Abstract
Objectives/Hypothesis
To identify patterns of airway collapse during preoperative drug-induced sleep endoscopy (DISE) as predictors of surgical failure following multilevel airway surgery for patients with obstructive sleep apnea-hypopnea syndrome (OSAHS).
Study Design
Retrospective clinical chart review.
Methods
Medical records of patients who underwent site-specific surgical modification of the upper airway for treatment of OSHAS were reviewed. Patients were included in this study if they had a preoperative airway evaluation with DISE as well as preoperative and postoperative polysomnography. Airway obstruction on DISE was described according to airway level, severity, and axis of collapse. Severe airway obstruction was defined as >75% collapse on endoscopy. Surgical success was described as a postoperative apnea-hypopnea index (AHI) of <20 and a >50% decrease in preoperative AHI.
Results
A total of 34 patients were included in this study. The overall surgical success rate was 56%. Surgical success (n = 19) and surgical failure (n = 15) patients were similar with regard to age, gender, body mass index, preoperative AHI, Friedman stage, adenotonsillar grades, and surgical management. DISE findings in the surgical failure group demonstrated greater incidence of severe lateral oropharyngeal wall collapse (73.3% vs. 36.8%, P = .037) and severe supraglottic collapse (93.3% vs. 63.2%, P = .046) as compared to the surgical success group.
Conclusions
The presence of severe lateral pharyngeal wall and/or supraglottic collapse on preoperative DISE is associated with OSAHS surgical failure. The identification of this failure-prone collapse pattern may be useful in preoperative patient counseling as well as in directing an individualized and customized approach to the treatment of OSHAS.
Keywords: Lateral oropharyngeal wall collapse, supraglottic collapse, obstructive sleep apnea-hypopnea syndrome, surgical failure
INTRODUCTION
Obstructive sleep apnea hypopnea syndrome (OSAHS) is a growing problem in the United States, affecting 9% to 24% of American adults and exacting a $16 billion annual toll to the US economy.1 Such cost is due to decreased productivity and greater likelihood of workplace and motor vehicle accidents, as well as the increased prevalence of cardiovascular and cerebrovascular disease.2,3 Despite recent medical and surgical advances, the prevalence of OSAHS is expected to continue to increase due to the obesity epidemic threatening the American public.4,5 Continuous positive airway pressure (CPAP) therapy remains the first-line medical therapy in the management of OSAHS, with a success rate of 78%.6 However, 46% to 85% of patients cannot tolerate CPAP and may thus potentially benefit from surgical interventions.7,8 Traditionally, CPAP-intolerant patients were offered an uvulopalatopharyngoplasty (UPPP) as the surgical procedure of choice. However, overall success rates for UPPP were found to be less than optimal (40.7%), as demonstrated in a meta-analysis by Sher et al. and supported by others.9,10 This poor surgical outcome following UPPP is thought to occur in part due to a failure to recognize patterns of airway collapse that are not addressed by palatal surgery.
The increased realization that upper airway obstruction in patients with OSAHS can occur at multiple airway levels was instrumental in improving patient selection and led to improved surgical outcomes. Patients with Fujita type I obstruction (retropalatal only) responded with an 83% success rate following a UPPP, whereas individuals with Fujita type II (retropalatal and retrolingual collapse) or type III obstruction (retrolingual collapse only) experience a success rate of only 19% after UPPP.9 Furthermore, the recognition of multilevel airway obstruction in OSAHS patients led to the introduction of numerous surgical techniques to address the retrolingual site of obstruction in addition to the retropalatal collapse. Surgical procedures, such as base of tongue resection, tongue suspension, hyoid myotomy and advancement, genioglossus advancement, bimaxillary advancement, among others, have now become part of the otolaryngologist’s armamentarium for the surgical management of OSAHS.11 The advent of these new techniques and the preoperative identification of airway obstruction levels have improved the success rate following site-directed multilevel upper airway surgery for OSAHS patients.12 However, despite these advances, patients with OSAHS still experience a significant failure rate that remains poorly understood.10,13
At our institution since 2006, routine preoperative drug-induced sleep endoscopy (DISE) has been conducted, along with other diagnostic techniques, as part of a standard preoperative evaluation to identify the site of airway obstruction in an attempt to guide the selection of the most appropriate surgical interventions. In this study, we aimed to carefully review our DISE findings in an attempt to identify any patterns of airway collapse associated with poor surgical outcome. The identification of failure-prone collapse patterns may be useful for preoperative patient counseling and may serve as an impetus for alteration of treatment strategies in this group of patients.
MATERIALS AND METHODS
Patient Selection
Institutional review board approval was obtained for a retrospective chart review of all patients (age >18 years) who underwent DISE at our institution from January 2006 through December 2010. This period coincided with the implementation of routine DISE for preoperative evaluation of OSAHS patients at our institution. Patients were included in this study if they had a preoperative DISE, had surgical procedures to address airway obstruction, and had preoperative and postoperative polysomnograms. All included patients were referred to our department for surgical evaluation due to the inability to tolerate CPAP therapy. Patients were excluded from this study if they had polysomnogram-proven central sleep apnea syndrome.
Patient Information
Detailed patient information was obtained as part of our standard obstructive sleep apnea (OSA) evaluation for all patients. Demographic data, past medical history, past surgical history, and social history were collected for all patients. Detailed physical examination records were also kept, including body mass index (BMI), Friedman palate position classification,14 and adenotonsillar grades. Finally, diagnostic testing, including preoperative and postoperative apnea-hypopnea index (AHI) as well as preoperative DISE findings, was carefully documented.
DISE
Sleep endoscopy was performed in the operating room by a single evaluating surgeon as part of a standard preoperative assessment. Sleep induction was achieved in the operating room according to a standard propofol titration protocol beginning at a rate of 50 to 75 μg/kg/min intravenous infusion. The use of benzodiazepines and other sedating medications were strictly prohibited. The target level of sedation was that of light sleep with arousal to tactile but not vocal stimulation. Once sedation was achieved, a flexible fiberoptic nasal endoscope was passed through the nose for inspection of the entire upper airway. Dynamic collapse was evaluated at the level of the retropalatal, retrolingual, and supraglottic airway segments and described in terms of a two-dimensional axial diameter change (i.e., lateral, anteroposterior [AP] or circumferential [both]). The degree of airway collapse at each level was quantified as mild (0%–25%), moderate (25%–75%), or severe (>75%) along each of the lateral and/or AP axes. DISE was chosen as the primary method of preoperative upper airway investigation in this study over other modalities, such as awake nasal laryngoscopy with Muller’s maneuver or lateral cephalometry. This preference relates to the senior author’s own experience and familiarity with DISE and does not serve to adjudicate DISE as the only diagnostic modality in the preoperative evaluation of OSAHS patients. In the senior author’s experience, DISE has proven to be a more reliable diagnostic and planning tool in the determination of the site (retropalatal, retrolingual, supraglottic), type (anteroposterior, lateral, circumferential), and severity of airway obstruction prior to upper airway surgerization.
Polysomnography
Standard polysomnography was performed on all patients as part of their preoperative OSAHS evaluation and postoperative follow-up. All studies were conducted and scored according to the standards of the American Academy of Sleep Medicine. During each sleep study, standard parameters were monitored, including electroencephalography, electrooculography, chin electromyography, and electrocardiography. Respiratory parameters included a nasal pressure transducer, oronasal thermistor, respiratory inductive plethysmography, and oximetry. The apnea-hypopnea index was the major sleep parameter used in this study as a measure of OSA severity and in the definition of surgical success or failure. Apneic episodes were defined as a complete cessation of airflow at the nose and mouth for at least 10 seconds, whereas hypopneas were defined as partial obstructive events with diminution of airflow by more than 30% for at least 10 seconds with an associated oxygen desaturation of 4% or more.
Surgical Therapy
All patients underwent surgical management of their obstructive sleep apnea by a single surgeon (H.-S.L.). The type and extent of surgical resection was based on the specific sites of airway collapse found during DISE, with the ultimate intent set on addressing all levels of severe airway collapse. This method of site-specific multilevel airway surgery is a generally well-accepted approach used by most sleep surgeons.11,12 Thus, a palatal procedure was selected to address severe collapse (as defined by >75% obstruction) at the retropalatal level, and a base of tongue procedure was used for the management of severe collapse at the retrolingual level. The selection of each particular type of procedure used in this study was entirely based on the experience and assessment by the senior author (H.-S.L.). Surgical procedures included a combination of the following: nasal surgery (including septoplasty and/or turbinate reduction), adenoidectomy, tonsillectomy, Pillar palatal implant, UPPP, hyoid advancement, genioglossus advancement, Repose tongue suspension, and base of tongue resection.
To address the nose and nasopharynx, nasal surgery and/or adenoidectomy were performed as needed. To address retropalatal collapse, either UPPP or Z-palatoplasty (ZPP) as described by Friedman et al.15,16 was selected. Sleep endoscopy findings dictated which procedure was chosen for management of the retropalatal airway. If DISE revealed only an anteroposterior form of collapse, then a UPPP was recommended. On the other hand, if sleep endoscopy findings showed a circumferential type of retropalatal collapse, then a ZPP was elected.
The approach to the surgical management of the retrolingual airway was similarly dictated by DISE findings. When the presence of significant lingual tonsils contributing to the collapse of the retrolingual airway was identified on DISE, a surgical base of tongue resection was employed. In patients with midline retrolingual collapse, the Repose tongue suspension or genioglossus advancement was used based on the patient’s preference after thorough discussion of the pros and cons of each procedure with the patient.
To address the presence of supraglottic airway collapse, hyoid suspension and advancement was employed and was frequently performed in conjunction with a tongue base procedure.
Patients were categorized as having one-, two-, three-, or four-level surgical management depending on the number of upper airway levels addressed at the time of surgery. Surgical success was strictly defined as a >50% decrease in preoperative AHI with a concurrent postoperative AHI of <20.
Statistical Analysis
Data analysis was carried out using XLSTAT software (Addinsoft, New York, NY). Numerical data sets were compared using the Mann-Whitney-Wilcoxon test and the Student t test as dictated by normality testing. Proportion testing for categorical variables was carried out using the Fisher exact test.
RESULTS
A total of 36 patients with preoperative DISE as well as preoperative and postoperative polysomnograms were identified for this retrospective study. Of these, two patients were excluded due to the presence of a significant central sleep apnea component. OSA severity ranged from mild (AHI 5–15, 18%), to moderate (AHI 15–30, 35%), to severe (AHI >30, 47%). The mean BMI was 34.4 kg/m2 (standard deviation [SD], 7.6 kg/m2; range, 23.0–58.5 kg/m2), with an average patient age of 48 years (SD, 12 years; range, 19–70 years). Our of all of the patients 73.5% were male. The overall surgical success rate was 56% (19/34).
There was no statistically significant difference between the surgical success (n = 19) and surgical failure (n = 15) groups in terms of age, preoperative severity of OSA, gender, BMI, prior airway surgical history, tobacco and alcohol use, and specific medical comorbidities (Table I). There was also no difference between the two groups in terms of physical exam findings, such as Friedman score and adenotonsillar grade (Table II). Finally, the surgical management for OSHAS was similar for both the surgical success and failure groups with regard to the type and location of upper airway surgery (Table III). The most common procedures performed were palatopharyngoplasty (79.4%) and base of tongue procedures (67.6%). Palatopharyngoplasty procedures were performed using either a standard UPPP (59.3%) or ZPP (40.7%) technique as described by Friedman et al.15,16 Base of tongue procedures included genioglossus advancement (4.4%),17 tongue Repose (39.1%),18 transoral base of tongue resection (34.8%),19 and lingual tonsillectomy (21.7%). Hyoid myotomy/advancement17 was the third most commonly performed procedure (47.1%). A temporary tracheotomy was performed in two subjects for prophylactic airway safeguarding, which was reversed prior to postoperative polysomnography. The majority of subjects had multilevel surgery, with most patients undergoing three-level surgical management.
TABLE I.
Demographics, Diagnostics, Relevant Past History, and Comparison Between Obstructive Sleep Apnea-Hypopnea Syndrome Surgical Success and Failure Groups.
| Characteristic | Success Group | Failure Group | P Value | Test |
|---|---|---|---|---|
| Sample size | n = 19 | n = 15 | ||
| Preoperative AHI, events/hr (SD) | 35.2 (20.4) | 49.5 (38.2) | .510 | Mann-Whitney |
| Mild OSAHS, no. (%) | 3 (15.8) | 3 (20.0) | .930 | Fisher exact |
| Moderate OSAHS, no. (%) | 7 (36.8) | 5 (33.3) | ||
| Severe OSAHS, no. (%) | 9 (47.4) | 7 (46.7) | ||
| Postoperative AHI, events/hr (SD) | 8.7 (5.1) | 34.7 (22.0) | >.0001 | Mann-Whitney |
| Age, yr (SD) | 48 (7.6) | 49 (15.6) | .948 | Student t |
| Male, no. (%) | 12 (63.2) | 13 (86.7) | .240 | Fisher exact |
| BMI, kg/m2 (SD) | 36.3 (9.4) | 31.6 (3.2) | .069 | Student t |
| Past surgical history, no. (%) | ||||
| Nasal | 3 (15.8) | 3 (20) | 1.000 | Fisher exact |
| UPPP | 0 | 3 (20) | .076 | |
| Tonsillectomy | 3 (15.8) | 6 (40) | .139 | |
| BOT procedure | 0 | 0 | 1.000 | |
| Past medical history, no. (%) | ||||
| Asthma | 2 (10.5) | 1 (6.7) | .430 | Fisher exact |
| COPD | 2 (10.5) | 1 (6.7) | .430 | |
| CHF | 1 (5.3) | 1 (6.7) | .510 | |
| GERD | 6 (31.6) | 3 (20) | .235 | |
| Tobacco use | 5 (26.3) | 4 (26.7) | 1.000 | Fisher exact |
| Alcohol use | 3 (15.8) | 0 | .672 | Fisher exact |
| Time from OR to PSG, mo (SD) | 6.1 (6.) | 7.7 (8.8) | .930 | Mann-Whitney |
AHI = apnea-hypopnea index; SD = standard deviation; OSAHS = obstructive sleep apnea-hypopnea syndrome; BMI = body mass index; UPPP = uvulopalatopharyngoplasty; BOT = base of tongue; COPD = chronic obstructive pulmonary disease; CHF = congestive heart failure; GERD = gastroesophageal reflux disease; OR = operating room; PSG = polysomnogram.
TABLE II.
Physical Examination Findings and Comparison Between Obstructive Sleep Apnea-Hypopnea Syndrome Surgical Success and Failure Groups.
| Characteristic | Success Group | Failure Group | P Value | Test |
|---|---|---|---|---|
| Base of tongue, no (%) | ||||
| Friedman I | 0 | 0 | .231 | Fisher exact |
| Friedman II | 7 (36.8) | 3 (20) | ||
| Friedman III | 10 (52.6) | 12 (80) | ||
| Friedman IV | 2 (10.5) | 0 | ||
| Palatine tonsils, no (%) | ||||
| Grade 0 | 5 (26.3) | 8 (53.3) | 1.000 | Fisher exact |
| Grade 1+ | 7 (36.8) | 4 (26.7) | ||
| Grade 2+ | 4 (21.1) | 1 (6.7) | ||
| Grade 3+ | 3 (15.8) | 2 (13.3) | ||
| Grade 4+ | 0 | 0 | ||
| Adenoids, no (%) | ||||
| Grade 0 | 9 (47.4) | 10 (66.7) | 1.000 | Fisher exact |
| Grade 1+ | 9 (47.4) | 4 (26.7) | ||
| Grade 2+ | 1 (5.3) | 0 | ||
| Grade 3+ | 0 | 1 (6.7) | ||
TABLE III.
Distribution of Airway Level-Specific Surgical Interventions for Obstructive Sleep Apnea-Hypopnea Syndrome Surgical Success and Failure Groups.
| Characteristic | Success Group | Failure Group | P Value | Test |
|---|---|---|---|---|
| Surgical procedures, % | ||||
| Nasal surgery | 26.30 | 55.30 | .160 | Fisher exact |
| UPPP/Z-plasty | 89.50 | 66.70 | .199 | |
| BOT procedure | 68.40 | 66.70 | 1.000 | |
| Hyoid advancement | 36.80 | 60 | .300 | |
| Pillar procedure | 5.30 | 20 | .288 | |
| Tracheostomy | 0 | 13.30 | .187 | |
| Adenoidectomy | 5.30 | 6.70 | 1.000 | |
| Other | 5.30 | 6.70 | 1.000 | |
| Surgical levels, no. (%) | ||||
| One | 6 (31.6) | 2 (13.3) | 1.000 | Fisher exact |
| Two | 5 (26.3) | 4 (26.7) | ||
| Three | 8 (42.1) | 6 (40) | ||
| Four | 0 | 3 (20) | ||
UPPP = uvulopalatopharyngoplasty; BOT = base of tongue.
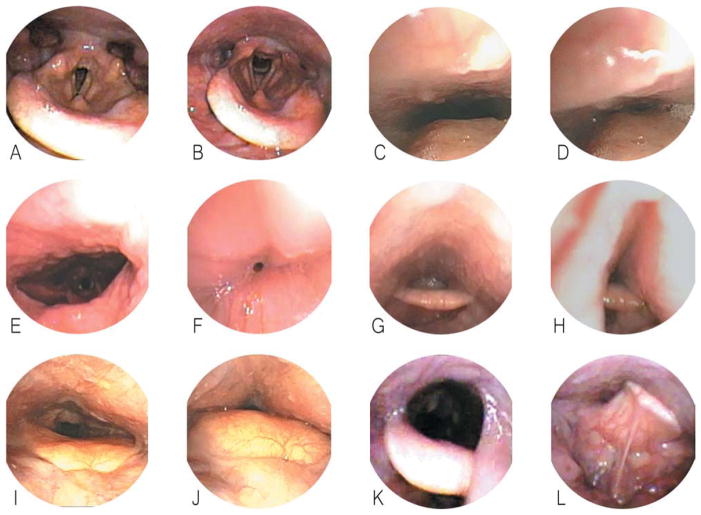
Obstructive patterns visible on sleep endoscopy are summarized in Figure 1. Common patterns of collapse included AP and circumferential retropalatal collapse (Fig. 1C–F), AP and lateral retrolingual collapse (Fig. 1G–J), and supraglottic laryngeal collapse (Fig. 1K–L). Of note, all patients undergoing preoperative sleep endoscopy were found to have severe retropalatal collapse in the AP dimension. The severity of airway collapse at other levels was more variable (Fig. 2). The incidence of severe collapse, defined as >75% reduction during inspiration, between the surgical success and failure groups is shown in Table IV. The incidence of severe collapse at the retropalatal level was not statistically different between the success and failure groups. In addition, although AP retrolingual collapse was more common in the surgical failure group, this was not statistically significant. In contrast, there was a statistically significant difference (P = .037) in the incidence of severe retrolingual lateral wall collapse between the two groups, with 73.3% of surgical failure subjects exhibiting >75% lateral wall collapse on sleep endoscopy compared to only 36.8% in the surgical success group. In addition, nearly all (93.3%) surgical failure patients displayed severe supraglottic collapse on sleep endoscopy compared to the surgical success group (63.2%, P = .046). The identification of severe lateral pharyngeal wall collapse was associated with a surgical success rate of only 38.9%, whereas its absence was favorably tied with a success rate of 75%. Similarly, the identification of severe supraglottic collapse was associated with a surgical success rate of 46.1%, whereas its absence was associated with an elevated success rate of 87.5%.
Fig. 1.
Patterns of airway collapse on drug-induced sleep endoscopy. Normal patient: preinspiration (A) and during inspiration (B). Antero-posterior retropalatal collapse: pre-inspiration (C) and during inspiration (D). Lateral retropalatal collapse: preinspiration (E) and during inspiration (F). Lateral retrolingual collapse: preinspiration (G) and during inspiration (H). Anteroposterior retrolingual collapse: preinspiration (I) and during inspiration (J). Supraglottic (epiglottic) collapse: preinspiration (K) and during inspiration (L).
Fig. 2.
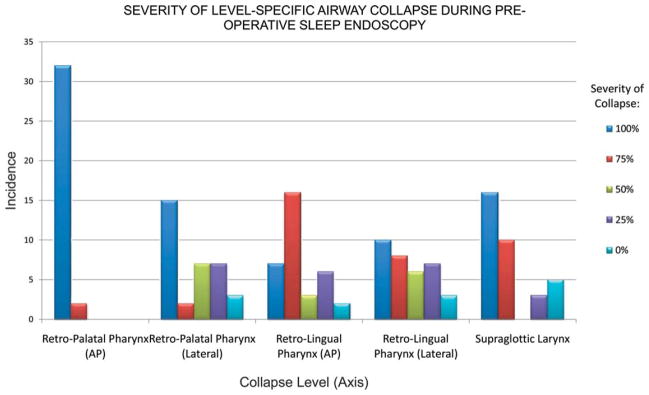
Severity of airway collapse for all patients according to airway level and severity of collapse. AP = anteroposterior.
TABLE IV.
Incidence of Severe Collapse (>75%) on Sleep Endoscopy and Comparison Between Obstructive Sleep Apnea-Hypopnea Syndrome Surgical Success and Failure Groups.
| Site/Axis of Collapse | Success Group, n = 19 | Failure Group, n = 15 | P Value (Fisher Exact Test) |
|---|---|---|---|
| Severe collapse (>75%), no. (%) | |||
| Retropalatal pharynx (anteroposterior) | 19 (100) | 15 (100) | 1.000 |
| Retropalatal pharynx (lateral) | 10 (53.6) | 7 (46.7) | .755 |
| Retrolingual pharynx (anteroposterior) | 11 (57.9) | 12 (80) | .159 |
| Retrolingual pharynx (lateral) | 7 (36.8) | 11 (73.3) | .037 |
| Supraglottic larynx | 12 (63.2) | 14 (93.3) | .046 |
A total of 18 subjects had evidence of severe retrolingual lateral wall collapse on preoperative sleep endoscopy (Table V). Compared to those without severe lateral wall collapse, those with significant lateral collapse were often male, a finding that almost reached statistical significance (P = .052). In contrast, no differences in BMI, age, and preoperative AHI were evident. A similar analysis of all of the patients displaying severe supraglottic collapse showed that there were no differences in BMI, age, gender, or preoperative AHI compared to those without severe supraglottic collapse. However, individuals with severe supraglottic collapse did show a statistically significant greater likelihood of having severe AP collapse at the retrolingual level (Table VI).
TABLE V.
Comparison of Patient Characteristics Between Individuals With Versus Without Severe Retrolingual Lateral Pharyngeal Wall Collapse.
| Characteristic | Severe Retrolingual Lateral Wall Collapse, n = 18 | No Severe Retrolingual Lateral Wall Collapse, n = 16 | P Value | Test |
|---|---|---|---|---|
| BMI, kg/m2 (SD) | 34.3 (6.5) | 34.1 (9.0) | .935 | Student t |
| Age, yr (SD) | 45 (11.9 | 52 (10.7) | .112 | Student t |
| Male, no (%) | 16 (89) | 9 (56) | .052 | Fisher exact |
| Preoperative AHI, events/hr (SD) | 45.4 (35.0) | 37.1 (23.4) | .876 | Mann-Whitney |
| Surgical success, % | 38.9 | 75.0 | .037 | Fisher exact |
BMI = body mass index; SD = standard deviation; AHI = apnea-hypopnea index.
TABLE VI.
Comparison of Patient Characteristics Between Individuals With Versus Without Severe Supraglottic Airway Collapse.
| Characteristic | Severe Supraglottic Collapse, n = 26 | No Severe Supraglottic Collapse, n = 8 | P Value | Test |
|---|---|---|---|---|
| BMI, kg/m2 (SD) | 34.5 (8.2) | 33.4 (6.0) | .737 | Student t |
| Age, yr (SD) | 49 (2.2) | 46 (10.5) | .522 | Student t |
| Male, no. (%) | 20 (76.9) | 5 (62.5) | .649 | Fisher exact |
| Retrolingual AP collapse, no. (%) | 21 (80.8) | 2 (33.3) | .007 | Fisher exact |
| Preoperative AHI, events/hr (SD) | 43.1 (32.0) | 36.3 (23.1) | .876 | Mann-Whitney |
| Surgical success, % | 46.2 | 87.5 | .046 | Fisher exact |
BMI = body mass index; SD = standard deviation; AP = anteroposterior; AHI = apnea-hypopnea index.
DISCUSSION
The role of lateral pharyngeal wall collapse as a significant contributor to airway obstruction in OSAHS patients has been speculated for decades but remains poorly understood. In 1990, Rodenstein et al. first noticed that the magnetic resonance imaging-scanned upper airway of patients with OSAHS was narrowed in the lateral dimension when compared to the airway of normal subjects.20 In 1995, Schwab et al. demonstrated that such lateral wall prominence and restriction was due to thickening of the lateral pharyngeal wall and increased parapharyngeal fat volume.21 The lateral pharyngeal walls of OSAHS patients were also shown to be more collapsible than in normal subjects.22 Stauffer et al. showed that airway volumes were identical between adult males with severe OSAHS and weight-and age-matched controls, but airway resistance was much greater in the OSAHS group.23 The importance of lateral oropharyngeal wall collapse was further demonstrated in a study by Schellenberg et al. of 420 subjects, wherein lateral pharyngeal wall narrowing was found to be the only statistically significant factor for OSAHS in males.24 However, a lack of functional analysis and visualization during sleep via sleep endoscopy diminished the objectivity of the study’s findings. These findings pointed to a morphologically different oropharyngeal airway that is both laterally restricted and predisposed to lateral collapse during inspiration in OSHAS patients.
Since the introduction of DISE at our institution, it has become evident that a large proportion of OSAHS patients display certain unexpected patterns of airway collapse. Specifically, although the majority of patients display severe retropalatal and anteroposterior retrolingual collapse, we found that 53% of patients have significant lateral oropharyngeal wall collapse at the retrolingual level, and 76% have significant laryngeal collapse at the supraglottic level. In this study, both of these patterns of airway collapse are found to be significantly more prevalent in the surgical failure group compared to the surgical success group. This remains evident despite there being no statistically significant identifiable differences between the two groups with regard to BMI, age, preoperative AHI, or type of surgical management. Severe retrolingual lateral wall collapse was evident in 73.3% of failures compared to 36.8% of successes (P = .037), whereas severe supraglottic collapse was seen in 93.3% of failures compared to 63.2% of successes (P = .046). These findings point toward a strong association between lateral oropharyngeal wall and supraglottic airway collapse and OSAHS multilevel surgical failure; the absence of either of these findings is predictive of surgical success with rates of 75% and 87.5%, respectively.
Lateral Oropharyngeal Wall Collapse
As noted in this study, patients with severe retrolingual lateral wall collapse were more likely to be male and have a higher AHI than those without such collapse. The association between male gender and retrolingual lateral wall collapse nearly reached statistical significance (P = .052) and confirms the findings by Schellenberg et al.24 Although the link between male gender and a greater tendency toward lateral oropharyngeal collapse is not fully understood, several theories are recognized. Females have been shown to have a lower volume of parapharyngeal fat and greater parapharyngeal dilator muscle activity than males,25,26 both of which would predispose males to more severe lateral oropharyngeal wall collapse.
Although the oropharynx is the most common site surgically addressed in the treatment of OSAHS, the lateral oropharyngeal walls often remain poorly managed even after UPPP. This has become evident despite the recent expansion in the number of surgical techniques targeting the tongue base and supraglottis, none of which physically target the lateral pharyngeal wall, especially at the retrolingual level. In 2003, Cahali described a lateral pharyngoplasty procedure designed to address the lateral oropharyngeal wall in patients with OSAHS.27 Despite the reported success compared to UPPP28 and few complications from this procedure, objective studies quantifying postoperative changes in lateral oropharyngeal collapse after either a UPPP or lateral pharyngoplasty are still lacking.
Supraglottic Laryngeal Collapse
Supraglottic collapse was characterized by the prolapse of the arytenoids or epiglottis toward the glottic inlet during inspiration, a finding that has been previously documented by Bachar et al. in a review of 55 DISE procedures.29 In our study, the presence of supraglottic laryngeal collapse was identified as a significant risk factor for surgical failure in patients undergoing multilevel surgical management of OSAHS. This is evident despite 60% of patients in the surgical failure group having undergone hyoid advancement, a finding that demonstrates limited effectiveness of this procedure in the management of epiglottic collapse.
Our study also finds a statistically significant association between severe supraglottic collapse and the presence of severe AP retrolingual collapse. This is likely due to the posterior displacement of the epiglottis resulting from a collapsed base of tongue. Under such circumstances, a predisposition toward supraglottic laryngeal collapse exists during inspiration. Thus, patients with severe retrolingual AP collapse are verifiably more prone to an additional supraglottic collapse and may therefore gain an additional benefit from a base of tongue procedure.
The rigidity of the supraglottic structures appears to be an essential component impacting the tendency of the supraglottic airway toward collapse. In normal subjects, the epiglottis and arytenoids remain erect during inspiration, whereas in individuals with a floppy epiglottis there is a demonstrable collapse during inspiration. The tendency for arytenoid prolapse is another well-documented form of supraglottic laryngeal collapse. In patients with these two types of supraglottic collapse, gastroesophageal reflux disease and adult laryngomalacia may be two key predisposing factors,30 although at this time no directed studies are yet available. These findings also suggest a possible role for supraglottoplasty in the management of adult OSAHS in selected patients.
CONCLUSION
To the best of our knowledge, this is the first study showing a statistically significant association between surgical failure in OSAHS patients and severe lateral oropharyngeal wall and/or supraglottic collapse. The identification of this failure-prone collapse pattern may be useful in preoperative patient counseling as well as in directing an individualized and customized approach to the treatment of OSAHS. In this study, the absence of lateral pharyngeal wall and supraglottic collapse is found to be predictive of surgical success, with rates of 75% and 87.5%, respectively. The development of novel surgical techniques to address these two problematic patterns of collapse should lead to improved surgical response rates in patients with OSAHS.
Footnotes
The authors have no funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med. 2010;182:269–277. doi: 10.1164/rccm.200911-1746OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sassani A, Findley LJ, Kryger M, Goldlust E, George C, Davidson TM. Reducing motor-vehicle collisions, costs, and fatalities by treating obstructive sleep apnea syndrome. Sleep. 2004;27:453–458. doi: 10.1093/sleep/27.3.453. [DOI] [PubMed] [Google Scholar]
- 4.Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med. 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crummy F, Piper AJ, Naughton MT. Obesity and the lung: 2. Obesity and sleep-disordered breathing. Thorax. 2008;63:738–746. doi: 10.1136/thx.2007.086843. [DOI] [PubMed] [Google Scholar]
- 6.Ballester E, Badia JR, Hernandez L, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome [published correction appears in: Am J Respir Crit Care Med 1999;159(5 pt 1):1688] Am J Respir Crit Care Med. 1999;159:495–501. doi: 10.1164/ajrccm.159.2.9804061. [DOI] [PubMed] [Google Scholar]
- 7.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–895. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 8.Sin DD, Mayers I, Man GC, Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121:430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 9.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–177. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 10.Caples SM, Rowley JA, Prinsell JR, et al. Surgical modifications of the upper airway for obstructive sleep apnea in adults: a systematic review and meta-analysis. Sleep. 2010;33:1396–1407. doi: 10.1093/sleep/33.10.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li KK. Surgical therapy for adult obstructive sleep apnea. Sleep Med Rev. 2005;9:201–209. doi: 10.1016/j.smrv.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin HC, Friedman M, Chang HW, Gurpinar B. The efficacy of multilevel surgery of the upper airway in adults with obstructive sleep apnea/hypopnea syndrome. Laryngoscope. 2008;118:902–908. doi: 10.1097/MLG.0b013e31816422ea. [DOI] [PubMed] [Google Scholar]
- 13.Richard W, Kox D, den Herder C, van Tinteren H, de Vries N. One stage multilevel surgery (uvulopalatopharyngoplasty, hyoid suspension, radio-frequent ablation of the tongue base with/without genioglossus advancement), in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2007;264:439–444. doi: 10.1007/s00405-006-0182-z. [DOI] [PubMed] [Google Scholar]
- 14.Friedman M, Ibrahim H, Bass L. Clinical staging for sleep-disordered breathing. Otolaryngol Head Neck Surg. 2002;127:13–21. doi: 10.1067/mhn.2002.126477. [DOI] [PubMed] [Google Scholar]
- 15.Friedman M, Schalch P. Z-palatoplasty. Oper Tech Otolaryngol. 2007;18:2–6. [Google Scholar]
- 16.Friedman M, Ibrahim HZ, Vidyasagar R, Pomeranz J, Joseph NJ. Z-palatoplasty (ZPP): a technique for patients without tonsils. Otolaryngol Head Neck Surg. 2004;131:89–100. doi: 10.1016/j.otohns.2004.02.051. [DOI] [PubMed] [Google Scholar]
- 17.Li K. Hypopharyngeal airway surgery. Otolaryngol Clin N Am. 2007;40:845–853. doi: 10.1016/j.otc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 18.Miller FR, Watson D, Malis D. Role of the tongue base suspension suture with The Repose System bone screw in the multilevel surgical management of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2002;126:392–398. doi: 10.1067/mhn.2002.123548. [DOI] [PubMed] [Google Scholar]
- 19.Vicini C, Dallan I, Canzi P, et al. Transoral robotic surgery of the tongue base in obstructive sleep Apnea-Hypopnea syndrome: anatomic considerations and clinical experience. Head Neck. 2011 Mar 11; doi: 10.1002/hed.21691. [DOI] [PubMed] [Google Scholar]
- 20.Rodenstein DO, Dooms G, Thomas Y, et al. Pharyngeal shape and dimensions in healthy subjects, snorers, and patients with obstructive sleep apnoea. Thorax. 1990;45:722–727. doi: 10.1136/thx.45.10.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152(5 pt 1):1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- 22.Ciscar MA, Juan G, Martinez V, et al. Magnetic resonance imaging of the pharynx in OSA patients and healthy subjects. Eur Respir J. 2001;17:79–86. doi: 10.1183/09031936.01.17100790. [DOI] [PubMed] [Google Scholar]
- 23.Stauffer JL, Zwillich CW, Cadieux RJ, et al. Pharyngeal size and resistance in obstructive sleep apnea. Am Rev Respir Dis. 1987;136:623–637. doi: 10.1164/ajrccm/136.3.623. [DOI] [PubMed] [Google Scholar]
- 24.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162(2 pt 1):740–748. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 25.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54:323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popovic RM, White DP. Influence of gender on waking genioglossal electromyogram and upper airway resistance. Am J Respir Crit Care Med. 1995;152:725–731. doi: 10.1164/ajrccm.152.2.7633734. [DOI] [PubMed] [Google Scholar]
- 27.Cahali MB. Lateral pharyngoplasty: a new treatment for obstructive sleep apnea hypopnea syndrome. Laryngoscope. 2003;113:1961–1968. doi: 10.1097/00005537-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Cahali MB, Formigoni GG, Gebrim EM, Miziara ID. Lateral pharyngoplasty versus uvulopalatopharyngoplasty: a clinical, polysomnographic and computed tomography measurement comparison. Sleep. 2004;27:942–950. doi: 10.1093/sleep/27.5.942. [DOI] [PubMed] [Google Scholar]
- 29.Bachar G, Feinmesser R, Shpitzer T, Yaniv E, Nageris B, Eidelman L. Laryngeal and hypopharyngeal obstruction in sleep disordered breathing patients, evaluated by sleep endoscopy. Eur Arch Otorhinolaryngol. 2008;265:1397–1402. doi: 10.1007/s00405-008-0637-5. [DOI] [PubMed] [Google Scholar]
- 30.Gessler EM, Simko EJ, Greinwald JH., Jr Adult laryngomalacia: an uncommon clinical entity. Am J Otolaryngol. 2002;23:386–389. doi: 10.1053/ajot.2002.126322. [DOI] [PubMed] [Google Scholar]