Abstract
Purpose
To retrospectively review our institutional experience with hypopharyngeal carcinoma with respect to treatment modality.
Methods and Materials
A total of 70 patients with hypopharyngeal cancer treated between 1999 and 2009 were analyzed for functional and survival outcomes. The treatments included surgery alone (n = 5), surgery followed by radiotherapy (RT) (n = 3), surgery followed by chemoradiotherapy (CRT) (n = 13), RT alone (n = 2), CRT alone (n = 22), induction chemotherapy followed by RT (n = 3), and induction chemotherapy followed by CRT (n = 22).
Results
The median follow-up was 18 months. The median overall survival and disease-free survival for all patients was 28.3 and 17.6 months, respectively. The 1- and 2-year local control rate for all patients was 87.1% and 80%. CRT, given either as primary therapy or in the adjuvant setting, improved overall survival and disease-free survival compared with patients not receiving CRT. The median overall survival and disease-free survival for patients treated with CRT was 36.7 and 17.6 months vs. 14.0 and 8.0 months, respectively (p <.01). Of the patients initially treated with an organ-preserving approach, 4 (8.2%) required salvage laryngectomy for local recurrence or persistent disease; 8 (16.3%) and 12 (24.5%) patients were dependent on a percutaneous gastrostomy and tracheostomy tube, respectively. The 2-year laryngoesophageal dysfunction-free survival rate for patients treated with an organ-preserving approach was estimated at 31.7%.
Conclusions
Concurrent CRT improves survival in patients with hypopharyngeal cancer. CRT given with conventional radiation techniques yields poor functional outcomes, and future efforts should be directed at determining the feasibility of pharyngeal-sparing intensity-modulated radiotherapy in patients with hypopharyngeal tumors.
Introduction
Hypopharyngeal cancer is a relatively uncommon malignancy, with less than 3,000 cases reported in the United States annually. Patients tend to present with locoregionally advanced disease, with one-half of patients having nodal metastasis at diagnosis (1). Management of this disease is complicated in part by its aggressive clinical course, as well as its anatomic location in relation to other critical structures.
Historically, the mainstay of treatment of tumors of the hypopharynx was surgical resection followed by adjuvant radiotherapy (RT). Organ-preserving therapy, with RT and chemotherapy, has been demonstrated to be a feasible and effective strategy in the management of locally advanced laryngeal cancer (2, 3). In hypopharyngeal cancer, larynx preservation with induction chemotherapy (IC) followed by definitive RT has been shown to produce equivalent survival to laryngectomy followed by RT (1). However, the evidence for chemoradiotherapy (CRT) in this disease site has been limited either to single-institutional experiences or small subset analyses of larger trials in which patients with hypopharyngeal tumors were a small minority (4–6).
Given the relative paucity of data regarding this disease site, our aim was to report our institutional experience with hypopharyngeal cancer, specifically analyzing the outcomes with respect to treatment modality.
Methods and Materials
Patient characteristics
A total of 70 patients with histologically proven squamous cell cancer of the hypopharynx, treated between January of 1999 and July of 2009, were included in the present analysis. The pretreatment evaluation included the history and physical examination, triple endoscopy with fiberoptic laryngoscopy, esophagoscopy, bronchoscopy, and computed tomography or magnetic resonance imaging of the neck in all patients. Positron emission tomography was not routinely performed. The patients' disease was staged retrospectively in accordance with the American Joint Committee on Cancer 2009 criteria. All patients were evaluated by a multidisciplinary team, including surgeons, medical oncologists, and radiation oncologists, before the initiation of treatment. Patient demographics are summarized in Table 1.
Table 1. Patient characteristics.
| Characteristic | No CRT (n = 13) | CRT (n = 57) | Total (n = 70) |
|---|---|---|---|
| Median age (y) | 64 | 57 | 59 |
| Gender (n) | |||
| Male | 7 (53.8) | 47 (82.5) | 54 (77.1) |
| Female | 6 (46.2) | 10 (17.5) | 16 (22.9) |
| Primary site (n) | |||
| Piriform sinus | 10 (76.9) | 47 (82.5) | 57 (81.4) |
| Pharyngeal wall | 2 (15.4) | 8 (14.0) | 10 (14.3) |
| Postcricoid pharynx | 1 (7.7) | 2 (3.5) | 3 (4.3) |
| T stage (n) | |||
| 1 | 3 (23.1) | 3 (5.3) | 6 (8.6) |
| 2 | 3 (23.1) | 16 (28.1) | 19 (27.1) |
| 3 | 3 (23.1) | 18 (31.6) | 21 (30.0) |
| 4 | 4 (30.8) | 20 (35.1) | 24 (34.3) |
| N stage (n) | |||
| 0 | 5 (38.5) | 10 (17.5) | 15 (21.4) |
| 1 | 1 (7.7) | 7 (12.3) | 8 (11.4) |
| 2 | 3 (23.1) | 29 (50.9) | 32 (45.7) |
| 3 | 4 (30.8) | 11 (19.3) | 15 (21.4) |
| AJCC stage (n) | |||
| I | 2 (15.4) | 0 (0.0) | 2 (2.9) |
| II | 1 (7.7) | 5 (8.8) | 6 (8.6) |
| III | 3 (23.1) | 10 (17.5) | 13 (18.6) |
| IVa | 2 (15.4) | 26 (45.6) | 28 (40.0) |
| IVb | 5 (38.5) | 16 (28.1) | 21 (30.0) |
Abbreviations: CRT = concurrent chemoradiotherapy; AJCC = American Joint Committee on Cancer.
Data in parentheses are percentages.
Treatment
Treatment consisted of either primary surgery, given with or without adjuvant treatment according to the pathologic features and stage, or organ preservation with either concurrent CRT or RT alone.
For patients undergoing surgery, the surgical approach was determined at the discretion of the treating surgeon and included total laryngopharyngectomy or partial pharyngectomy with total or supraglottic laryngectomy. Neck dissection was routinely performed, along with surgical resection of the primary tumor, in patients with clinically evident nodal metastases. Six patients received up-front neck dissection alone, without resection of the primary tumor, and were included in the organ-preservation group. Of 16 patients receiving adjuvant therapy, 13 received postoperative CRT and 3 patients received RT alone, as described in the following paragraphs.
Of the patients treated with an organ-preserving approach, IC was given before definitive RT or CRT in 25 patients and consisted of either docetaxel, cisplatin, and 5-fluorouracil (TPF) or cisplatin and 5-fluorouracil (PF). The TPF regimen consisted of docetaxel (75 mg/m2) given as a 1-h infusion, followed by intravenous cisplatin (100 mg/m2) given within 3 h, and 5-fluorouracil (1,000 mg/m2) given continuously for 4 days. The PF regimen consisted of intravenous cisplatin (100 mg/m2) followed by 5-fluorouracil (1,000 mg/m2) given continuously for 4 days. IC was given every 3 weeks for two to three cycles, depending on the clinical response. Patients with a complete response received two cycles, and those with a partial response received a third cycle. Those with no response subsequently underwent either salvage laryngectomy or definitive radiotherapy, depending on their resectability status.
Radiotherapy was administered to all patients in the organ-preserving group and to those with high-risk pathologic findings after surgery. Most patients were treated with 6-MV photons using a three-field technique to cover the primary tumor and draining lymphatics, including the bilateral retropharyngeal lymph nodes. The three-field technique consisted of opposed lateral fields matched to an anterior field covering the low neck and supraclavicular regions using a half-beam block. The lateral fields were reduced at 4,000 cGy to avoid the spinal cord, at which point the anterior neck was treated with opposed lateral fields and the posterior neck supplemented with direct posterior electron fields. The median dose delivered was 7,000 cGy to gross disease, 6,000–6,400 cGy to the postoperative high-risk regions, and 5,000 cGy to the elective nodal regions, including the low neck and supraclavicular field. Three patients were treated with intensity-modulated RT (IMRT). Two patients with bulky Stage N3 disease were treated with mixed photon-neutron RT.
A total of 57 patients (81.4%) received CRT as a component of their therapy. This group included 13 postoperative patients, 22 patients after IC, and 22 patients who received CRT alone. The agents used for concurrent treatment included cisplatin (79%), carboplatin (14%), and cetuximab (7%). Cisplatin was typically administered intravenously at a dose of 100 mg/m2 during Weeks 1, 4, and 7. Carboplatin was given as a weekly intravenous infusion at an area under the curve of 1.5 during Weeks 1–7. Erbitux was administered intravenously, with a loading dose of 400 mg/m2 given1week before RT, followed by weekly infusions of 250 mg/m2 during Weeks 1–7.
A summary of the treatment characteristics is provided in Table 2.
Table 2. Treatment characteristics.
| Treatment type | Patients (n) |
|---|---|
| Surgery | 21 (30.0) |
| Surgery alone | 5 (7.1) |
| Surgery followed by RT | 3 (4.3) |
| Surgery followed by CRT | 13 (18.6) |
| Organ preservation | 49 (70.0) |
| RT alone | 2 (2.9) |
| IC followed by RT | 3 (4.3) |
| IC followed by CRT | 22 (31.4) |
| CRT alone | 22 (31.4) |
| Overall population | 70 (100) |
| No CRT | 13 (18.6) |
| Surgery alone | 5 (7.1) |
| Surgery + RT | 3 (4.3) |
| RT alone | 2 (2.9) |
| IC + RT | 3 (4.3) |
| CRT | 57 (81.4) |
| Surgery + CRT | 13 (18.6) |
| IC + CRT | 22 (31.4) |
| CRT alone | 22 (31.4) |
Abbreviations: RT = radiotherapy; CRT = concurrent chemoradiotherapy; IC = induction chemotherapy.
Data in parentheses are percentages.
Follow-up
After treatment completion, the patients were typically seen on a monthly basis for the first year, every 2–3 months the following year, and every 3–6 months thereafter. The evaluation consisted of history and physical examination, which included flexible laryngoscopy. Computed tomography of the neck and chest were routinely performed within 6 months of treatment completion and annually thereafter. Patients who had suspicious findings, either radiographically or on physical examination, underwent directed biopsy. Patients with pathologically confirmed local recurrences were evaluated for salvage laryngectomy. Those patients who were not deemed surgical candidates, or who had synchronous distant metastases, received palliative chemotherapy or best supportive care.
Statistical analysis
The rates of overall survival (OS), disease-free survival (DFS), and laryngoesophageal dysfunction-free survival (LEDFS) were estimated using the Kaplan-Meier method. The events included in LEDFS were death, local recurrence, laryngectomy, tracheostomy at ≥2 years, and feeding tube placement at ≥2 years (7). For multi-variate analysis, the Cox proportional hazard function was used to estimate the hazard ratios (HRs) and to test for significant covariates. The following covariates were included in the initial Cox regression model fit for both OS and DFS outcomes: age, gender, stage, laryngectomy, any chemotherapy, IC, concurrent CRT, any RT, N stage, and T stage. Differences in the mean values or relative frequencies of the covariates between tumor stages were determined using the t test or Fisher's exact test, respectively.
Results
Patient population
Of the 70 patients included in the analysis, 54 were men and 16 were women, with a median age of 59 years (range, 41–90). The primary site was in the piriform sinus in 57 patients (81.4%), pharyngeal wall in 10 (14.3%), and postcricoid pharynx in 3 (4.3%). The disease stage was Stage I in 2 patients (2.8%), Stage II in 6 (8.5%), Stage III in 13 (18.6%), Stage IVa in 28 (40%), and Stage IVb in 21 (30%).
Survival
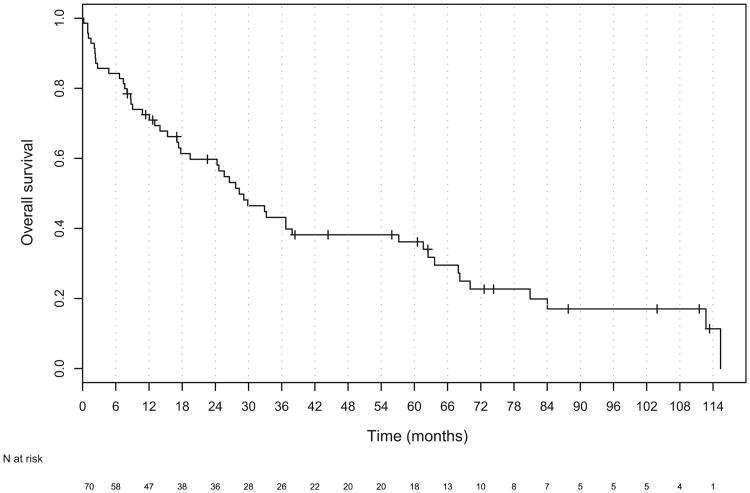
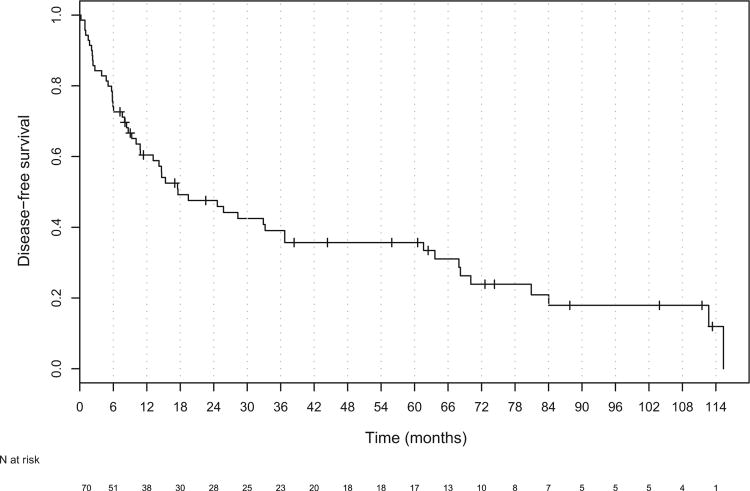
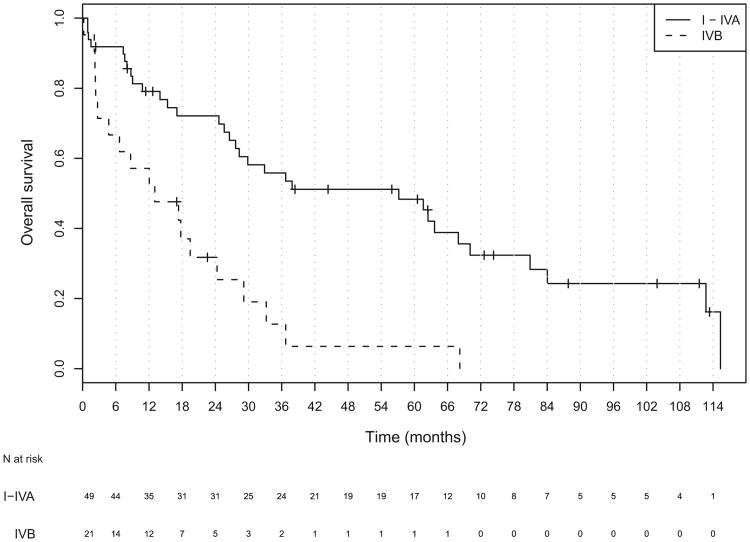
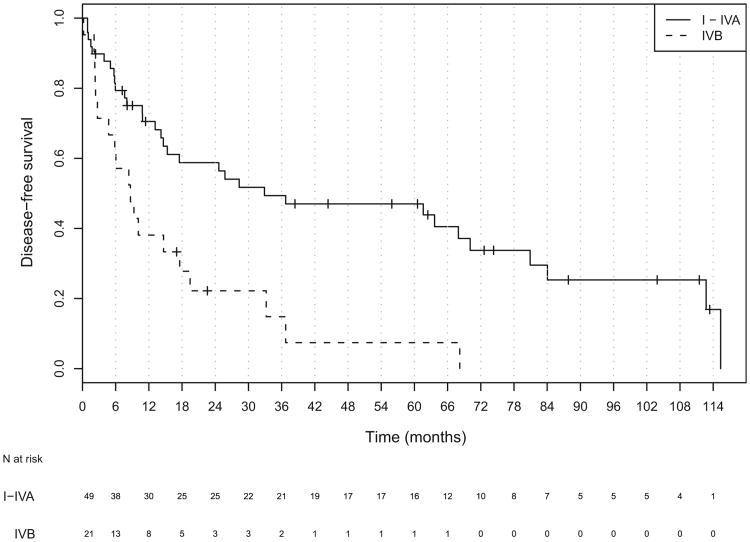
The median follow-up for all patients was 18 months (range, 1–114). Of the 70 patients, 19 patients were alive at last follow-up, and the median follow-up for all living patients was 44 months (range, 2–114). The median OS and DFS for all patients was 28.3 months (95% confidence interval [CI], 19.4–61.6) and 17.6 months (95% CI, 10.8–36.7), respectively (Figs. 1 and 2). The median OS was significantly worse in patients with unresectable (Stage IVb) disease than in those with resectable (Stage I-IVa) disease (HR, 3.30; 95% CI, 1.78–6.09; Table 3 and Fig. 3). The DFS was also worse in patients with unresectable tumors (hazard ratio, 2.72; 95% CI, 1.49–4.99; Table 3 and Fig. 4).
Fig. 1.
Kaplan-Meier plot of overall survival for all patients.
Fig. 2.
Kaplan-Meier plot of disease-free survival for all patients.
Table 3. Median overall and disease-free survival (in months) stratified by overall stage.
| Variable | Median OS | HR | Median DFS | HR |
|---|---|---|---|---|
| All stages | 28.3 (19.4–61.6) | – | 17.6 (10.8–36.7) | – |
| Stage I-IVa | 57.2 (28.3–80.9) | 1.0 | 32.9 (15.3–80.9) | 1.0 |
| Stage IVb | 13.0 (4.7–29.1) | 3.30 (1.78–6.09)* | 0.72 (0.39–2.77) | 2.72 (1.49–4.99)* |
Abbreviations: OS = overall survival; HR = hazard ratio; DFS = disease-free survival.
Data in parentheses are 95% confidence interval.
HR statistically significant at 0.001 level using Wald test.
Fig. 3.
Kaplan-Meier plot of overall survival for all patients stratified by disease stage.
Fig. 4.
Kaplan-Meier plot of disease-free survival for all patients stratified by disease stage.
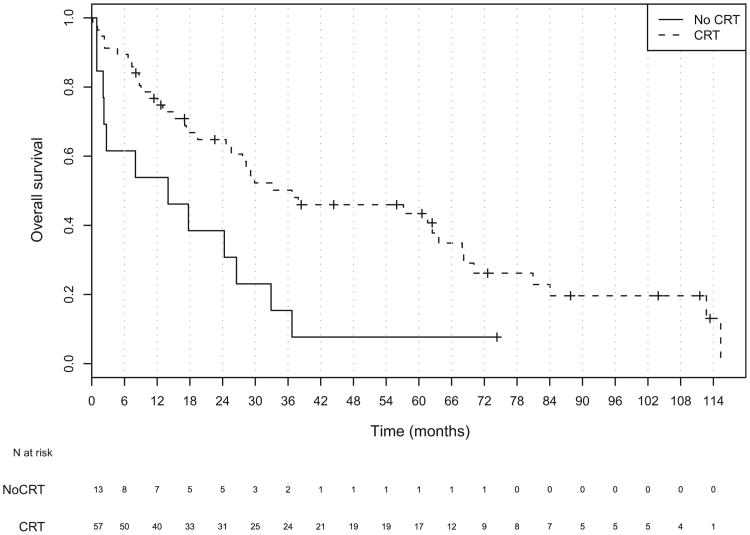
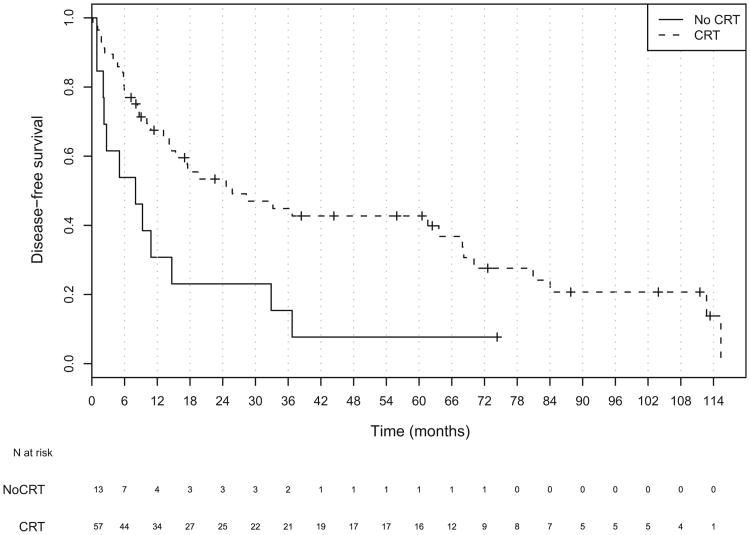
No statistically significant differences were found in tumor stage, nodal stage, overall stage, or use of IC between those receiving CRT compared with those not receiving CRT. The patients receiving CRT were younger and more frequently treated with organ-preserving therapy (Table 2). The addition of concurrent CRT, either as definitive or adjuvant treatment, improved overall survival in all stages. The median OS for patients receiving CRTas a part of their therapy was 36.7 months compared with 14 months for those who did not (hazard ratio, 0.41; 95% CI, 0.21–0.81; Table 4 and Fig. 5). Patients with Stage I-IVa disease who received CRT had a median survival of 63.6 months vs. 20.3 months for those who did not receive CRT (HR, 0.38; 95% CI, 0.16– 0.90). No statistically significant survival benefit was found for CRT in patients with unresectable disease (HR, 0.46; 95% CI, 0.16–1.35). The use of CRT improved disease-free survival among the entire population (Fig. 6). The median DFS in patients receiving CRT was 17.6 months vs. 8.0 months (HR, 0.41; 95% CI, 0.21–0.80). When analyzed by resectability status, only those patients with Stage I-IVa disease showed a DFS benefit (HR, 0.40; 95% CI, 0.17– 0.95; Table 4).
Table 4. Median overall and disease-free survival (in months) stratified by overall stage and CRT status.
| Variable | Median OS | HR | Median DFS | HR |
|---|---|---|---|---|
| All stages | ||||
| CRT | 36.7 (25.6–68.0) | 0.41 (0.21–0.81)* | 17.6 (10.8–36.7) | 0.41 (0.21–0.80)* |
| No CRT | 14.0 (2.2–NA) | 1.00 | 8.0 (2.2–NA) | 1.00 |
| Stage I-IVa | ||||
| CRT | 63.6 (17.5–NA) | 0.40 (0.17–0.95)† | 62.5 (29.9–NA) | 0.38 (0.16–0.90)† |
| No CRT | 20.3 (8.0–NA) | 1.00 | 9.4 (5.1–NA) | 1.00 |
| Stage IVb | ||||
| CRT | 15.2 (6.7–NA) | 0.46 (0.16–1.35) | 9.4 (5.9–NA) | 0.38 (0.12–1.13) |
| No CRT | 2.7 (2.2–NA) | 1.00 | 2.7 (2.2–NA) | 1.00 |
Abbreviation: NA = not available (confidence interval could not be estimated); abbreviations as in Table 3.
Data in parentheses are 95% confidence interval.
HR statistically significant at 0.01 level using Wald test.
HR statistically significant at 0.05 level using Wald test.
Fig. 5.
Kaplan-Meier plot of overall survival for all patients stratified by chemoradiotherapy status.
Fig. 6.
Kaplan-Meier plot of disease-free survival for all patients stratified by chemoradiotherapy status.
No statistically significant difference was found in OS or DFS between patients treated with laryngectomy or organ-preserving therapy. On multivariate analysis, the only significant predictors of survival were stage and the addition of CRT.
Disease control
Of the 70 patients, 15 developed local failure, 13 of which occurred in patients treated with organ-preserving therapy. The median time to local failure was 10 months. The 1- and 2-year local control rate was 87.1% and 80%, respectively. Of the 13 failures that occurred in the organ-preserving group, 7 were isolated local recurrences, 4 of which were deemed resectable, and salvage laryngectomy was performed. The patients with isolated local recurrence who underwent salvage laryngectomy had a median survival of 22.5 months while those who did not have surgical salvage had a median survival of 3 months. There were 15 distant failures, with 11 occurring in patients treated with organ-preserving treatment and 4 in those treated with laryngectomy. The median interval to distant failure was 10 months. The 1- and 2-year distant control rate was 87.1% and 81.4%, respectively.
Induction chemotherapy
A total of 25 patients received IC before definitive RT or CRT, with 22 receiving PF and 3 receiving TPF. In the group receiving PF, a complete response was observed in 6, a partial response in 13, and no response in 3. In the TPF group, 1 patient had a complete response and 2 patients had a partial response. Of the patients with no response to IC, 1 underwent laryngectomy, 1 underwent mixed photon-neutron RT, and 1 underwent definitive CRT.
Larynx preservation
Overall, 45 (91.8%) of the 49 patients treated without laryngectomy had an intact larynx at the last follow-up visit or death. A total of 8 patients (16.3%) required a permanent gastrostomy tube and 12 (24.5%) required a permanent tracheostomy tube. The LEDFS rate was estimated at 45.8% and 31.7% at 1 and 2 years, respectively.
Treatment compliance
A total of 4 patients did not complete their prescribed treatment. Of the 4 patients, 2 discontinued treatment against medical advice, 1 patient was placed in hospice, and 1 patient died of complications from congestive heart failure during CRT.
Discussion
The ideal treatment strategy for the management of hypo-pharyngeal cancer is unclear. In early-stage disease, organ preservation with primary RT alone has been shown to yield acceptable results, with local control rates of 70–90% (8–10). The management of locally advanced disease, however, varies by institution.
In 1996, Lefebvre et al (1) published the results of an European Organization of Research and Treatment of Cancer trial comparing surgery and adjuvant RT with organ-preserving therapy consisting of IC followed by definitive RT. The results of this trial demonstrated equivalence between the operative and nonoperative approaches. The median survival of those patients treated on the organ-preserving arm was reported at 44 months, with the larynx preservation rate estimated to be 35% at 5 years (1).
Concurrent CRT has emerged as a mainstay in the treatment of hypopharyngeal cancer, mostly based on the extrapolation of evidence from other head-and-neck subsites. The Radiation Therapy Oncology Group 91-11 trial, a three-arm trial comparing different organ-preserving approaches in glottic or supraglottic laryngeal cancer, demonstrated the superior efficacy of concurrent CRT compared with IC followed by definitive RT or RT alone (3). Trials specifically investigating the role of CRT for hypopharyngeal tumors, however, are limited. Bensadoun et al. reported the results of a French randomized trial investigating twice-daily RT, given with or without chemotherapy, in unresectable pharyngeal and hypopharyngeal tumors. The results of the that trial demonstrated a significant survival advantage with the addition of chemotherapy, with a 2-year overall survival rate of 37.8%. As expected, treatment-related toxicity was increased in the concurrent arm, with a more frequent need for gastrostomy tube placement (11). More recently, the results of a Phase III trial comparing concurrent CRT with IC followed by definitive RT was reported by Prades et al. This trial was restricted to tumors of the piriform sinus with a fixed hemilarynx. The primary endpoint of this trial was laryngeal preservation, which was significantly improved in the concurrent arm. Despite this benefit, no difference was present in survival between the two treatment arms (12).
Our institutional results demonstrate a significant DFS and OS benefit with the use of concurrent CRT, either as definitive treatment or in the postoperative setting in the treatment of hypopharyngeal carcinoma. No difference was found in survival between those patients treated with primary surgery vs. an organ-preserving approach. For patients with potentially resectable tumors treated with CRT, the median survival of 63.6 months compares very favorably with historical values. Our analysis is certainly limited by the factors that limit any retrospective review but is consistent with the results from other reported series. Yoon et al. published their results demonstrating the superiority of concurrent CRT compared with IC followed by definitive RT, in terms of both survival and larynx preservation (13). Similar survival results were observed in a study that included both laryngeal and hypopharyngeal primary tumors, but with significant long-term toxicity. Only 52% of patients reported in this series were alive with complete response and a functional larynx (14).
The interpretation of laryngeal function rates across studies is difficult, because the definition has varied dramatically. In the present study, we elected to report the endpoint of LEDFS, as defined by the recently reported international consensus panel summary (7). To our knowledge, this is the first study to report laryngeal function in these terms, and, as such, it is unclear how our results compare with the historical rates. Regardless, our 2-year LEDFS rate of 31.7% certainly highlights the need for improvement of the long-term functional outcome after CRT. This is especially true of tumors involving the hypopharynx, which correlated negatively with functional preservation (15). Modern techniques of RT delivery might improve patient outcomes. Eisbruch et al. identified the pharyngeal constrictor muscles, along with the glottis and supraglottic larynx, as the dysphagia-related structures for which avoidance using IMRT might improve long-term function (16). A subsequent prospective study published by that group showed that increasing the dose to the constrictors did indeed correlate with poorer swallowing function and that dose sparing to these structures was feasible (17). In the case of hypopharyngeal tumors, however, sparing of these structures is technically difficult owing to its close proximity to the constrictors. Nevertheless, several institutional experiences with IMRT for hypopharyngeal cancer have been reported. Lee et al. reported on a small population of patients treated with concomitant chemotherapy and IMRT, with 20 laryngeal and 11 hypopharyngeal tumors. The 2-year OS rate for patients with hypopharyngeal tumors was 53%. The functional larynx rate was not specifically reported in that study; however, the rate of percutaneous gastrostomy dependence was 31%. Although IMRT resulted in favorable outcomes in terms of xerostomia, the survival and laryngeal function reported in this study were not appreciably improved from historic values (18). More recently, Studer et al. updated the Swedish experience with IMRT in laryngeal and hypopharyngeal tumors. That study, which used simultaneous integrated boost (i.e., dose painting), reported a 2-year OS, DFS, and local control rate of 83%, 75%, and 82%, respectively. In an effort to reduce late toxicity, the dose per fraction delivered to the gross tumor volume was reduced from 2.2 Gy to 2.0 Gy in patients with tumors that involved a large portion of the pharynx; 75% of all patients had an intact, functional larynx at the last follow-up visit, significantly greater than our reported rate (19).
Conclusions
We have reported the mature results of a relatively large population of patients with hypopharyngeal cancer demonstrating the OS and DFS benefit of concurrent CRT. Despite our favorable survival outcomes, the preservation of laryngoesophageal function remained unacceptably low. Recently published studies have indicated the possibility of a role for pharyngeal-sparing RT in this subsite. Future studies aimed at determining the feasibility of pharyngeal constrictor-sparing IMRT for hypopharyngeal cancer are needed.
Footnotes
Presented in poster format at the 2010 Annual Meeting of the American Society for Therapeutic Radiology and Oncology, San Diego, CA.
Conflict of interest: none.
References
- 1.Lefebvre JL, Chevalier D, Luboinski B, et al. Larynx preservation in pyriform sinus cancer: Preliminary results of a European Organization for Research and Treatment of Cancer phase III trial. J Natl Cancer Inst. 1996;88:890–899. doi: 10.1093/jnci/88.13.890. [DOI] [PubMed] [Google Scholar]
- 2.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991;324:1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein DJ, Adams GL, Wagner H, et al. An Intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol. 2003;21:92–98. doi: 10.1200/JCO.2003.01.008. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Albers ME, Fisher SR, et al. Hyperfractionated irradiation with or without concurrent chemotherapy for locally advanced head and neck cancer. N Engl J Med. 1998;338:1798–1804. doi: 10.1056/NEJM199806183382503. [DOI] [PubMed] [Google Scholar]
- 6.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 7.Lefebvre JL, Ang KK. Larynx preservation clinical trial design: Key issues and recommendations—A consensus panel summary. Int J Radiat Oncol Biol Phys. 2009;73:1293–1303. doi: 10.1016/j.ijrobp.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 8.Garden AS, Morrison WH, Clayman GL, et al. Early squamous cell carcinoma of the hypopharynx: Outcomes of treatment with radiation alone to the primary disease. Head Neck. 1996;18:317–322. doi: 10.1002/(SICI)1097-0347(199607/08)18:4<317::AID-HED2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Amdur RJ, Mendenhall WM, Stringer SP, et al. Organ preservation with radiation therapy for T1-T2 carcinoma of the pyriform sinus. Head Neck. 2001;23:353–362. doi: 10.1002/hed.1044. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura RY, Kagami Y, Ito Y, et al. Outcomes in patients with early-stage hypopharyngeal cancer treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77:1017–1023. doi: 10.1016/j.ijrobp.2009.06.066. [DOI] [PubMed] [Google Scholar]
- 11.Bensadoun RJ, Benezery K, Dassonville O, et al. French multicenter phase III randomized study testing concurrent twice-a-day radiotherapy and cisplatin/5-fluorouracil chemotherapy (BiRCF) in unresectable pharyngeal carcinoma: Results at two years (FNCLCC-GORTEC) Int J Radiat Oncol Biol. 2006;64:983–994. doi: 10.1016/j.ijrobp.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 12.Prades JM, Lallemant B, Garrel R, et al. Randomized phase III trial comparing induction chemotherapy followed by radiotherapy to concomitant chemoradiotherapy for laryngeal preservation in T3M0 pyriform sinus carcinoma. Acta Otolaryngol. 2010;130:150–155. doi: 10.3109/00016480902914080. [DOI] [PubMed] [Google Scholar]
- 13.Yoon MS, Chung WK, Ahn SJ, et al. Concurrent chemoradiotherapy with cisplatin and fluorouracil for locally advanced hypopharyngeal cancer. Acta Otolaryngol. 2008;128:590–596. doi: 10.1080/00016480701596021. [DOI] [PubMed] [Google Scholar]
- 14.Lambert L, Fortin B, Soulieres D, et al. Organ preservation with concurrent chemoradiation for advanced laryngeal cancer: Are we succeeding? Int J Radiat Oncol Biol. 2010;76:398–402. doi: 10.1016/j.ijrobp.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 15.Lee WT, Akst LM, Adelstein DJ, et al. Risk factors for hypo-pharyngeal/upper esophageal stricture formation after concurrent chemoradiation. Head Neck. 2006;28:808–812. doi: 10.1002/hed.20427. [DOI] [PubMed] [Google Scholar]
- 16.Eisbruch A, Schwartz M, Rasch C, et al. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: Which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol. 2004;60:1425–1439. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 17.Feli FY, Kim HM, Lyden TH, et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dyspha-gia: Early dose–effect relationships for the swallowing structures. Int J Radiat Oncol Biol. 2007;68:1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 18.Lee NY, O'Meara W, Chan K, et al. Concurrent chemotherapy and intensity-modulated radiotherapy for locoregionally advanced laryngeal and hypopharyngeal cancers. Int J Radiat Oncol Biol. 2007;69:459–468. doi: 10.1016/j.ijrobp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Studer G, Peponi E, Kloeck S, et al. Surviving hypopharynx-larynx carcinoma in the era of IMRT. Int J Radiat Oncol Biol. 2010;77:1391–1396. doi: 10.1016/j.ijrobp.2009.07.005. [DOI] [PubMed] [Google Scholar]