Abstract
Background
The appropriate management of the neck in patients with regionally advanced head and neck cancer remains controversial. The purpose of this study was to retrospectively analyze our institutional experience with up-front neck dissection followed by definitive chemoradiotherapy.
Methods
Fifty-five patients with radiographic evidence of large or necrotic lymph nodes underwent up-front neck dissection followed by definitive chemoradiation.
Results
The 5-year overall survival (OS) and progression-free survival (PFS) rates were estimated at 71.3% and 64.7%, respectively. There were 2 failures in the dissected neck, for a control rate of 96.7%. There were 7 locoregional failures and 12 distant failures, for locoregional and distant control rates of 87.3% and 78.2%, respectively.
Conclusion
Up-front neck dissection followed by chemoradiotherapy resulted in excellent locoregional control, OS, and PFS. Utilization of this strategy should be considered in carefully selected patients with regionally advanced head and neck cancer.
Keywords: neck dissection, chemoradiation, head and neck cancer, regional metastasis, oropharyngeal cancer
The appropriate management of the node-positive neck in advanced head and neck cancer remains controversial. Historically, the clinically positive neck was addressed surgically along with the primary tumor. As the treatment paradigm has shifted toward organ-preserving treatment, however, the role for neck dissection has become less clear. The efficacy of planned neck dissection, after either concurrent chemoradiation or radiotherapy alone, has been reported in a number of series.1–8 Reports of postoperative complications after dissection of the previously irradiated neck are varied, with severe late toxicity rates reported as high as 55%.9,10
Up-front neck dissection of the clinically positive neck is an alternative to planned postradiotherapy dissection that confers the advantage of avoidance of surgery on an irradiated neck, as well as the removal of areas of hypoxic nodal metastases and bulky tumor volume, which may be less responsive to chemoradiotherapy. Excellent control rates in the dissected neck, along with minimal delay of definitive treatment, have been reported previously in multiple series.11–14
At our institution, up-front neck dissection is typically recommended for patients with bulky (>3 cm) lymphadenopathy or radiographic evidence of necrosis. The purpose of this study was to report our institutional experience utilizing this strategy in patients with regionally advanced head and neck cancer.
Materials and Methods
Patients with previously untreated node-positive head and neck cancer who underwent up-front neck dissection from March 2000 to November 2009 were included in the analysis. Pretreatment evaluation included medical history and physical examination, radiographic imaging of the neck with CT, MRI, and triple endoscopy with fiber-optic laryngoscopy, esophagoscopy, and bronchoscopy with directed biopsies. Positron emission tomography (PET)/CT was not routinely performed as part of the staging workup. All patients were evaluated by a multidisciplinary team including surgeons, medical oncologists, and radiation oncologists before the initiation of treatment. Staging was performed in accordance with the American Joint Committee on Cancer (AJCC) 7th edition criteria. Tumor classification was based on physical examination, direct visualization, and radiographic imaging. Nodal classification was based on pathologic review of the dissection specimens, along with clinical and radiographic findings of the contralateral neck, if not dissected. Patient characteristics are summarized in Table 1. Institutional review board approval for this retrospective chart review was obtained before the initiation of the study.
Table 1.
Patient population.
| Characteristic | No. of patients (%) |
|---|---|
| Sex | |
| Male | 47 (85.5) |
| Female | 8 (14.5) |
| Primary site | |
| Oral cavity | 3 (5.5) |
| Oropharynx | 38 (69.1) |
| Hypopharynx | 7 (12.7) |
| Larynx | 7 (12.7) |
| T classification | |
| T1 | 19 (34.5) |
| T2 | 16 (29.1) |
| T3 | 18 (32.7) |
| T4 | 2 (3.6) |
| N classification | |
| N0 | 1 (1.8) |
| N1 | 3 (5.5) |
| N2 | 41 (74.5) |
| N2a | 10 (18.2) |
| N2b | 21 (38.2) |
| N2c | 9 (16.4) |
| N3 | 11 (20.0) |
| AJCC stage | |
| I | 0 (0.0) |
| II | 1 (1.8) |
| III | 3 (5.5) |
| IVa | 38 (69.1) |
| IVb | 13 (23.6) |
Abbreviation: AJCC, American Joint Committee on Cancer.
Patients with radiographic evidence of bulky (>3 cm) or necrotic lymphadenopathy were routinely offered up-front neck dissection before chemoradiation. Patients with evidence of deep muscle invasion or encasement of the carotid artery were considered unresectable and were not offered up-front neck dissection. Neck dissection was routinely performed along with any necessary dental extractions in order to minimize the delay of definitive therapy. Choice of surgical procedure was determined by the treating surgeon on the basis of clinical nodal burden. Procedures performed included either radical neck dissection (RND) or modified radical neck dissection (MRND). MRND routinely included dissection of nodal levels I to V, with preservation of CN XI and sometimes the sternocleidomastoid muscle. Radical neck dissection was reserved for patients with gross tumor involvement of CN XI. Selective neck dissection was not routinely performed, but was done so in several cases at the discretion of the treating surgeon.
Definitive treatment consisted of radiotherapy given concurrently with chemotherapy. Patients who were deemed medically unfit for concurrent therapy were treated with radiation alone. Induction chemotherapy after neck dissection was utilized in 4 patients, usually due to bulky or locally advanced primary disease, at the discretion of the medical oncologist. Induction chemotherapy regimens included docetaxel, cisplatin, and 5-fluorouracil, cisplatin and 5-fluorouracil, and docetaxel, cetuximab, and carboplatin.
Radiation was delivered using 6 mV photons, either via 3-dimensional (3D) conformal or intensity-modulated radiation therapy (IMRT), to the primary tumor and draining lymphatics. The 3D conformal radiation consisted of a 3-field technique utilizing opposed lateral fields matched to an anterior field to cover the lower neck and supraclavicular regions using a half-beam block. The lateral fields were reduced at 4000 centi-Gray to avoid excessive dose to the spinal cord and direct posterior electron fields were used to supplement the neck bilaterally during the off-cord portion of treatment. IMRT planning was volume-based, with the gross tumor volume defined as any gross disease, including primary tumor and residual nodal disease, the clinical target volume including the gross tumor volume with a margin and areas of suspected microscopic spread including elective nodal regions, and the planning target volume including the clinical target volume with a margin for patient setup error. The primary tumor was treated to a dose of 66 to 70 gray (Gy), the dissected neck received 60 to 66 Gy, and the elective nodal regions received 50 Gy, all in 2 Gy fractions given once daily, 5 days per week.
Systemic agents used for concurrent treatment included cisplatin, carboplatin, and cetuximab. The agent used was at the discretion of the medical oncologist. Cisplatin was typically administered intravenously at a dose of 100 mg/m2 during weeks 1, 4, and 7. Carboplatin was given as a weekly intravenous infusion at an area under the curve of 1.5 during weeks 1 to 7. Cetuximab was administered intravenously, with a loading dose of 400 mg/m2 given 1 week before radiotherapy (RT), followed by weekly infusions of 250 mg/m2 during weeks 1 to 7. A summary of treatment is provided in Table 2.
Table 2.
Treatment summary.
| Treatment type | No. of patients (%) |
|---|---|
| Modified radical neck dissection | 44 (80.0) |
| Unilateral MRND alone | 39 (70.9) |
| Unilateral MRND with contralateral SND | 4 (7.3) |
| Bilateral MRND | 1 (1.8) |
| Radical neck dissection | 11 (20.0) |
| Unilateral RND | 11 (20.0) |
| Bilateral RND | 0 (0.0) |
| Induction chemotherapy | 4 (7.3) |
| TPF | 1 (1.8) |
| PF | 2 (3.6) |
| TPE | 1 (1.8) |
| Radiotherapy | 55 (100) |
| CRT | 53 (96.4) |
| Cisplatin | 46 (83.6) |
| Carboplatin | 4 (7.3) |
| Cetuximab | 3 (5.5) |
| RT alone | 2 (3.6) |
| Conventional radiotherapy | 41 (74.5) |
| IMRT | 14 (25.5) |
Abbreviations: MRND, modified radical neck dissection; SND, selective neck dissection; RND, radical neck dissection; TPF, docetaxel, cisplatin, and 5-fluorouracil; PF, cisplatin and 5-fluorouracil; TPE, docetaxel, cisplatin, and cetuximab; CRT, chemoradiotherapy; RT, radiotherapy; IMRT, intensity-modulated radiation therapy.
Follow-Up
After the completion of treatment, patients were typically seen on a monthly basis for the first year, then every 2 to 3 months the following year, then every 3 to 6 months thereafter. Evaluation consisted of history and physical examination, which included flexible laryngoscopy. CT of the neck and chest were routinely performed within 6 months of the completion of treatment, then annually thereafter. Patients who had suspicious findings, either radiographically or on physical examination, underwent directed biopsy. Patients with isolated local or locoregional recurrences were evaluated for salvage surgery. Those patients whose disease was not deemed to be resectable, or who had synchronous distant metastases, received palliative chemotherapy or best supportive care.
Statistics
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan–Meier method. Events recorded in PFS included death due to any cause, progression of disease, and recurrence, either local or distant. Locoregional failure was defined as recurrence within the neck or in the primary site. Stratified analysis of OS and PFS was performed with the following stratification variables: site of primary tumor, nodal classification (N0–2 vs N3), and overall stage (II–IVa vs IVb). The log-rank test was used to compare survival distributions for each variable. Treatment delay was calculated from postoperative day 1 to the first day of radiotherapy or induction chemotherapy.
Results
Patient population
Fifty-five patients fit the eligibility criteria and were included in the analysis. The median age was 53 years (range, 40–74 years). The primary site was located in the oropharynx in 69.1%, the hypopharynx in 12.7%, the larynx in 12.7%, and the oral cavity in 5.5%. Fifty-four patients (98.2%) were stage III to IV. One patient had radiographic evidence of nodal disease but was subsequently found to have a pathologically negative dissection, and thus this patient's disease was stage II (T2 N0).
Pathologic findings
Fifty-five patients underwent surgery with 60 neck specimens available for pathologic review. Four (6.7%) dissections yielded negative pathologic specimens: 3 were negative dissections after previous excisional nodal biopsy and 1 was a negative dissection performed on a patient with radiographic evidence of cervical adenopathy on preoperative CT. The median number of lymph nodes yielded per neck dissection was 40. Extracapsular extension was identified in 39 specimens (65.0%) and necrosis was identified in 33 specimens (55.0%). The median largest lymph node size was 4.3 cm (range, 0–9 cm).
Survival
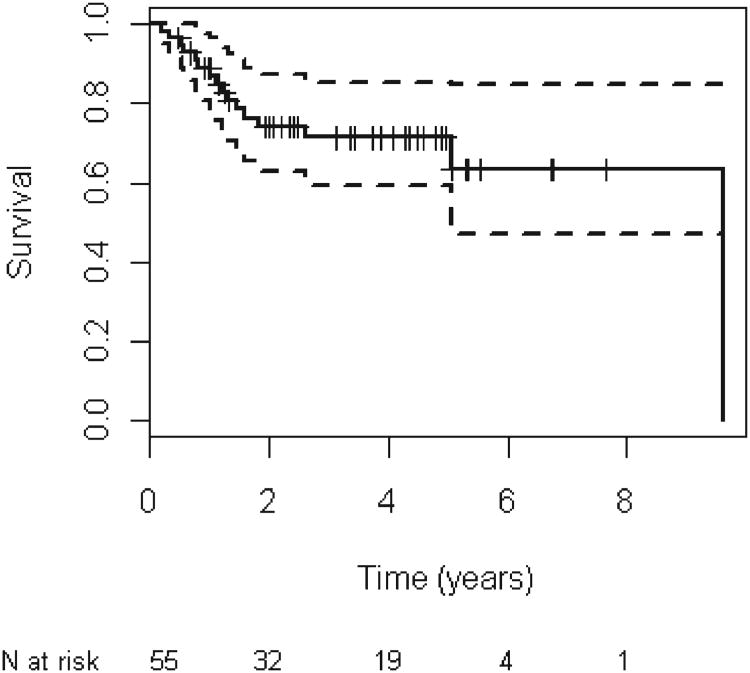
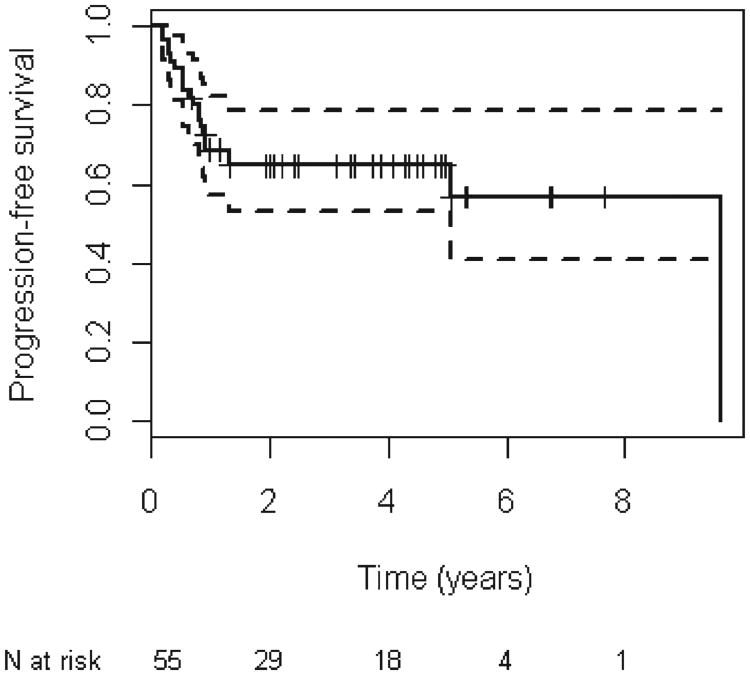
The median follow-up period for all patients was 3.9 years (range, 0–9.6 years). There were 16 total deaths recorded at last follow-up. The 5-year OS was estimated at 71.3% (95% confidence interval [CI], 59.4–85.5; Figure 1). The 5-year PFS was estimated at 64.7% (95% CI, 53.0–78.9; Figure 2). There was no statistically significant difference in OS or PFS based on disease site, nodal stage, or overall stage (Table 3). However, there was a significant difference in OS (hazard ratio, 2.8; 95% CI, 1.0–7.7; p = .05) and PFS (hazard ratio, 2.6; 95% CI, 1.1–6.2; p = .04) when performing a combined analysis of patients with hypopharyngeal or laryngeal primaries compared with patients with oropharyngeal or oral cavity tumors.
Figure 1.
Kaplan–meier analysis of overall survival for all patients.
Figure 2.
Kaplan–meier analysis of progression-free survival for all patients.
Table 3.
Analysis of overall and progression-free survival stratified by disease site, nodal stage, and overall stage.
| Variable | 5-y PFS % (95% CI) | p value | 5-y OS % (95% CI) | p value |
|---|---|---|---|---|
| Disease site | .14 | .20 | ||
| Hypopharynx | 29 (9–92) | 36 (12–100) | ||
| Larynx | N/A (N/A) | N/A (N/A) | ||
| Oral cavity | 67 (30–100) | 100 (0–100) | ||
| Oropharynx | 76 (64–91) | 81 (69–95) | ||
| N classification | .81 | .78 | ||
| N0–N2 | 66 (53–81) | 72 (59–88) | ||
| N3 | 62 (39–100) | 70 (46–100) | ||
| AJCC stage | .7044 | .52 | ||
| II–IVa | 66 (53–82) | 73 (60–89) | ||
| IVb | 60 (38–95) | 67 (44–100) |
Abbreviations: PFS, progression-free survival; CI, confidence interval; OS, overall survival; N/A, not estimable because no patients reached this endpoint, either because of death or censoring; AJCC, American Joint Committee on Cancer.
Disease control
There were 2 failures in the dissected neck, for an overall neck control rate of 96.7%. Neither of the recurrences was isolated, with both of them occurring synchronously with distant metastasis. One failure occurred in a patient with N3 disease, while the other occurred in a patient with N2b nodal disease. The pathologic findings in both patients revealed the presence of extracapsular extension and necrosis. Both patients received palliative chemotherapy alone. There were 7 locoregional failures, including the 2 patients noted above, for a locoregional control rate of 87.3%. Three of the locoregional failures were isolated, with 2 of these patients undergoing composite resection. The third patient was deemed to have unresectable disease and was treated with cryotherapy to the accessible recurrent lesions, followed by palliative chemotherapy. The other 4 patients with locoregional failure were found to have distant metastatic disease, and were thus treated palliatively. There were a total of 12 distant failures, yielding a distant control rate of 78.2%.
Treatment delay and toxicity
The median delay between surgery and the initiation of definitive treatment was 21 days (range, 10–48 days). Eight patients experienced a delay of greater than 30 days. There was 1 incidence of a significant postoperative wound complication, consisting of persistent drainage from the incisional site, which delayed treatment for 42 days. There was 1 occurrence of grade 3 wound toxicity after radiotherapy, consisting of a persistent tracheocutaneous fistula, which was not surgically addressed due to recurrent disease and subsequent death of the patient. There were no grade ≥4 postradiotherapy wound complications. There were 2 instances of non-wound-related grade 4 toxicity after radiotherapy, both consisting of osteoradionecrosis of the mandible requiring surgical debridement. There were no treatment-related mortalities.
Discussion
The proper management of the clinically node-positive neck in the era of organ preservation is controversial. Failure in the neck after definitive chemoradiotherapy without lymph node dissection remains a significant problem. The report of European Organization for Research and Treatment of Cancer 24954, a prospective trial comparing sequential chemotherapy and radiotherapy or alternating chemoradiotherapy in patients with advanced laryngeal and hypopharyngeal cancer, showed long-term rates of failure in the neck of 16.5% in the sequential arm and 20.8% in the alternating arm15. The Groupe d'Oncologie Radiothérapie Tête et Cou 94–01 trial, which compared standard radiotherapy with concurrent chemoradiotherapy in stage III to IV oropharyngeal cancer, similarly showed a neck failure rate of 19% in the combined modality arm.16
The addition of planned neck dissection after chemoradiotherapy has been shown to improve local control within the neck. Sewall et al,7 in a retrospective review of 93 patients with stage III to IV head and neck cancer treated with neck dissection after chemoradiotherapy, reported a 93% regional control rate. These results are supported by Brizel et al,5 who report not only a local control benefit, but an OS benefit of postchemoradiotherapy neck dissection compared with chemoradiotherapy alone in patients with N2 to N3 head and neck cancer.
There is evidence to support the notion that neck dissection for all node-positive patients may be unnecessary and that the decision to operate should be dictated by radiographic response. Proponents of this approach will note that the rate of pathologically positive dissection after chemoradiotherapy ranges from 20% to 43%, meaning that approximately two/thirds of patients will undergo an unnecessary surgery.4,6,7,17,18 Langerman et al,19 in a retrospective review of 49 patients undergoing chemoradiotherapy followed by planned neck dissection, demonstrated that 100% of patients with negative CT findings after chemoradiation had negative pathologic dissection specimens, while 50% of patients with residual disease on CT had positive pathologic findings, illustrating the specificity of post-RT CT. Lau et al20 reported a 9% neck failure rate in patients who achieved complete response, either radiographically or on physical examination, after chemoradiotherapy without neck dissection. Additionally, Clayman et al21 showed in their retrospective review that patients with complete response after sequential chemotherapy and radiation derived no benefit from planned neck dissection, although incomplete responders did demonstrate a survival benefit from neck dissection.
Although failures in the neck after chemoradiation may be relatively infrequent, those patients who do fail are left with a dismal prognosis. The rate of successful surgical salvage after definitive radiation has been shown to be very poor. In a series of 116 patients with neck failure after primary radiation therapy, Bernier et al22 reported that 14 patients underwent an attempted surgical resection, with only 1 successful salvage operation. In another report of 51 patients with neck recurrence, of whom 33 (65%) underwent attempted salvage treatment, only 1 patient achieved durable local control.23 Similarly, Liauw et al24 identified 13 patients with isolated neck failures after definitive radiotherapy that underwent salvage treatment, of whom only 4 patients (30.8%) were successfully salvaged.
It is likely that some patients with advanced head and neck cancer benefit from planned dissection of the neck. The challenge lies in the accurate and reliable identification of this subset of patients. There are multiple reports within the literature demonstrating that central necrosis, along with large nodal volume, are poor prognostic factors.25–27 In a study correlating radiographic findings of pathologic lymph nodes on radiation planning CT with clinical outcomes, nodal volume and central necrosis were both found to be predictive of locoregional failure.27 There is evidence to the contrary, however, with Cho et al28 reporting that neither necrosis nor nodal size >3 cm were predictive of failure in patients undergoing chemoradiotherapy.
There is also a growing body of evidence that outcomes in head and neck cancer can be predicted by standardized uptake values (SUVs) of the primary tumor on pretreatment PET scan.29–32 In the study by Cho et al28 mentioned above, only SUV uptake was predictive of nodal failure. Similarly, Inokuchi et al32 recently demonstrated that a nodal SUV >6 may predict for failure and could be a valuable metric with which to select patients for planned neck dissection. A PET scan was not routinely performed as part of the preoperative workup in our patient population and the decision of whether or not to proceed with neck dissection was based mainly on nodal size, along with radiographic evidence of necrosis. However, a future prospective trial could incorporate SUV as part of the selection criteria, with patients who planned dissection reserved for patients with high uptake values.
For those patients who are most likely to have failure in the neck, the question of how best to sequence neck dissection with definitive treatment has yet to be answered. As outlined previously, planned neck dissection after the completion of chemoradiotherapy results in high rates of local control. This approach, however, is limited by relatively high rates of postoperative wound complications and severe late toxicities.9,10 Certainly, many of the late complications associated with planned neck dissection, such as shoulder dysfunction, neck pain, fistula formation, and chyle leak, are all applicable in the setting of up-front neck dissection.
There are several concerns that are raised regarding up-front neck dissection. Aside from the possibility of an unnecessary surgery and the subsequent morbidity caused by it, there is the perception that the delay of definitive treatment due to the procedure may negatively impact local control of the primary tumor. We have reported a 21-day median delay between surgery and initiation of definitive treatment, a finding that is supported by other series, which have reported similar intervals of delay.12–14 The present study shows excellent rates of local control within the dissected neck, as well as rates of overall locoregional control. As a comparison, the largest prospective trials including this population of patients treated with definitive chemoradiotherapy alone have reported locoregional control rates ranging from 67% to 78%.16,34 Given that many patients must undergo dental extraction before the initiation of radiotherapy, and the standard delay from this procedure is 10 to 14 days, the addition of 1 to 2 weeks of further delay does not seem to adversely affect outcomes. These findings are similar in nature to other series reporting the use of up-front neck dissection, which are summarized in Table 4.
Table 4.
Previously published reports of up-front neck dissection.
| Study | Patients | Primary site | Neck control | Locoregional control | Survival |
|---|---|---|---|---|---|
| Byers et al,11 1992 | 35 | Multiple | 89% | 71% | 5-y OS: 55% |
| Smeele et al,25 2000 | 37 | Multiple | 86% | 43% | 2-y DSS: 49% |
| Reddy et al,12 2005 | 16 | Oropharynx | 100% | 94% | OS: 100% |
| Cupino et al,13 2007 | 25 | Oropharynx | 100% | 88% | 3-y OS: 92% |
| Prades et al,14 2008 | 76 | Hypopharynx | 90% | 84% | 2-y OS: 43% |
| Present study | 56 | Multiple | 96% | 87% | 5-y OS: 70% |
Abbreviations: OS, overall survival; DSS, disease-specific survival.
The limitations to this study are mainly related to its retrospective nature. The selection criteria were not uniform, which led to a relatively heterogenous patient population, making the interpretation of our results somewhat more difficult. As noted above, a PET scan was not routinely performed and thus no conclusions regarding its utility in patient selection or outcomes can be drawn. Long-term complications of neck dissection, including neck pain and shoulder dysfunction, were inconsistently recorded and thus not reportable. Finally, human papillomavirus (HPV) testing was not performed, which raises the question regarding the appropriateness of this strategy as it relates to HPV status. This is an important point, as there is very clear evidence that patients with HPV-positive head and neck cancers have significantly improved outcomes after chemoradiotherapy compared with patients who are HPV negative, as illustrated in the recently published Radiation Therapy Oncology Group 0122 trial.35 In addition, there is evidence that HPV-positive cancers may present with large or cystic lymph nodes that would have fit our selection criteria, despite the likelihood of an excellent response to chemoradiotherapy alone.36 Thus, future efforts to identify patients that may benefit from up-front neck dissection should certainly include the results of HPV screening as part of the decision-making process.
Conclusion
We report here one of the largest series utilizing up-front neck dissection for patients with regionally advanced head and neck cancer, and have demonstrated excellent locoregional control with very encouraging survival rates. We feel that there is a role for up-front neck dissection in the management of regionally advanced head and neck cancer. Further studies to better identify the population of patients most appropriate for this approach are needed.
References
- 1.Mendenhall WM, Million RR, Cassisi NJ. Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int J Radiat Oncol Biol Phys. 1986;12:733–740. doi: 10.1016/0360-3016(86)90030-1. [DOI] [PubMed] [Google Scholar]
- 2.Parsons JT, Mendenhall WM, Cassisi NJ, Stringer SP, Million RR. Neck dissection after twice-a-day radiotherapy: morbidity and recurrence rates. Head Neck. 1989;11:400–404. doi: 10.1002/hed.2880110504. [DOI] [PubMed] [Google Scholar]
- 3.Boyd TS, Harari PM, Tannehill SP, et al. Planned postradiotherapy neck dissection in patients with advanced head and neck cancer. Head Neck. 1998;20:132–137. doi: 10.1002/(sici)1097-0347(199803)20:2<132::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Robbins KT, Wong FSH, Kumar P, et al. Efficacy of targeted chemoradiation and planned selective neck dissection to control bulky nodal disease in advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 1999;125:670–675. doi: 10.1001/archotol.125.6.670. [DOI] [PubMed] [Google Scholar]
- 5.Brizel DM, Prosnitz RG, Hunter S, et al. Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;58:1418–1423. doi: 10.1016/j.ijrobp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Frank DK, Hu KS, Culliney BE, et al. Planned neck dissection after concomitant radiochemotherapy for advanced head and neck cancer. Laryngoscope. 2005;115:1015–1020. doi: 10.1097/01.MLG.0000162648.37638.76. [DOI] [PubMed] [Google Scholar]
- 7.Sewall GK, Palazzi–Churas KL, Richards GM, Hartig GK, Harari PM. Planned postradiotherapy neck dissection: rationale and clinical outcomes. Laryngoscope. 2007;117:121–128. doi: 10.1097/01.mlg.0000246709.93530.72. [DOI] [PubMed] [Google Scholar]
- 8.Igidbashian L, Fortin B, Guertin L, et al. Outcome with neck dissection after chemoradiation for N3 head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:414–420. doi: 10.1016/j.ijrobp.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Davidson BJ, Newkirk KA, Harter KW, Picken CA, Cullen KJ, Sessions RB. Complications from planned, posttreatment neck dissections. Arch Otolaryngol Head Neck Surg. 1999;121:401–405. doi: 10.1001/archotol.125.4.401. [DOI] [PubMed] [Google Scholar]
- 10.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byers RM, Clayman GL, Guillamondequi OM, Peters LJ, Goepfert H. Resection of advanced cervical metastasis prior to definitive radiotherapy for primary squamous carcinomas of the upper aerodigestive tract. Head Neck. 1992;14:133–138. doi: 10.1002/hed.2880140210. [DOI] [PubMed] [Google Scholar]
- 12.Reddy AN, Eisele DW, Forastiere AA, Lee DJ, Westra WH, Califano JA. Neck dissection followed by radiotherapy or chemoradiotherapy for small primary oropharynx carcinoma with cervical metastasis. Laryngoscope. 2005;115:1196–1200. doi: 10.1097/01.MLG.0000162643.91849.79. [DOI] [PubMed] [Google Scholar]
- 13.Cupino A, Axelrod R, Anne R, et al. Neck dissection followed by chemoradiotherapy for stage IV (N+) oropharynx cancer. Otolaryngol Head Neck Surg. 2007;137:416–421. doi: 10.1016/j.otohns.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Prades JM, Timoshenko AP, Schmitt TH, et al. Planned neck dissection before combined chemoradiation for pyriform sinus carcinoma. Acta Otolaryngol. 2008;128:324–328. doi: 10.1080/00016480701477669. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre JL, Rolland F, Tesselaar M, et al. Phase 3 randomized trial on larynx preservation comparing sequential vs alternating chemotherapy and radiotherapy. J Natl Cancer Inst. 2009;101:142–152. doi: 10.1093/jnci/djn460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advancedstage oropharynx carcinoma. J Clin Oncol. 2004;22:69–76. doi: 10.1200/JCO.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Stenson KM, Huo D, Blair E, et al. Planned post-chemoradiation neck dissection: significance of radiation dose. Laryngoscope. 2006;116:33–36. doi: 10.1097/01.mlg.0000185846.27617.fe. [DOI] [PubMed] [Google Scholar]
- 18.Goguen LA, Posner MR, Tishler RB, et al. Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2006;132:526–531. doi: 10.1001/archotol.132.5.526. [DOI] [PubMed] [Google Scholar]
- 19.Langerman A, Plein C, Vokes E, et al. Neck response to chemoradiotherapy: complete radiographic response correlates with pathologic complete response in locoregionally advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1133–1136. doi: 10.1001/archoto.2009.154. [DOI] [PubMed] [Google Scholar]
- 20.Lau H, Phan T, MacKinnon J, Matthews W. Absence of planned neck dissection for the N2-N3 neck after chemoradiation for locally advanced squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 2008;134:257–261. doi: 10.1001/archoto.2007.49. [DOI] [PubMed] [Google Scholar]
- 21.Clayman GL, Johnson CJ, Morrison W, Ginsberg L, Lippman SM. The role of neck dissection after chemoradiotherapy for oropharyngeal cancer with advanced nodal disease. Arch Otolaryngol Head Neck Surg. 2001;127:135–139. doi: 10.1001/archotol.127.2.135. [DOI] [PubMed] [Google Scholar]
- 22.Bernier J, Bataini JP. Regional outcome in oropharyngeal and pharyngolaryngeal cancer treated with high dose per fraction radiotherapy Analysis of neck disease response in 1646 cases. Radiother Oncol. 1986;6:87–103. doi: 10.1016/s0167-8140(86)80015-9. [DOI] [PubMed] [Google Scholar]
- 23.Mabanta SR, Mendenhall WM, Stringer SS, Cassisi NJ. Salvage treatment for neck recurrence after irradiation alone for head and neck squamous cell carcinoma with clinically positive neck nodes. Head Neck. 1999;21:591–594. doi: 10.1002/(sici)1097-0347(199910)21:7<591::aid-hed1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 24.Liauw SL, Amdur RJ, Morris CG, Werning JW, Villaret DB, Mendenhall WM. Isolated neck recurrence after definitive radiotherapy for node-positive head and neck cancer: salvage in the dissected or undissected neck. Head Neck. 2007;29:715–719. doi: 10.1002/hed.20580. [DOI] [PubMed] [Google Scholar]
- 25.Munck JN, Cvitkovic E, Piekarski JD, et al. Computed tomographic density of metastatic lymph nodes as a treatment-related prognostic factor in advanced head and neck cancer. J Natl Cancer Inst. 1991;83:569–575. doi: 10.1093/jnci/83.8.569. [DOI] [PubMed] [Google Scholar]
- 26.Grabenbauer GG, Steininger H, Meyer M, et al. Nodal CT density and total tumor volume as prognostic factors after radiation therapy of stage III/IV head and neck cancer. Radiother Oncol. 1998;47:175–183. doi: 10.1016/s0167-8140(98)00016-4. [DOI] [PubMed] [Google Scholar]
- 27.Vergeer MR, Doornaert P, Leemans CR, Buter J, Slotman BJ, Langendijk JA. Control of nodal metastases in squamous cell head and neck cancer treated by radiation therapy or chemoradiation. Radiother Oncol. 2006;79:39–44. doi: 10.1016/j.radonc.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Cho AH, Shah S, Ampil F, Bhartur S, Nathan CA. N2 disease in patients with head and neck squamous cell cancer treated with chemoradiotherapy: is there a role for posttreatment neck dissection? Arch Otolaryngol Head Neck Surg. 2009;135:1112–1118. doi: 10.1001/archoto.2009.148. [DOI] [PubMed] [Google Scholar]
- 29.Allal AS, Slosman DO, Kebdani T, Allaoua M, Lehmann W, Dulguerov P. Prediction of outcome in head-and-neck cancer patients using the standadized uptake value of 2-[18F]fluoro-2-deoxy-D-glucose. Int J Radiat Oncol Biol Phys. 2004;59:1295–1300. doi: 10.1016/j.ijrobp.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 30.Roh JL, Pae KH, Kim JS, et al. 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography as guidance for primary treatment in patients with advanced-stage resectable squamous cell carcinoma of the larynx and hypopharynx. Eur J Surg Oncol. 2007;33:790–795. doi: 10.1016/j.ejso.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Roh JL, Kim MR, et al. Use of 18F-FDG PET for primary treatment strategy in patients with squamous cell carcinoma of the oropharynx. J Nucl Med. 2007;48:752–757. doi: 10.2967/jnumed.107.039610. [DOI] [PubMed] [Google Scholar]
- 32.Inokuchi H, Kodaira T, Tachibana H, et al. Clinical usefulness of [(18)F] fluoro-2-deoxy-D-glucose uptake in 178 head-and-neck cancer patients with nodal metastasis treated with definitive chemoradiotherapy: consideration of its prognostic value and ability to provide guidance for optimal selection of patients for planned neck dissection. Int J Radiat Oncol Biol Phys. 2011;79:747–755. doi: 10.1016/j.ijrobp.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 33.Smeele LE, Leemans MD, Reid CB, Tiwari R, Snow GB. Neck dissection for advanced lymph node metastasis before definitive radiotherapy for primary carcinoma of the head and neck. Laryngoscope. 2000;110:1210–1214. doi: 10.1097/00005537-200007000-00027. [DOI] [PubMed] [Google Scholar]
- 34.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemoradiotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. New Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 35.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldenberg D, Begum S, Westra W, et al. Cystic lymph node metastasis in patients with head and neck cancer: an HPV-associated phenomenon. Head Neck. 2008;30:898–903. doi: 10.1002/hed.20796. [DOI] [PubMed] [Google Scholar]