically normal in mandibulofacial dysostosis with microcephaly, the phenotypic overlap with Nager syndrome is likely due in part to a perturbation of the same biological process. Nager syndrome is the best known example of the group of disorders collectively termed acrofacial dsyostosis which encompasses malformations of the craniofacial skeleton and also the limbs [Halal et al., 1983; Opitz, 1987]. Similar to Treacher Collins syndrome, one of the defining features of Nager syndrome is downward slanting of the palpebral fissures. In addition to this, affected individuals exhibit micrognathia, midface retrusion, cleft palate and external ear anomalies. Limb defects usually affect the radial elements of the forelimbs and include hypoplasia or agenesis of the radius, or radioulnar synostosis. Furthermore, hypoplasia or agenesis of the thumbs, or triphalangeal thumbs have been observed as part of the condition. Phocomelia of the upper limbs and occasionally lower-limb defects have also been reported [Le Merrer et al., 1989]. The presentation of anterior forelimb anomalies as opposed to posterior forelimb anomalies and the general lack of hindlimb malformations distinguishes Nager syndrome from Miller syndrome, another rare acrofacial disorder.
Distinguishing Nager syndrome from other mandibulofacial disorders is challenging based on phenotype alone. Contributing to this is the fact that Nager syndrome is very rare, with less than 100 reported cases by 2012 [Schlieve et al., 2012]. Although autosomal recessive [Chemke et al., 1988; Kennedy and Teebi, 2004] and autosomal dominant [Aylsworth et al., 1991; Hall, 1989; McDonald and Gorski, 1993] modes of inheritance have been reported, most cases of Nager syndrome arise spontaneously. Consequently this has made identification of the causal gene(s) challenging through conventional gene-discovery approaches.
Recently, whole exome sequencing positively identified mutations in the SF3B4 gene as causative mutations underlying the etiology of Nager syndrome [Bernier et al., 2012]. A total of 18 unique mutations were identified, and these included 14 frameshift, two nonsense, one splicing, and one missense mutation. All of the SF3B4 variants identified are predicted to encode a truncated polypeptide chain or an elongated polypeptide with an altered 3’ end in the absence of nonsense- mediated RNA decay [Bernier et al., 2012]. Taken together with the observation that a child with the characteristics of Nager syndrome harbors a deletion of region 1q12–q21.1 or q21.3 [Waggoner et al., 1999], which encompasses SF3B4, this strongly suggests that Nager syndrome results from haploinsufficiency of SF3B4. In agreement with this, another whole exome sequencing study confirmed the SF3B4 gene is mutated in about half of the patients with the clinical diagnosis of Nager syndrome and provided further support for the genetic heterogeneity for this condition [Czeschik et al., 2013]. SF3B4 encodes a spliceosomal protein and is one of seven core proteins that constitute the SF3B complex. SF3B4 binds to pre-mRNA and U2 snRNPS, as part of the U2SNP prespliceosomal complex [Champion-Arnaud and Reed, 1994] which is one of a number of complexes that removes introns and ligates exons during splicing. SF3B4 is expressed during mouse embryogenesis from at least E10 in the optic eminence, optic vesicle, hindbrain and somites [Ruiz-Lozano et al., 1997]. Although it is currently not known if SF3B4 is expressed in neural crest cells, the defects observed in individuals with Nager syndrome could be due to aberrant splicing of genes involved in craniofacial and limb development. This would place Nager syndrome in a category of emerging disorders which includes mandibulofacial dysostosis with microcephaly which are caused by mutations in genes encoding subunits of the spliceosome or that affect splicing. However, mutations in SF3B4 might cause Nager syndrome via a mechanism unrelated to a role in mediating splicing. Consistent with this, SF3B4 was recovered in a yeast two-hybrid screen to identify downstream targets of BMP signaling. BMP2 and BMP4 play key signaling roles developing limbs and during chondrogenesis [Robert, 2007]. Coimmunoprecipitation and immunoblot analysis revealed an interaction between SF3B4 and the BMP receptor BMPR-1A [Watanabe et al., 2007]. Interestingly, SF3B4 inhibits BMP-2-mediated osteogenic and chondrogenic differentiation which implies that in addition to any role in mRNA splicing, SF3B4 may also specifically inhibit BMP mediated osteochondral cell differentiation [Watanabe et al., 2007]. What is needed now however are animal models to investigate the cellular pathogenesis of Nager syndrome alongside characterization of the in vivo biochemical function of SF3B4.
MILLER SYNDROME
Miller syndrome, also referred to as Genee-Wiedemann and Wildervanck-Smith syndromes, represents several independent reports of a clinically recognizable acrofacial dysostosis syndrome with a combination of craniofacial and limb anomalies. This includes micrognathia, orofacial clefts, malar hypoplasia, aplasia of the medial lower lid eyelashes, coloboma of the lower eyelid and cup-shaped ears combined with postaxial limb deformities, including apparent absence of either the fifth or both the fourth and fifth rays of the hands and feet, with or without ulnar and fibular hypoplasia [Genee, 1969; Miller et al., 1979; Wiedemann, 1973]. Many of these characteristics are very similar to Treacher Collins syndrome.
Miller syndrome was the first Mendelian disorder whose molecular basis was identified via whole-exome sequencing and shown to correlate with mutations in dihydroorotate dehydrogenase (DHODH) [Ng et al., 2010]. Recently additional biallelic mutations in DHODH were identified in a further four unrelated families with typical clinical features of Miller syndrome [Rainger et al., 2012]. To date, a total of 14 distinct mutations in the coding region of DHODH, including 2 nonsense mutations, have now been identified. Surprisingly, all affected individuals have compound heterozygous mutations in the DHODH coding region. The lack of homozygous mutations and the paucity of nonsense or frameshift alleles are unusual in a rare autosomal recessive disorder [Rainger et al., 2012].
DHODH is an enzyme associated with the mitochondrial electron transport chain and is required for de novo pyrimidine synthesis. In vivo and in vitro assays of disease-associated DHODH alleles predict affected individuals have a deficiency of de novo pyrimidine synthesis but with significant residual function. However, recently, treatment of zebrafish with inhibitors of DHODH such as leflunomide, resulted in an almost complete abrogation of neural crest cell development principally by blocking the transcriptional elongation of critical neural crest cell genes [White et al., et al., 2011]. This suggests the cellular basis of Miller syndrome may lie in deficient neural crest cell formation and the failure to generate sufficient numbers of migrating neural crest cells, which is analogous to the pathogenesis of Treacher Collins syndrome. Furthermore, similar to TCOF1, DHODH has also been implicated in oxidative stress [Hail et al., 2010], suggesting there may be some considerable mechanistic overlap in the pathogenesis of Miller and Treacher Collins syndromes. In the future it will be critical to explore the in vivo biochemical function of DHODH and the cellular pathogenesis of Miller syndrome through the generation of Dhodh loss-of-function mammalian animal models.
SUMMARY OF BASIC BIOLOGY OF FACIAL DYSOSTOSIS
Treacher Collins syndrome, mandibulofacial dysostosis with microcephaly, Nager and Miller syndromes share a number of overlapping phenotypic characteristics and are all considered examples of facial dyostoses. Considerable progress has been made in determining the etiology and cellular pathogenesis of each of these congenital birth defects and although Treacher Collins syndrome has been shown to be preventable in animal models, we are still a long way from realistic clinical prevention. Therefore, considerable ongoing effort exists to continually improve the care of individuals affected with facial dysostosis.
Clinical Management of Facial Dysostosis
As discussed, the clinical phenotype of Treacher Collins syndrome is similar to mandibulofacial dysostosis with microcephaly, Miller and Nager syndrome (Fig 1). To a lesser degree Goldenhar, Pierre Robin, agnathia-otocephaly, and craniofacial microsomia also resemble and overlap these syndromes. Depending on their severity, the craniofacial anomalies present at birth may be life-threatening. The typical clinical manifestations of these syndromes can be categorized by anatomic subunits as follows:
(1) Mandible- micrognathia, glossoptosis, choanal atresia, airway obstruction and occasional cleft palate (2) Ear- external and internal ear anomalies and hearing loss, (3) midface skeletal anomalies- maxillary/zygomatic/malar hypoplasia, and (4) Eye- orbital hypoplasia and dystopia, eyelid colobomas and malposition, and epibulbar dermoids (5) Soft tissue- facial nerve weakness, masticatory muscle agenesis, spine malformations, hand anomalies (Nager syndrome) and kidney disorders [Kumar and Bradley, 2008]. The medical and surgical management for patients with any type of facial dysotosis are usually similar and continue from early infancy into adulthood. Surgical experience and advances in technology have allowed for dramatic aesthetic transformations and exceptional functional outcomes in most patients.
Neonatal Period
The treatment of these syndromes begins at birth. The most life-threatening congenital anomaly is airway obstruction. In these patients, choanal atresia presents or the posterior tongue limits or in severe cases, occludes airflow in the oropharynx. When severe and the problem is identified in-utero, an EXIT (Ex-Utero Intrapartum Treatment) procedure is required at the time of delivery to secure an airway. During this rare procedure, the neonate’s head and neck are partially delivered by cesarean while the neonate is still receiving maternal circulation and an oral intubation or tracheostomy is performed. Once the airway is secured, delivery is completed and placenta/umbilical separation performed. Even if delivered successfully by conventional methods (vaginally or by cesarean), infants often “tire out” secondary to increased respiratory efforts and require mechanical ventilation. When this occurs, anatomic by-pass of the oropharyngeal tongue base obstruction is necessary and endotracheal intubation is considered first-line therapy. However, this procedure is often difficult or impossible due to the inability to visualize the larynx and place an endotracheal tube.
Tracheostomy
Tracheostomy is the “gold standard” for establishing a secure airway in any child with oropharyngeal obstruction and facial dysostosis. This surgical procedure requires a neck incision and exposure of the airway below the vocal cords. The airway is then opened and a tracheostomy tube is inserted and secured to the skin. The advantage of this procedure is the upper airway is by-passed and the child breathes thru their neck alleviating the airway obstruction caused by glossoptosis. The disadvantage of this procedure is neonatal tracheostomy has a mortality less than 10% [Zenk et al., 2009]. Once placed it is often difficult to “decanulate” or remove the tracheostomy until the child is 5 years of age. Airway scarring and stenosis, speech delays, swallowing difficulties, recurrent tracheitis, and sudden death can all occur as a result of tracheostomy.
Tongue-Lip Adhesion
Tongue-lip adhesion is a procedure in which the anterior tongue is surgically attached/sutured to the lower lip. First described by Shukowsky in 1911, the technique has been revised and modified by many with varying success [Shukowski, 1911]. Publications of this techniques success at alleviating of airway obstruction are variable and range from 36-100% [Rogers et al., 2011]. The surgical concept of tongue lip adhesion is airway obstruction resulting from glossoptosis which is temporary and quickly outgrown in the first year of life. Suturing the tongue anteriorly to the lower lip anatomically reduces the airway obstruction in the oropharynx. However, this procedure can only be maintained until dental eruption begins at approximately one year of age. Furthermore, it requires a second procedure to reverse or undo the tongue-lip adhesion prior to one year of age. Feeding and swallowing abnormalities are common after this procedure but they are often transient.
Mandibular Distraction Osteogenesis
Neonatal mandibular distraction is the most recent surgical technique to correct micrognathia and alleviate upper airway obstruction. This technique borrows principles described by Illizarov for long bone lengthening and was first introduced to craniomaxillofacial surgery by McCarthy in 1992 [McCarthy et al., 1992]. This procedures requires an osteotomy be made in the mandibular ramus and a screw driven device be applied to each side of the osteotomies. The device is then sequentially turned and the screw spreads the mandibular osteotomy 1-2 mm/day until a suitable advancement is obtained (Fig 3). As the distal mandible is advanced, the base of the tongue is repositioned and pulled anteriorly enlarging the oropharyngeal airway. Indications for mandibular distraction differ among craniofacial centers with many centers reporting excellent success when the proper candidates are selected [Andrews et al., 2013]. The advantage of this procedure is the oropharyngeal airway can be “normalized” in a relatively short time period (1-2 weeks) even in the most severe cases. Disadvantages include the need to establish a secure airway until the advancement is complete and a second procedure is required to remove the distractor 2-3 months after its application and bone consolidation has occurred at the osteotomy/advancement site. Future growth of the mandible is also a concern and its long-term effects on future mandibular development remain to be assessed for this rather new technique.
Fig 3. Neonatal Mandibular Distraction Osteogenesis.
Pre-op (left) and post-op (right)
Adolescence
Midface and orbit reconstruction
Construction of a bony skeletal platform provides the framework for mid-face reconstruction in patients with facial dysostosis. Tessier pioneered mid-face reconstruction with his use of the autologous cranial and rib grafts to augment the facial skeleton [Tessier, 1982]. The most common procedure involves harvesting split parietal cranial bone grafts and rigidly fixating this bone to augment the lateral orbit and zygomatic arch and malar eminence. The advent of liposuction and fat grafting has provided a powerful adjuvant to these techniques. In some cases, soft tissue augmentation with fat grafting alone is enough to reconstruct the malar deficiencies in these patients (Fig 4).
Fig 4. Cheek Augmentation with Fat Grafting.
Pre-op (left) and 1-week post-op (right)
Ear Construction for Microtia/Anotia
There are many classifications to describe congenital ear malformations in patients with facial dysostosis. The Meurmen classification system is the most used and simplest to reproduce [Meurman, 1957]. In this system, type 1 corresponds to the external ear being small; however, the auricle retains most of its normal structure. Additionally, the external auditory meatus is usually present. In type 2, the external ear is moderately anomalous. There are often one or more identifiable structures; however, the majority of the ear framework is malformed. Type 3 is a rudimentary soft tissue structure with little or no cartilage and the auricle has little resemblance of an ear other than a lobule. Type 4 is also termed anotia and presents with all external ear structures being absent. Type 1 microtia is often not altered as attempts to construct a “more normal” ear often are worse than the presenting anomaly. The size of the contralateral ear is reduced by means of a wedge resection to match the Type 1 microtia.
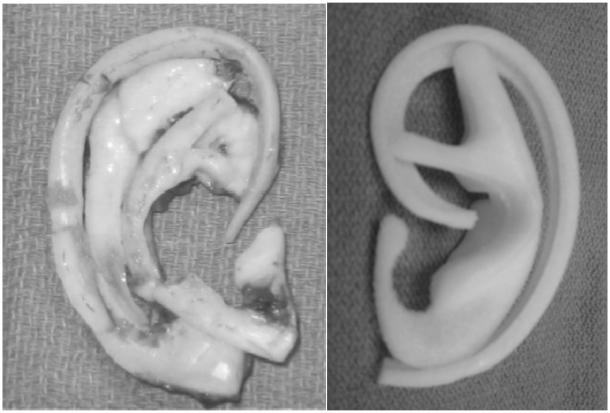
Type 2, 3, and 4 microtia/anotia require complete or near complete ear construction. There are two types of ear construction: autologous (costochondral grafts) and alloplastic (implant based) (Fig 5). Tanzer popularized the use of autologous tissue ear construction utilizing costochondral (rib) cartliage [Tanzer, 1959]. This technique has been modified by many surgeons and is usually performed as a two or three stage procedure. Many alloplastic materials have been used for ear construction. Porous polyethylene (Medpor©) is the most commonly used material. Preformed implants are available and the morbidity at the donor site is avoided with their usage. However, implant constructions are less tolerant of trauma and may require removal if exposure occurs.
Fig 5. 5 Ear Construct Framework.
Costochondral cartilage (left) and Medpor© (right)
Ear construction is typically deferred to a minimum of 6 years of age. This is because the ear reaches 80% of its adult size by this age. Autologous ear constructions will often grow in proportion to normal facial development. Additionally, rib donor site availability is usually limited before 6 years of age. Some surgeons prefer to delay this surgery until 9 or 10 years of age because of concerns for adequate rib cartilage to shape an appropriate sized ear. Ear construction surgery is always multi-staged no matter what style is performed. Typically, the construction of an external auditory canal is left until last and is dependent on the presence or absence of certain anatomic structures within the middle ear [Jahrsdoerfer, 1980]. The creation of an external auditory canal serves to correct the maximal conductive hearing loss (60 dB) that is present with external auditory canal atresia. The advent of the bone anchored hearing aide (BAHA) has eliminated the need for external auditory canal construction in many patients. This hearing aid vibrates the cranial bones directly causing ossicular chain stimulation. The hearing aid is worn with a head band until age 5 at which time a permanent cranial abutment is placed post-auricularly. Treacher Collins children who utilized BAHAs compared with conventional bone anchored hearing aides experience better auditory outcomes [Marsella et al., 2011].
Eyelid Reconstruction
Lower eyelid malformation and the downward slanting of the palpebral fissure is one of the hallmarks of the mandibulofacial dysostosis phenotype. Correction of this is often delayed until adolescence, as adequate tissue is often not present prior to this age. Jackson described the most commonly utilized technique for reconstruction [Jackson, 1981]. In this technique, redundant upper eyelid skin is used to reconstruction the deficient lower eyelid and coloboma after it is released (Fig 6). A lateral canthopexy is also performed to correct the downslanting palpebral fissure by securing the lateral canthus to a more cephalad position on the lateral orbit.
Fig 6. Eyelid Switch Reconstruction.
Pre-op (left) and 2-weeks post-op (right) showing elevation of the lateral canthus and soft tissue augmentation of the lower eyelids
Adulthood
Jaw (Orthognathic) Reconstruction
For most children and adults with facial dysostoses, orthodontics (ie. braces) are required to align the teeth and dental arches and establish a normal occlusion. Even with braces, proper alignment of the teeth may be difficult or impossible. Orthognathic surgery may be performed as an adjuvant to orthodontics to help establish a proper bite. Prior to surgery, facial relationships and proportions are measured and compared to normative values in a process called cephalometric analysis. These measurements are then used to plan precise surgical movements that will correct both the malocclusion and reestablish facial balance.
Genioplasty (or chin reconstruction) is the simplest and easiest of all the jaw procedures. This technique can be performed by surgically repositioning the mention (anterior inferior most chin point) of the mandible. In the majority of cases, the mandible needs to be both advanced and have its height increased. For this reason, the sliding genioplasty is the ideal procedure in most patients as it performs both of these movements. However, adequate mandibular bone stock is often not present and an alloplastic implant such as Medpor© is required in these patients.
CONCLUSIONS AND PERSEPECTIVES
Facial dysostoses comprise a group of rare, clinically and etiologically heterogeneous craniofacial disorders. Proper healthcare for patients with facial dysotoses comes at a great cost over many years and results are often variable, and rarely fully corrective. These disorders have serious functional, aesthetic and social consequences that require a lifetime of medical and surgical management. A multidisciplinary team involving plastic surgery, oral surgery, orthodontics, pediatrics, otolaryngology, genetics, dentistry, psychology, audiology, speech and language pathology, social work and nursing is required to treat and ameliorate a lifetime of specific problems.
As a result, better techniques for tissue repair and regeneration need to be developed and therapeutic avenues of prevention broadened to eliminate the devastating consequences of craniofacial birth anomalies. This requires a comprehensive understanding of the normal events controlling craniofacial development during embryogenesis. Central to this is a better appreciation of the distinct signals, switches and mechanism that regulate the formation, migration and differentiation of neural crest cells, which give rise to most of the bone and cartilage of the head and face.
The integration of human and mouse genetics, cell biology, biochemistry and experimental embryology has recently provided novel insights into the etiology and pathogenesis of facial dysostoses such as Treacher Collins syndrome, mandibulofacial dysostosis with microcephaly, Nager and Miller syndromes. Furthermore with respect to Treacher Collins syndrome, the pathogenesis of this disorder is now so well understood that potential avenues for therapeutic prevention have been identified. In fact, the prevention of Treacher Collins syndrome craniofacial anomalies represents one of the first successful embryologic animal model “rescues” of a congenital neurocristopathy (Fig. 2). Additionally, it provides an attractive model for the prevention of other craniofacial birth defects of similar etiology and pathogenesis.
Although tremendous progress has been made, the etiology and pathogenesis of many other facial dysostoses remains unknown. Furthermore, the challenges of translating any potential preventative approaches into the clinic application still remain. Nonetheless, with continued advances and integration between clinical genetics and basic biomedical science, the future looks bright for managing, treating and preventing congenital craniofacial disorders such as facial dysostosis.
ACKNOWLEDGMENTS
Research in the Trainor laboratory is supported by the Stowers Institute for Medical Research, and National Institute of Dental and Craniofacial Research (RO1 DE 016082).
Biographies
Dr Paul Trainor, PhD is an Investigator at the Stowers Institute for Medical Research in Kansas City, Missouri and an adjunct Professor at the University of Kansas Medical Center. Dr Trainor leads a research team that focuses on understanding the mechanisms that govern normal embryo development and the process that go awry in the pathogenesis of congenital anomalies, with a particular emphasis on craniofacial development and neurocristopathies. Dr Trainor did his PhD training through the University of Sydney in Australia, at the Children’s Medical Research Institute, followed by post-doctoral training at the National Institute for Medical Research in London, UK.
Dr Brian Andrews, M.D. is the Director of Cleft and Craniofacial Surgery and an Assistant Professor at the University of Kansas Medical Center in Kansas City, Kansas Dr. Andrews collaborates with a team of expert physicians trained in advanced treatments for people with cleft and craniofacial disorders and also facilitates clinical and basic science research in areas such as speech development, language development, and long-term hearing outcomes. Dr. Andrews completed a craniofacial fellowship program at UCLA in Los Angeles and prior to that, a plastic surgery residency at Harvard in Boston, Massachusetts, and an Otolaryngology residency at the University of Iowa in Iowa City, Iowa.
REFERENCES
- Andrews BT, Fan KL, Roostaeian J, Federico C, Bradley JP. Incidence of concomitant airway anomalies when using the University of California, Los Angeles, protocol for neonatal mandibular distraction. Plast Reconstr Surg. 2013;131(5):1116–1123. doi: 10.1097/PRS.0b013e3182865da0. [DOI] [PubMed] [Google Scholar]
- Aylsworth AS, Lin AE, Friedman PA. Nager acrofacial dysostosis: male-to-male transmission in 2 families. Am J Med Genet. 1991;41(1):83–88. doi: 10.1002/ajmg.1320410121. [DOI] [PubMed] [Google Scholar]
- Bernier FP, Caluseriu O, Ng S, Schwartzentruber J, Buckingham KJ, Innes AM, Jabs EW, Innis JW, Schuette JL, Gorski JL, Byers PH, Andelfinger G, Siu V, Lauzon J, Fernandez BA, McMillin M, Scott RH, Racher H, FORGE Canada Consortium. Majewski J, Nickerson DA, Shendure J, Bamshad MJ, Parboosingh JS. Haploinsufficiency of SF3B4, a component of the pre-mRNA spliceosomal complex, causes Nager syndrome. Am J Hum Genet. 2012;90(5):925–933. doi: 10.1016/j.ajhg.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champion-Arnaud P, Reed R. The prespliceosome components SAP 49 and SAP 145 interact in a complex implicated in tethering U2 snRNP to the branch site. Genes Dev. 1994;8(16):1974–1983. doi: 10.1101/gad.8.16.1974. [DOI] [PubMed] [Google Scholar]
- Chemke J, Mogilner BM, Ben-Itzhak I, Zurkowski L, Ophir D. Autosomal recessive inheritance of Nager acrofacial dysostosis. J Med Genet. 1988;25(4):230–232. doi: 10.1136/jmg.25.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong DK, Murray DJ, Britto JA, Tompson B, Forrest CR, Phillips JH. A cephalometric analysis of maxillary and mandibular parameters in Treacher Collins syndrome. Plast Reconstr Surg. 2008;121(3):77e–4e. doi: 10.1097/01.prs.0000299379.64906.2e. [DOI] [PubMed] [Google Scholar]
- Cohen J, Ghezzi F, Goncalves L, Fuentes JD, Paulyson KJ, Sherer DM. Prenatal sonographic diagnosis of Treacher Collins syndrome: a case and review of the literature. Am J Perinatol. 1995;12(6):416–419. doi: 10.1055/s-2007-994511. [DOI] [PubMed] [Google Scholar]
- Czeschik JC, Voigt C, Alanay Y, Albrecht B, Avci S, Fitzpatrick D, Goudie DR, Hehr U, Hoogeboom AJ, Kayserili H, Simsek-Kiper PO, Klein-Hitpass L, Kuechler A, López-González V, Martin M, Rahmann S, Schweiger B, Splitt M, Wollnik B, Lüdecke HJ, Zeschnigk M, Wieczorek D. Clinical and mutation data in 12 patients with the clinical diagnosis of Nager syndrome. Hum Genet. 2013 doi: 10.1007/s00439-013-1295-2. [DOI] [PubMed] [Google Scholar]
- Dauwerse JG, Dixon J, Seland S, Ruivenkamp CA, van Haeringen A, Hoefsloot LH, Peters DJ, Boers AC, Daumer-Haas C, Maiwald R Zweier C, Kerr B, Cobo AM, Toral JF, Hoogeboom AJ, Lohmann DR, Hehr U, Dixon MJ, Breuning MH, Wieczorek D. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet. 2011;43(1):20–22. doi: 10.1038/ng.724. [DOI] [PubMed] [Google Scholar]
- Dixon J, Dixon MJ. Genetic background has a major effect on the penetrance and severity of craniofacial defects in mice heterozygous for the gene encoding the nucleolar protein Treacle. Dev Dyn. 2004;229(4):907–914. doi: 10.1002/dvdy.20004. [DOI] [PubMed] [Google Scholar]
- Dixon J, Jones NC, Sandell LL, Jayasinghe SM, Crane J, Rey JP, Dixon MJ, Trainor PA. Tcof1/Treacle is required for neural crest cell formation and proliferation deficiencies that cause craniofacial abnormalities. Proceedings Of The National Academy Of Sciences Of The United States Of America; 2006. pp. 13403–13408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Trainor P, Dixon MJ. Treacher Collins syndrome. Orthod Craniofac Res. 2007;10(2):88–95. doi: 10.1111/j.1601-6343.2007.00388.x. [DOI] [PubMed] [Google Scholar]; Dixon MJ, Marres HA, Edwards SJ, Dixon J, Cremers CW. Treacher Collins syndrome: correlation between clinical and genetic linkage studies. Clin Dysmorphol. 1994;3(2):96–103. [PubMed] [Google Scholar]
- Duan X, Kelsen SG, Clarkson AB, Jr., Ji R, Merali S. SILAC analysis of oxidative stress-mediated proteins in human pneumocytes: new role for treacle. Proteomics. 2010;10(11):2165–2174. doi: 10.1002/pmic.201000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SJ, Fowlie A, Cust MP, Liu DT, Young ID, Dixon MJ. Prenatal diagnosis in Treacher Collins syndrome using combined linkage analysis and ultrasound imaging. J Med Genet. 1996;33(7):603–606. doi: 10.1136/jmg.33.7.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards SJ, Gladwin AJ, Dixon MJ. The mutational spectrum in Treacher Collins syndrome reveals a predominance of mutations that create a premature-termination codon. American Journal of Human Genetics. 1997;60(3):515–524. [PMC free article] [PubMed] [Google Scholar]
- Fang J, Uchiumi T, Yagi M, Matsumoto S, Amamoto R, Saito T, Takazaki S, Kanki T, Yamaza H, Nonaka K, Kang D. Protein instability and functional defects caused by mutations of dihydro- orotate dehydrogenase in Miller syndrome patients. Biosci Rep. 2012;32(6):631–639. doi: 10.1042/BSR20120046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschetti A, Klein D. The mandibulofacial dysostosis; a new hereditary syndrome. Acta Ophthalmol (Copenh) 1949;27(2):143–224. [PubMed] [Google Scholar]
- Genee E. [An extensive form of mandibulo-facial dysostosis] J Genet Hum. 1969;17(1):45–52. [PubMed] [Google Scholar]; Gladwin AJ, Dixon J, Loftus SK, Edwards S, Wasmuth JJ, Hennekam RC, Dixon MJ. Treacher Collins syndrome may result from insertions, deletions or splicing mutations, which introduce a termination codon into the gene. Human Molecular Genetics. 1996;5(10):1533–1538. doi: 10.1093/hmg/5.10.1533. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Cohen MM, Levin LS. Oxford University Press; Oxford, UK: 1990. Syndromes of the Head and Neck. [Google Scholar]
- Guion-Almeida ML, Zechi-Ceide RM, Vendramini S, Tabith A., Junior A new syndrome with growth and mental retardation, mandibulofacial dysostosis, microcephaly, and cleft palate. Clin Dysmorphol. 2006;15(3):171–174. doi: 10.1097/01.mcd.0000220603.09661.7e. [DOI] [PubMed] [Google Scholar]
- Hail N, Jr., Chen P, Kepa JJ, Bushman LR, Shearn C. Dihydroorotate dehydrogenase is required for N-(4-hydroxyphenyl)retinamide-induced reactive oxygen species production and apoptosis. Free Radic Biol Med. 2010;49(1):109–116. doi: 10.1016/j.freeradbiomed.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halal F, Herrmann J, Pallister PD, Opitz JM, Desgranges MF, Grenier G. Differential diagnosis of Nager acrofacial dysostosis syndrome: report of four patients with Nager syndrome and discussion of other related syndromes. Am J Med Genet. 1983;14(2):209–224. doi: 10.1002/ajmg.1320140203. [DOI] [PubMed] [Google Scholar]
- Hall BD. Nager acrofacial dysostosis: autosomal dominant inheritance in mild to moderately affected mother and lethally affected phocomelic son. Am J Med Genet. 1989;33(3):394–397. doi: 10.1002/ajmg.1320330321. [DOI] [PubMed] [Google Scholar]
- Isaac C, Marsh KL, Paznekas WA, Dixon J, Dixon MJ, Jabs EW, Meier UT. Characterization of the nucleolar gene product, treacle, in Treacher Collins syndrome. Mol Biol Cell. 2000;11(9):3061–3071. doi: 10.1091/mbc.11.9.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson IT. Reconstruction of the lower eyelid defect in Treacher Collins syndrome. Plast Reconstr Surg. 1981;67(3):365–368. doi: 10.1097/00006534-198103000-00017. [DOI] [PubMed] [Google Scholar]
- Jahrsdoerfer R. Congenital malformations of the ear. Analysis of 94 operations. Ann Otol Rhinol Laryngol. 1980;89:348–352. doi: 10.1177/000348948008900410. 4 Pt 1. [DOI] [PubMed] [Google Scholar]
- Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, Rey JP, Glynn EF, Ellington L, Du C, Dixon J, Dixon MJ, Trainor PA. Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nature Medicine. 2008;14(2):125–133. doi: 10.1038/nm1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy SJ, Teebi AS. Newly recognized autosomal recessive acrofacial dysostosis syndrome resembling Nager syndrome. Am J Med Genet A. 2004;129A(1):73–76. doi: 10.1002/ajmg.a.30113. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bradley JP. Informa Healthcare; New York: 2008. Treacher-Collins Syndrome. [Google Scholar]
- Le Merrer M, Cikuli M, Ribier J, Briard ML. Acrofacial dysostoses. Am J Med Genet. 1989;33(3):318–322. doi: 10.1002/ajmg.1320330307. [DOI] [PubMed] [Google Scholar]
- Lindstrom MS, Deisenroth C, Zhang Y. Putting a finger on growth surveillance: insight into MDM2 zinc finger-ribosomal protein interactions. Cell Cycle. 2007;6(4):434–437. doi: 10.4161/cc.6.4.3861. [DOI] [PubMed] [Google Scholar]
- Lines MA, Huang L, Schwartzentruber J, Douglas SL, Lynch DC, Beaulieu C, Guion-Almeida ML, Zechi-Ceide RM, Gener B, Gillessen-Kaesbach G, Nava C, Baujat G, Horn D, Kini U, Caliebe A, Alanay Y, Utine GE, Lev D, Kohlhase J, Grix AW, Lohmann DR, Hehr U, Böhm D, FORGE Canada Consortium. Majewski J, Bulman DE, Wieczorek D, Boycott KM. Haploinsufficiency of a spliceosomal GTPase encoded by EFTUD2 causes mandibulofacial dysostosis with microcephaly. Am J Hum Genet. 2012;90(2):369–377. doi: 10.1016/j.ajhg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YH, Tang Z, Kundu RK, Wu L, Luo W, Zhu D, Sangiorgi F, Snead ML, Maxson RE. Msx2 gene dosage influences the number of proliferative osteogenic cells in growth centers of the developing murine skull: a possible mechanism for MSX2-mediated craniosynostosis in humans. Dev Biol. 1999;205(2):260–274. doi: 10.1006/dbio.1998.9114. [DOI] [PubMed] [Google Scholar]
- Luquetti DV, Hing AV, Rieder MJ, Nickerson DA, Turner EH, Smith J, Park S, Cunningham ML. "Mandibulofacial dysostosis with microcephaly" caused by EFTUD2 mutations: expanding the phenotype. Am J Med Genet A. 2013;161A(1):108–113. doi: 10.1002/ajmg.a.35696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marres HA, Cremers CW, Dixon MJ, Huygen PL, Joosten FB. The Treacher Collins syndrome. A clinical, radiological, and genetic linkage study on two pedigrees. Arch Otolaryngol Head Neck Surg. 1995;121(5):509–514. doi: 10.1001/archotol.1995.01890050009002. [DOI] [PubMed] [Google Scholar]
- Marsella P, Scorpecci A, Pacifico C, Tieri L. Bone-anchored hearing aid (Baha) in patients with Treacher Collins syndrome: tips and pitfalls. Int J Pediatr Otorhinolaryngol. 2011;75(10):1308–1312. doi: 10.1016/j.ijporl.2011.07.020. [DOI] [PubMed] [Google Scholar]; McCarthy JG, Schreiber J, Karp N, Thorne CH, Grayson BH. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg. 1992;89(1):1–8. discussion 9-10. [PubMed] [Google Scholar]
- McDonald MT, Gorski JL. Nager acrofacial dysostosis. J Med Genet. 1993;30(9):779–782. doi: 10.1136/jmg.30.9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Bochukova EG, Brugger SM, Ishii M, Pilz DT, Wall SA, Lyons KM, Wilkie AO, Maxson RE., Jr. Cell mixing at a neural crest-mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Human Molecular Genetics. 2006;15(8):1319–1328. doi: 10.1093/hmg/ddl052. [DOI] [PubMed] [Google Scholar]
- Meurman Y. Congenital microtia and meatal atresia; observations and aspects of treatment. AMA Arch Otolaryngol. 1957;66(4):443–463. doi: 10.1001/archotol.1957.03830280073008. [DOI] [PubMed] [Google Scholar]
- Miller M, Fineman R, Smith DW. Postaxial acrofacial dysostosis syndrome. J Pediatr. 1979;95(6):970–975. doi: 10.1016/s0022-3476(79)80285-1. [DOI] [PubMed] [Google Scholar]
- Milligan DA, Harlass FE, Duff P, Kopelman JN. Recurrence of Treacher Collins' syndrome with sonographic findings. Mil Med. 1994;159(3):250–252. [PubMed] [Google Scholar]
- Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, Bamshad MJ, Shendure J. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42(1):30–35. doi: 10.1038/ng.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden DM, Trainor PA. Relations and interactions between cranial mesoderm and neural crest populations. Journal Of Anatomy. 2005;207(5):575–601. doi: 10.1111/j.1469-7580.2005.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz JM. Nager "syndrome" versus "anomaly" and its nosology with the postaxial acrofacial dysostosis syndrome of Genee and Wiedemann. Am J Med Genet. 1987;27(4):959–963. doi: 10.1002/ajmg.1320270425. [DOI] [PubMed] [Google Scholar]
- Phelps PD, Poswillo D, Lloyd GA. The ear deformities in mandibulofacial dysostosis (Treacher Collins syndrome) Clin Otolaryngol. 1981;6(1):15–28. doi: 10.1111/j.1365-2273.1981.tb01782.x. [DOI] [PubMed] [Google Scholar]
- Poswillo D. The pathogenesis of the Treacher Collins syndrome (mandibulofacial dysostosis) Br J Oral Surg. 1975;13(1):1–26. doi: 10.1016/0007-117x(75)90019-0. [DOI] [PubMed] [Google Scholar]
- Rainger J, Bengani H, Campbell L, Anderson E, Sokhi K, Lam W, Riess A, Ansari M, Smithson S, Lees M, Mercer C, McKenzie K, Lengfeld T, Gener Querol B, Branney P, McKay S, Morrison H, Medina B, Robertson M, Kohlhase J, Gordon C, Kirk J, Wieczorek D, Fitzpatrick DR. Miller (Genee-Wiedemann) syndrome represents a clinically and biochemically distinct subgroup of postaxial acrofacial dysostosis associated with partial deficiency of DHODH. Hum Mol Genet. 2012;21(18):3969–3983. doi: 10.1093/hmg/dds218. [DOI] [PubMed] [Google Scholar]
- Robert B. Bone morphogenetic protein signaling in limb outgrowth and patterning. Dev Growth Differ. 2007;49(6):455–468. doi: 10.1111/j.1440-169X.2007.00946.x. [DOI] [PubMed] [Google Scholar]
- Rogers GF, Murthy AS, LaBrie RA, Mulliken JB. The GILLS score: part I. Patient selection for tongue-lip adhesion in Robin sequence. Plast Reconstr Surg. 2011;128(1):243–251. doi: 10.1097/PRS.0b013e318217420d. [DOI] [PubMed] [Google Scholar]
- Rubbi CP, Milner J. Disruption of the nucleolus mediates stabilization of p53 in response to DNA damage and other stresses. Embo Journal. 2003;22(22):6068–6077. doi: 10.1093/emboj/cdg579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Lozano P, Doevendans P, Brown A, Gruber PJ, Chien KR. Developmental expression of the murine spliceosome-associated protein mSAP49. Dev Dyn. 1997;208(4):482–490. doi: 10.1002/(SICI)1097-0177(199704)208:4<482::AID-AJA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Sakai D, Dixon J, Dixon MJ, Trainor PA. Mammalian neurogenesis requires Treacle-Plk1 for precise control of spindle orientation, mitotic progression, and maintenance of neural progenitor cells. PLoS Genet. 2012;8(3):e1002566. doi: 10.1371/journal.pgen.1002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlieve T, Almusa M, Miloro M, Kolokythas A. Temporomandibular joint replacement for ankylosis correction in Nager syndrome: case report and review of the literature. J Oral Maxillofac Surg. 2012;70(3):616–625. doi: 10.1016/j.joms.2011.02.053. [DOI] [PubMed] [Google Scholar]
- Shukowski WP. Zur atiologie des stridor inspiratorius congenitus. Jahrb Kinderheilk. 1911;73:459–474. [Google Scholar]
- Splendore A, Silva EO, Alonso LG, Richieri-Costa A, Alonso N, Rosa A, Carakushanky G, Cavalcanti DP, Brunoni D, Passos-Bueno MR. High mutation detection rate in TCOF1 among Treacher Collins syndrome patients reveals clustering of mutations and 16 novel pathogenic changes. Hum Mutat. 2000;16(4):315–322. doi: 10.1002/1098-1004(200010)16:4<315::AID-HUMU4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Stovin JJ, Lyon JA, Jr., Clemmens RL. Mandibulofacial dysostosis. Radiology. 1960;74:225–231. doi: 10.1148/74.2.225. [DOI] [PubMed] [Google Scholar]
- Tanzer RC. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. 1959;23(1):1–15. doi: 10.1097/00006534-195901000-00001. [DOI] [PubMed] [Google Scholar]
- Teber OA, Gillessen-Kaesbach G, Fischer S, Bohringer S, Albrecht B, Albert A, Arslan-Kirchner M, Haan E, Hagedorn-Greiwe M, Hammans C, Henn W, Hinkel GK, König R, Kunstmann E, Kunze J, Neumann LM, Prott EC, Rauch A, Rott HD, Seidel H, Spranger S, Sprengel M, Zoll B, Lohmann DR, Wieczorek D. Genotyping in 46 patients with tentative diagnosis of Treacher Collins syndrome revealed unexpected phenotypic variation. Eur J Hum Genet. 2004;12(11):879–890. doi: 10.1038/sj.ejhg.5201260. [DOI] [PubMed] [Google Scholar]
- Tessier P. Autogenous bone grafts taken from the calvarium for facial and cranial applications. Clin Plast Surg. 1982;9(4):531–538. [PubMed] [Google Scholar]
- Treacher Collins E. Case with symmetrical congenital notches in the outer part of each lower lid and defective development of the malar bones. Trans Opthalmol Soc UK. 1900;20:90. [Google Scholar]; TreacherCollinsSyndromeCollaborativeGroup Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. Nat Genet. 1996;12(1):130–136. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci U S A. 2004;101(29):10709–10714. doi: 10.1073/pnas.0402492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner DJ, Ciske DJ, Dowton SB, Watson MS. Deletion of 1q in a patient with acrofacial dysostosis. Am J Med Genet. 1999;82(4):301–304. doi: 10.1002/(sici)1096-8628(19990212)82:4<301::aid-ajmg5>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Shionyu M, Kimura T, Kimata K, Watanabe H. Splicing factor 3b subunit 4 binds BMPR-IA and inhibits osteochondral cell differentiation. J Biol Chem. 2007;282(28):20728–20738. doi: 10.1074/jbc.M703292200. [DOI] [PubMed] [Google Scholar]
- White RM, Cech J, Ratanasirintrawoot S, Lin CY, Rahl PB, Burke CJ, Langdon E, Tomlinson ML, Mosher J, Kaufman C, Chen F, Long HK, Kramer M, Datta S, Neuberg D, Granter S, Young RA, Morrison S, Wheeler GN, Zon LI. DHODH modulates transcriptional elongation in the neural crest and melanoma. Nature. 2011;471(7339):518–522. doi: 10.1038/nature09882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann HR. [Malformation-retardation syndrome with bilateral absence of the 5th rays in both hands and feets, cleft palate, malformed ears and eyelids, radioulnar synostosis (author's transl)] Klin Padiatr. 1973;185(3):181–186. [PubMed] [Google Scholar]
- Zenk J, Fyrmpas G, Zimmermann T, Koch M, Constantinidis J, Iro H. Tracheostomy in young patients: indications and long-term outcome. Eur Arch Otorhinolaryngol. 2009;266(5):705–711. doi: 10.1007/s00405-008-0796-4. [DOI] [PubMed] [Google Scholar]