Abstract
Background
Antihypertensive drugs that block the renin-angiotensin system (angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin receptor blockers) are recommended for patients with chronic kidney disease (CKD). A low blood pressure (BP) goal (BP, <130/80 mm Hg) is also recommended. The objective of this study was to determine the long-term effects of currently recommended BP therapy in 1094 African Americans with hypertensive CKD.
Methods
Multicenter cohort study following a randomized trial. Participants were 1094 African Americans with hypertensive renal disease (glomerular filtration rate, 20–65 mL/min/1.73 m2). Following a 3×2-factorial trial (1995–2001) that tested 3 drugs used as initial antihypertensive therapy (ACEIs, calcium channel blockers, and β-blockers) and 2 levels of BP control (usual and low), we conducted a cohort study (2002–2007) in which participants were treated with ACEIs to a BP lower than 130/80 mm Hg. The outcome measures were a composite of doubling of the serum creatinine level, end-stage renal disease, or death.
Results
During each year of the cohort study, the annual use of an ACEI or an angiotensin receptor blocker ranged from 83.7% to 89.0% (vs 38.5% to 49.8% during the trial). The mean BP in the cohort study was 133/78 mm Hg (vs 136/82 mm Hg in the trial). Overall, 567 participants experienced the primary outcome; the 10-year cumulative incidence rate was 53.9%. Of 576 participants with at least 7 years of follow-up, 33.5% experienced a slow decline in kidney function (mean annual decline in the estimated glomerular filtration rate, <1 mL/min/1.73 m2).
Conclusion
Despite the benefits of renin-angiotensin system–blocking therapy on CKD progression, most African Americans with hypertensive CKD who are treated with currently recommended BP therapy continue to progress during the long term.
Hypertensive chronic kidney disease (CKD) is a major public health problem in the United States, especially among African Americans. An estimated 4.5 million Americans have hypertensive CKD, and another 110 000 persons have end-stage renal disease (ESRD) attributed to hypertension.1 According to the US Renal Data System,2 37% of ESRD cases in African Americans can be attributed to hypertension, whereas the corresponding figure in persons of white race/ethnicity is 19%. In studies3,4 conducted before renin-angiotensin system (RAS)–blocking therapy, African Americans with hypertensive CKD experienced continued CKD progression compared with persons of white race/ethnicity, who experienced little or no progression while receiving antihypertensive drug therapy.
RAS-blocking therapy (angiotensin-converting enzyme inhibitors [ACEIs] or angiotensin receptor blockers [ARBs]) and a low blood pressure (BP) goal, generally less than 130/80 mm Hg, are recommended in most patients with CKD.5,6 The recommendation for RAS-blocking therapy is based largely on trials that were shorter than 4 years in duration.7 Data on the long-term effects of recommended therapy are sparse. In a posttrial follow-up study8 of patients with proteinuric nondiabetic CKD, the ACEI ramipril seemed to stabilize the glomerular filtration rate (GFR) and to prevent ESRD during 4.5 years.
In the African American Study of Kidney Disease and Hypertension (AASK),9–11 we previously reported that treatment with ramipril reduced the risk of clinical renal events (50% or 25 mL/ min/1.73 m2 decline in GFR, ESRD, or death) by 48%compared with the calcium channel blocker amlodipine besylate and by 22% compared with the β-blocker metoprolol succinate; a low BP goal conferred no additional benefit over a conventional BP goal. On completion of the AASK Clinical Trial, participants were invited to enroll in a posttrial cohort study in which they received treatment with ACEIs to a BP lower than 130/80 mm Hg.12,13 Using data from the trial and cohort phases of AASK, we report the long-term effects of ACEI therapy with a low BP goal.
METHODS
Detailed descriptions of study methods and trial results have been published.9–12 Protocols for the trial phase and cohort study were approved by institutional review boards at each center. Participants provided written informed consent. Independent scientific advisory committees reviewed and approved the study protocols and monitored progress.
STUDY PARTICIPANTS
Participants were eligible for the trial (Trial Registration: clinicaltrials.gov Identifier: NCT00582777) if they were self-identified African Americans, aged 18 to 70 years, with hypertensive CKD as defined by a diastolic BP higher than 95 mm Hg and a GFR between 20mL/min/1.73 m2 and 65mL/min/1.73 m2, measured by isothalamate sodium iodide I 125 clearance. Individuals were excluded if there was an apparent cause for CKD other than hypertension. Specific exclusion criteria were (1) a fasting glucose level greater than 140 mg/dL (to convert glucose level to millimoles per liter, multiply by 0.0555), a random glucose level greater than 200 mg/dL, or receipt of drug therapy for diabetes mellitus; (2) a urinary protein to urinary creatinine ratio (UP/ Cr) greater than 2.5; (3) the presence of accelerated or malignant hypertension; (4) the presence of secondary hypertension; (5) the presence of serious systemic disease; (6) the presence of congestive heart failure; or (7) the presence of a specific indication for or contraindication to a study drug or procedure.
AASK TRIAL PHASE
The AASK trial phase had a 3×2-factorial design. Between April 7, 1995, and September 28, 1998, 1094 participants were randomized to initial treatment with an ACEI (ramipril, 2.5–10 mg/d), a sustained-release β-blocker (metoprolol, 50–200 mg/d), or a dihydropyridine calcium channel blocker (amlodipine, 5–10 mg/d) and to 1 of 2 BP goals (a usualmean arterial pressure [MAP] goal of 102–107 mm Hg or a low MAP goal of <92 mm Hg). A MAP of 107mmHg corresponds to the conventional BP goal of 140/90mmHg, while a MAP of 92mmHg is slightly lower than the BP goal of 130/80 mm Hg currently recommended for individuals with CKD.5 If the BP goal could not be achieved by the highest tolerated dose of the randomized drug, other antihypertensive drugs (furosemide, doxazosin mesylate, clonidine hydrochloride, hydralazine hydrochloride, or minoxidil) were sequentially added. The primary outcome was the GFR slope as assessed by isothalamate sodium iodide I 125 clearance. A secondary outcome was a composite of a GFR reduction by 50% or by 25 mL/min/1.73 m2 from baseline, ESRD, or death.
Participants randomized to ACEIs had a slower rate of CKD progression than those assigned to the other 2 drug regimens, particularly in the setting of a baseline UP/Cr greater than 0.22, a level that corresponds to approximately 300 mg of UP/d. Despite a sustained 10–mm Hg difference in MAP between the low and usual MAP groups, CKD progression was similar in both groups. In observational analyses, participants with a baseline UP/Cr greater than 0.22 had more rapid CKD progression than those with a UP/Cr of 0.22 or less.
AASK COHORT STUDY
Trial participants who had not reached ESRD were invited to enroll in the AASK cohort study, during which BP was monitored, antihypertensive drug therapy adjusted, and adherence promoted. Between the end of the trial (September 30, 2001) and the start of the cohort study (April 1, 2002), there was a brief transition period during which the cohort study was designed and participants were switched from randomized therapy to ACEIs.
A major objective of the cohort study was to determine the long-term course of hypertensive CKD in the setting of recommended BP therapy. Antihypertensive therapy in the cohort study was largely based on AASK trial phase results and prevailing recommendations. Ramipril was first-line therapy; if ramipril was not tolerated, an ARB was used. If the BP goal was not achieved with ramipril, 10 mg/d, additional drugs were added (furosemide, β-blockers, calcium channel blockers, centrally acting α-adrenergic blockers, and direct vasodilators). All drugs were open label. Consistent with AASK trial phase results, the initial BP goal in the cohort study was less than 140/90 mm Hg. However, the goal was reduced to less than 130/80 mm Hg after national guidelines recommended this target.5
Participants were seen at least twice per year and more often if needed to achieve BP control. Antihypertensive medications were provided free. Efforts to achieve high adherence included the use of pill organizers, home visits, and reports to personal care physicians. A clinical management subcommittee monitored achieved BP levels and the use of specific antihypertensive therapy at the clinical centers.
OUTCOMES
The primary outcome was a composite end point defined by doubling of the serum creatinine level from trial baseline (roughly equivalent to halving the GFR), ESRD, or death. Other outcomes were those events directly related to CKD(doubling of the serum creatinine level or ESRD, censoring at death) and clinical events (ESRD or death). Because these event rates are affected by the initial level of kidney function and its pattern of change over time, another outcome variable is the mean annual change in the estimated GFR, derived from a validated estimating equation that used age, sex, and the serum creatinine level.14,15 The serum creatinine level was centrally measured at the same laboratory in both phases of the study using the rate Jaffe method with an alkaline picrate assay. During the trial phase and cohort study, the serum creatinine level was measured twice at baseline and then every 6 months. It was not measured during the transition period. End-stage renal disease was defined by the start of dialysis or by the occurrence of renal transplantation.
STATISTICAL ANALYSIS
Selected patient characteristics were summarized at trial baseline for all 1094 participants and at selected follow-up visits during the trial phase and cohort study in those who remained at risk for the primary composite outcome. Blood pressure levels were summarized at approximate 1-year intervals.
Event rates for each composite, expressed as the number of events per 100 patient-years, were computed as the ratio of the number of patients reaching events divided by the total patient-years of follow-up before an event or until censoring. For the trial phase, follow-up time started at the date of randomization. For the cohort study, follow-up time started at the end of the trial (September 30, 2001) and included the transition period. The maximum duration of follow-up was 12.2 years, which corresponds to the interval between the start of trial enrollment (April 7, 1995) and the end of outcome ascertainment (June 30, 2007).
Kaplan-Meier curves16 were constructed to display the cumulative probability of study outcomes. A competing risk approach was used to examine the extent to which the cumulative probabilities of the overall clinical composite reflected renal events or death at each follow-up time. Using the competing risk formulation, 17,18 cumulative incidence curves were constructed to display the cumulative probability of doubling of the serum creatinine level or ESRD (without censoring death) and of death before a renal event (without censoring the renal events). Chronic kidney disease progression was also examined in selected subgroups defined by sex and trial baseline values of age (below and above the median age), UP level (UP/Cr ≤0.22 and >0.22), and the GFR (>40 and ≤40 mL/min/1.73 m2).
For analyses of change in renal function, separate mixed-effects models were used to estimate the mean rate of change in the estimated GFR among the 1094 trial phase participants and among all enrollees in the cohort study, irrespective of whether the participant remained at risk for the composite outcome. Because antihypertensive drugs can lead to large acute changes in the GFR that do not reflect long-term progression, we calculated the change in the GFR from 3 months after randomization rather than from baseline, as was done previously.9,10
RESULTS
PARTICIPANT FLOW AND CHARACTERISTICS
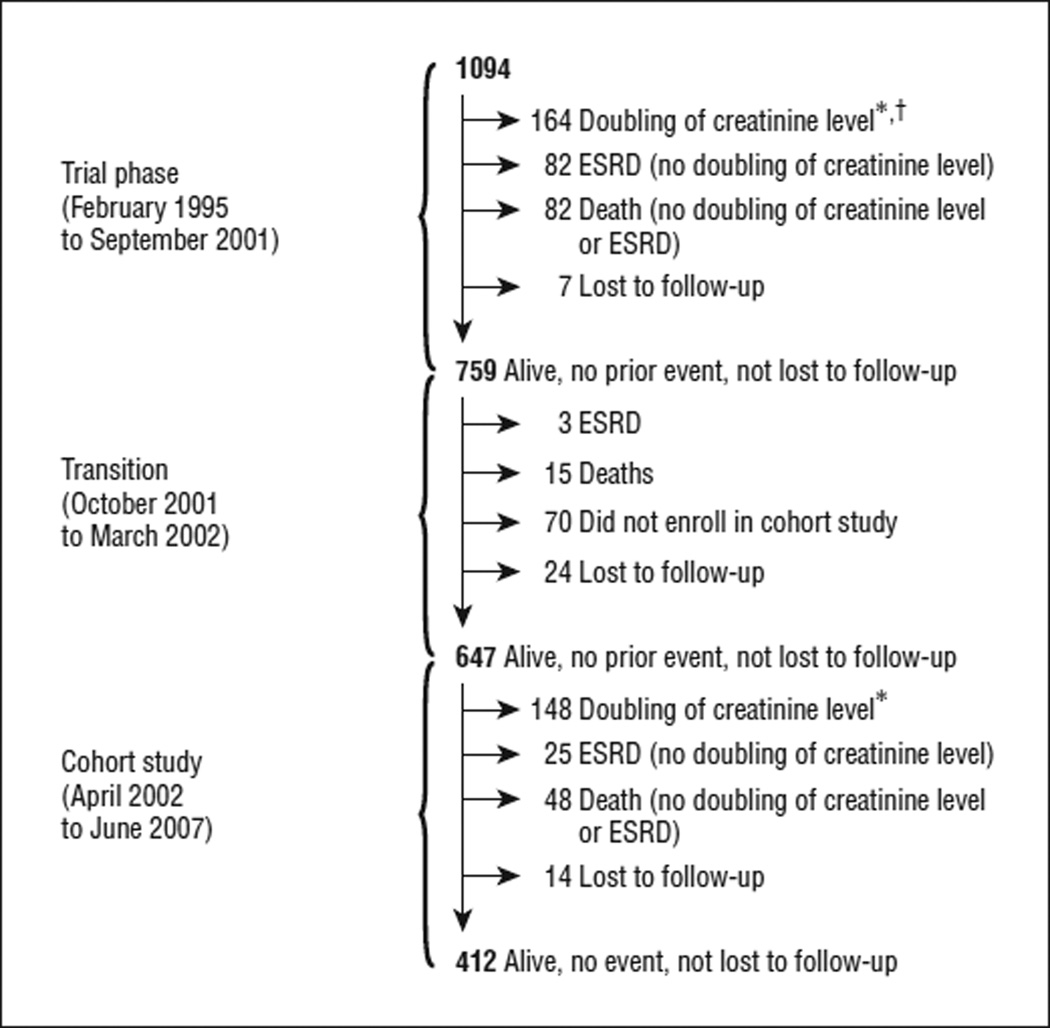
In the trial, 328 outcomes occurred (Figure 1). Of 164 persons with a doubling of their serum creatinine level, 134 (81.7%) subsequently developed ESRD, and 14 (8.5%) died. The remainder (16 participants) were alive without ESRD. At the end of the trial, only 7 trial participants were lost to follow-up. Of 759 persons who had not experienced an outcome during the trial, 18 (2.4%) experienced an outcome (15 deaths and 3 ESRD events) during the transition period, and a total of 94 (12.4%) either did not enroll in the cohort study (70 participants) or were lost to follow-up (24 participants). On average, nonenrollees were older than those who enrolled (mean age at the end of the trial, 62.8 vs 59.7 years; P=.009), but the levels of kidney function (serum creatinine level, GFR, and proteinuria) were similar in the 2 groups (data not shown).Demographic and clinical characteristics of the trial phase and cohort study participants are given in Table 1.
Figure 1.
Participant flow in the trial phase and the cohort study of the African American Study of Kidney Disease and Hypertension. ESRD indicates end-stage renal disease. *Doubling of the creatinine level from trial baseline was the first event. †Those individuals who experienced a doubling of their serum creatinine level in the trial phase were enrolled in the cohort study. However, for the present study they did not contribute follow-up time in the cohort study because the event occurred in the trial phase.
Table 1.
Characteristics of All Participants at the Start of the Trial Phase and of Participants at the Start of the Cohort Study Without a Prior Outcomea
| Characteristic | Trial Phase (n=1094) |
Cohort Studyb (n=759) |
|---|---|---|
| Age, y | 54.6 (10.7) | 60.2 (10.2) |
| Male sex | 61.2 | 61.9 |
| Current smokers | 29.3 | 26.5 |
| Less than a high school degree | 40.7 | 40.8 |
| Weight, kg | 89.5 (20.7) | 92.0 (22.1) |
| Body mass indexc | 30.6 (6.59) | 31.5 (7.10) |
| Glomerular filtration rate, mL/min/1.73 m2 | ||
| Overall | 47.5 (13.9) | 47.4 (15.1) |
| Men | 49.0 (14.1) | 48.7 (14.9) |
| Women | 45.0 (13.4) | 45.2 (15.2) |
| Serum creatinine level, mg/dL | ||
| Overall | 2.0 (0.7) | 2.0 (0.8) |
| Men | 2.2 (0.7) | 2.1 (0.8) |
| Women | 1.8 (0.6) | 1.7 (0.7) |
| Urinary protein level, g/d | ||
| Overall | 0.53 (0.94) | 0.40 (0.79) |
| Men | 0.61 (1.05) | 0.42 (0.78) |
| Women | 0.41 (0.73) | 0.38 (0.80) |
| Urinary protein–urinary creatinine ratio, mg/mg | ||
| Overall | 0.33 (0.52) | 0.27 (0.49) |
| Men | 0.33 (0.51) | 0.25 (0.44) |
| Women | 0.33 (0.53) | 0.30 (0.56) |
| % With urinary protein–urinary creatinine ratio >0.22 | ||
| Overall | 32.8 | 27.1 |
| Men | 34.3 | 27.1 |
| Women | 30.4 | 27.2 |
SI conversion factor: To convert creatinine level to micromoles per liter, multiply by 88.4.
Data are given as mean (SD) or as percentages. Characteristics of those who did not experience the composite outcome during the trial phase and hence who remained “at risk” for the composite outcome (doubling of the serum creatinine level, end-stage renal disease, or death) during the cohort study.
Data are drawn from the last year of the trial rather than the initial cohort visit because those trial participants who died, reached end-stage renal disease, or did not enroll in the cohort study did not attend the initial cohort visit.
Calculated as weight in kilograms divided by height in meters squared.
BP LEVELS AND THE USE OF ACEIs OR ARBs
Blood pressure was well controlled in both phases (Table 2). During the trial phase, the mean follow-up BP was 136/82mmHg (130/78mmHg among those randomized to the low BP goal and 142/86 mm Hg among those randomized to the usual BP goal). During the cohort study, the mean achieved BP was 133/78 mm Hg. Throughout both phases, few participants had a mean systolic BP higher than 160 mm Hg or a mean diastolic BP higher than 100 mm Hg. The mean numbers of BP management visits per participant were 20.4 during the trial phase and 13.7 during the cohort study.
Table 2.
Achieved Blood Pressure Levels and the Use of Angiotensin-Converting Enzyme Inhibitor (ACEI) and Angiotensin Receptor Blocker (ARB) Therapy During the Trial Phase and During the Cohort Studya
| Blood Pressure, mm Hg, Mean (SD) |
Blood Pressure, % | Therapy, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Systolic <130 and Diastolic <80 mm Hg |
Systolic <140 and Diastolic <90 mm Hg |
Systolic >160 or Diastolic >100 mm Hg |
ACEI | ARB | ACEI or ARB |
||||
| Variable | No. | Systolic | Diastolic | ||||||
| Trial Phase | |||||||||
| Baseline | 1094 | 150 (24) | 96 (14) | 7.6 | 19.7 | 20.3 | 39.0 | 1.6 | 40.6 |
| Year 1 | 993 | 135 (20) | 83 (13) | 26.8 | 52.8 | 4.4 | 37.5 | 1.1 | 38.5 |
| Year 2 | 862 | 133 (20) | 82 (13) | 31.6 | 58.0 | 3.5 | 38.6 | 1.1 | 39.7 |
| Year 3 | 773 | 134 (19) | 81 (12) | 32.0 | 55.1 | 3.1 | 41.0 | 2.1 | 43.0b |
| Year 4 | 538 | 133 (20) | 80 (12) | 35.9 | 58.6 | 3.0 | 41.9 | 3.1 | 44.6b |
| Year 5 | 326 | 133 (19) | 79 (12) | 35.6 | 58.0 | 2.8 | 46.0 | 4.1 | 49.8b |
| Cohort Study | |||||||||
| Baseline | 608b | 135 (22) | 81 (12) | 28.3 | 56.9 | 4.4 | 74.1 | 10.51 | 83.7 |
| Year 1 | 547 | 132 (19) | 78 (13) | 34.2 | 68.9 | 2.6 | 75.5 | 11.9 | 85.9 |
| Year 2 | 494 | 129 (19) | 76 (12) | 42.3 | 74.7 | 1.8 | 77.3 | 14.6 | 89.0 |
| Year 3 | 434 | 128 (19) | 75 (13) | 48.4 | 74.4 | 1.4 | 73.9 | 18.2 | 87.3 |
| Year 4 | 386 | 127 (18) | 74 (11) | 52.3 | 76.7 | 0.5 | 72.0 | 19.6 | 85.6 |
Blood pressure measurements were censored after occurrence of doubling of the serum creatinine level.
The increased use of ACEI and ARB in years 3 to 5 reflects early termination of the amlodipine arm of the trial and subsequent transition to ACEI therapy.
The number is not 759 because not all participants reached the baseline of the cohort and blood pressure was unavailable for a few others.
A total of 436 trial participants (39.9%) were randomized to ACEI therapy. Considering dropouts and dropins, the annual percentage of participants receiving ACEIs ranged from 37.5% to 46.0% during the trial phase; of those receiving ramipril, at least 68.8% were taking 10 mg/d, which was the highest recommended dosage while the trial was conducted. The corresponding percentage who reported taking ACEIs or ARBs ranged from 38.5% to 49.8%. During the cohort study, 72.0% to 77.3% of participants were prescribed ACEIs, and 83.7% to 89.0% took ACEIs or ARBs; of those receiving ramipril, at least 70.4% of the participants were taking 10 mg/d.
CKD PROGRESSION
The median follow-up time before the occurrence of the primary composite outcome or administrative censoring was 7.1 years during both phases of the study. The corresponding number of patient-years was 7658.
Overall, 567 participants (52.0%) experienced a doubling of the serum creatinine level from trial baseline, developed ESRD, or died; the corresponding 10-year cumulative incidence rate was 53.9% (7.4 events/100 patient-years). During the trial phase, 30.0% of participants experienced a doubling of their serum creatinine level from trial baseline, ESRD, or death, while 31.5% of the persons who remained at risk at the start of the cohort study experienced this composite outcome (Table 3). Corresponding event rates were 7.2 and 7.8 events per 100 person-years in the trial phase and cohort study, respectively. A similar pattern was evident for the secondary outcomes of renal events and clinical events (Table 2). The distribution of composite outcomes (renal outcomes and death) was similar over time (Figure 2).
Table 3.
Event Rates per 100 Person-Years During the Trial Phase and During the Cohort Study
| Both Phases | Trial Phase | Cohort Study | ||||
|---|---|---|---|---|---|---|
| Variable | No. at Risk (No. of Events, [%]) |
Event Rate |
No. at Risk (No. of Events, [%]) |
Event Rate |
No. at Risk (No. of Events, [%]) |
Event Rate |
| Doubling of Serum Creatinine Level, ESRD, or Death | ||||||
| Overall | 1094 (567 [51.8]) | 7.4 | 1094 (328 [30.0]) | 7.2 | 759 (239 [31.5]) | 7.8 |
| Randomized to ACEI and low BP goal | 215 (113 [52.6]) | 7.4 | 215 (62 [28.8]) | 6.9 | 151 (51 [33.8]) | 8.0 |
| Age, y | ||||||
| ≤55 | 524 (302 [57.6]) | 8.5 | 524 (178 [34.0]) | 8.4 | 343 (124 [36.2]) | 8.8 |
| >55 | 570 (265 [46.5]) | 6.4 | 570 (150 [26.3]) | 6.1 | 416 (115 [27.6]) | 6.9 |
| Baseline urinary protein–urinary creatinine ratio, mg/mg | ||||||
| ≤0.22 | 733 (280 [38.2]) | 4.8 | 733 (125 [17.1]) | 3.8 | 602 (155 [25.7]) | 6.0 |
| >0.22 | 357 (285 [79.8]) | 16.1 | 357 (202 [56.6]) | 15.9 | 154 (83 [53.9]) | 16.6 |
| Sex | ||||||
| Female | 425 (215 [50.6]) | 7.2 | 425 (126 [29.6]) | 7.1 | 297 (89 [30.0]) | 7.4 |
| Male | 669 (352 [52.6]) | 7.5 | 669 (202 [30.2]) | 7.2 | 462 (150 [32.5]) | 8.0 |
| Baseline glomerular filtration rate, mL/min/1.73 m2 | ||||||
| >40 | 718 (276 [38.4]) | 4.8 | 718 (129 [18.0]) | 4.0 | 583 (147 [25.2]) | 5.9 |
| ≤40 | 376 (291 [77.4]) | 15.1 | 376 (199 [52.9]) | 14.7 | 176 (92 [52.3]) | 16.1 |
| Doubling of Serum Creatinine Level or ESRD (Renal Event) | ||||||
| Overall | 1094 (422 [38.6]) | 5.5 | 1094 (246 [22.5]) | 5.4 | 759 (176 [23.2]) | 5.7 |
| Randomized to ACEI and low BP goal | 215 (86 [40.0]) | 5.6 | 215 (48 [22.3]) | 5.3 | 151 (38 [25.2]) | 5.9 |
| ESRD or Death (Clinical Event) | ||||||
| Overall | 1094 (494 [45.2]) | 6.0 | 1094 (264 [24.1]) | 5.6 | 823 (230 [27.9]) | 6.6 |
| Randomized to ACEI and low BP goal | 215 (92 [42.8]) | 5.6 | 215 (47 [21.9]) | 5.1 | 166 (45 [27.1]) | 6.2 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; BP, blood pressure; ESRD, end-stage renal disease.
Figure 2.
Cumulative incidence of renal outcome (doubling of the serum creatinine level from trial baseline or end-stage renal disease [ESRD]), death, and a renal outcome or death.
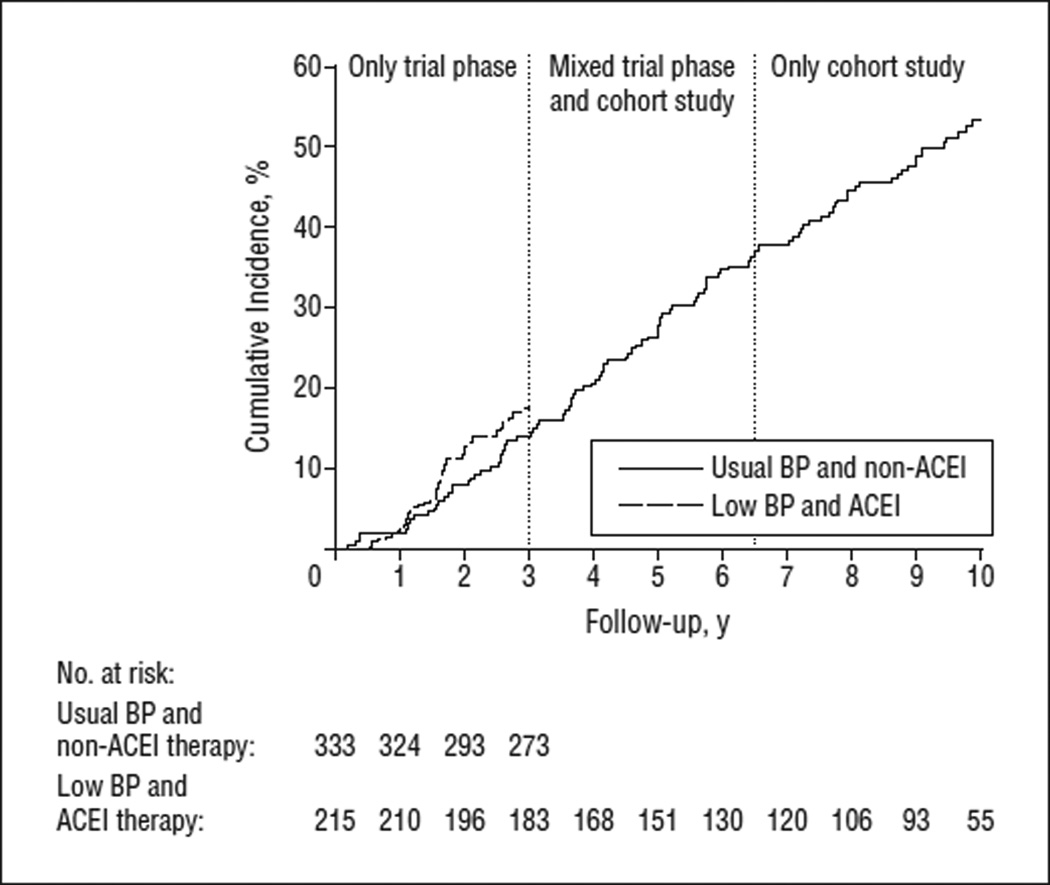
In analyses restricted to those randomized to ACEI therapy with the low BP goal, the 10-year cumulative incidence was 53.5%. Event rates for the primary outcome were 6.9 and 8.0 events per 100 person-years in the trial phase and cohort study, respectively (Table 3). This group received recommended BP therapy during both phases. Figure 3 shows the cumulative incidence of events during the trial phase only among those assigned to the usual BP goal and the non-ACEI therapies (β-blockers or calcium channel blockers).
Figure 3.
Cumulative incidence of composite outcome (doubling of the serum creatinine level from trial baseline, end-stage renal disease, or death) separately for those assigned to a low blood pressure (BP) goal and angiotensin-converting enzyme inhibitor (ACEI) therapy during the trial phase and the cohort study and for those assigned to the usual BP goal and non-ACEI therapies (β-blockers or calcium channel blockers) during the trial phase. All participants had at least 3 years of follow-up in the trial phase. The period between 3 and 6.5 years is a mixed period and corresponds to the trial phase for early enrollees and to the cohort study for late enrollees. The last 3.5 years (6.5–10 years) include cohort data only.
In subgroups defined by variables collected before randomization in the trial, results tended to be similar (Table 3). Absolute event rates were highest among participants with more advanced CKD. Of those with a baseline UP/Cr greater than 0.22 (median UP level, 1 g/d), 79.8% experienced a doubling of the serum creatinine level, developed ESRD, or died across the trial and cohort phases. Among those with a GFR of 40 mL/min/ 1.73 m2 or less, 77.4% experienced this outcome.
The mean (SE) annual rates of decline in the estimated GFR were −2.11 (0.11) mL/min/1.73 m2 and −1.5 (0.11) mL/min/1.73 m2 during the trial phase and cohort study, respectively; among participants randomized to ACEI therapy and the low BP group, the corresponding mean (SE) annual estimates were −2.10 (0.25) mL/min/1.73 m2 and −1.36 (0.22) mL/min/1.73 m2. Of 576 participants with at least 7 years of follow-up after their first 3 months in the study, 33.5% experienced a slow decline in kidney function (ie, a mean annual decline in the estimated GFR of <1 mL/min/1.73 m2); among those randomized to ACEI therapy and the low BP goal, the percentage who were slow progressors was 43.2%.
COMMENT
Antihypertensive drugs that block the RAS are recommended therapy in patients with CKD. Herein, we documented that most African Americans with hypertensive CKD experience continued progression during the long term, despite the use of RAS-blocking therapy and the achievement of BP levels close to recommended goals. The cumulative 10-year incidence of doubling of the serum creatinine level, developing ESRD, or death was 53.9%. A similar event rate was observed in those participants who were randomized to ACEI therapy and the low BP goal (ie, a group that received recommended therapy throughout the 2 phases of AASK). Just 33.5% of participants experienced a slow annual decline in the estimated GFR of less than 1 mL/min/1.73 m2.
An important strength of this study is the provision of recommended antihypertensive therapy. In the cohort study, more than 80% of participants received RAS-blocking therapy, mostly ACEIs, and the mean achieved BP was close to the recommended goal of 130/80mmHg. Another strength is the study’s long duration of follow-up, up to 11.5 years. In previous studies19,20 of patients with nondiabetic CKD, follow-up was typically less than 4 years. Another salient feature is our study population, namely, African Americans with hypertensive CKD. This group has been underrepresented (<10%) in previous studies, despite the fact that African Americans contribute disproportionately to the number of patients who reach ESRD. In the meta-analysis by Jafar et al,7 only 6% of participants were African American. In the Modification of Diet in Renal Disease Study Group,21 only 8% of participants were African American.
A limitation of our study is the nonrandomized design of the posttrial cohort study. In view of our trial findings indicating a benefit of ACEI therapy, it would have been inappropriate to continue non-ACEI arms in the long term. Likewise, because national guidelines recommended a BP goal of less than 130/80mmHg,5,6 it would have been inappropriate to continue the high BP arm. Second, in the cohort study, isothalamate sodium iodide I 125 clearances were not obtained. However, doubling of the serum creatinine level is a well-accepted validated surrogate outcome that has been commonly used in trials of CKD progression.14,15 The clinical relevance of this surrogate marker is underscored by our finding that 81.7% of participants with a doubling of the serum creatinine level during the trial subsequently developed ESRD. Third, AASK trial phase and the cohort study adjusted antihypertensive therapy based on traditional office BP readings rather than on ambulatory BP readings. Sustained nocturnal BP, which cannot be detected by office measurements, is commonplace in the setting of CKD and may lead to rapid CKD progression.22 Fourth, comparisons of event rates in the trial phase and cohort study should be interpreted with caution because events in the cohort phase defined by a doubling of the serum creatinine level from trial baseline likely reflect a smaller absolute reduction in the GFR than corresponding events during the trial phase. For this reason, we emphasize cumulative event rates across both phases of the study.
Findings from previous studies suggest that the excess burden of ESRD among African Americans results from rapid progression of CKD to ESRD rather than an excess of CKD23 or a higher prevalence of hypertension or diabetes mellitus among African Americans.24,25 In a case series by Rostand et al3 and in the Multiple Risk Factor Intervention Trial,4 African Americans had faster CKD progression than persons of white race/ethnicity, despite similar levels of BP control; however, these studies were conducted before ACEI therapy and at a time when a conventional BP goal was implemented. In a recent observational analysis that combined data from the Third National Health and Nutrition Examination Survey and the US Renal Data System, the prevalence of CKD was similar among African Americans and among persons of white race/ethnicity, but estimated progression rates among those with CKD were 5-fold higher among African Americans.23 Because AASK did not enroll persons of white race/ethnicity, our study cannot directly compare CKD progression rates by race/ethnicity. However, it seems reasonable to speculate that the substantial burden of ESRD among African Americans results in part from continued progression of CKD to ESRD, which still occurs in the setting of recommended antihypertensive drug therapy.
Our results do not alter current recommendations for the use of ACEIs (or ARBs) and a low BP goal in persons with hypertensive CKD. The rate of CKD progression would likely have been even greater without RAS-blocking therapy. Although the benefit of a low BP goal on CKD progression is not supported by results from major clinical trials, including AASK, recommendations for a low BP goal are prudent given the substantial burden of vascular disease in patients with CKD26 and the direct relationship between BP and CKD in observational investigations.27 Still, our data reveal a sobering picture. In the setting of a research study that provided RAS-blocking therapy and that achieved BP levels close to recommended goals, CKD continued to progress in most African Americans with hypertensive CKD. Rates of progression would likely be greater in the community setting, where RAS-blocking therapy is used less frequently28and where achieved BP levels are much higher.29 On the other hand, 33.5% of participants experienced an annual slow decline in the estimated GFR of less than 1 ml/min/1.73 m2. Additional analyses may provide insights into factors associated with preserved kidney function.
In conclusion, our study demonstrated that most African Americans with hypertensive CKD who are treated with currently recommended BP therapy continue to progress during the long term. These results highlight the importance of preventing initial kidney damage, the critical need to identify modifiable risk factors, and the requirement to test promising therapies at the earliest stages of CKD. Candidate therapies include nocturnal BP reduction,30 aldosterone blockade,31 combined ACEIARB therapy,32 fish oil supplementation,33 bicarbonate therapy,34 and sodium reduction,35,36 which reduces pro-inflammatory profibrosis cytokines, chemokines, or oxidant stress.
Acknowledgments
Funding/Support: This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases, by the National Center for Minority Health and Health Disparities, and by King Pharmaceuticals Company.
Financial Disclosures: Dr Wright reports research grants from AstraZeneca, GlaxoSmithKline, Novartis, and Pfizer; consultant relationships with AstraZeneca, GlaxoSmith Kline, Merck, Novartis, Pfizer, King Pharmaceuticals, Encysive, Bayer, and Bristol-Myers Squibb; and honoraria from GlaxoSmithKline, Novartis, and Pfizer. Dr Lewis reports research grants from Bristol-Myers Squibb, Sanofi, Amgen, Roche, Keryx, Genzyme, Pfizer, Boehringer-Ingelheim, AstraZeneca, Bayer, Wyeth-Ayerst, Scios Nova, and Hoechst-Roussel and honoraria from Bristol-Myers Squibb, Sanofi, Merck, AstraZeneca, and Boehringer-Ingelheim. Dr Norris reports consultant and speaker relationships with King Pharmaceuticals, Abbott, Amgen, Roche, and Merck. Dr Bakris reports consultant, speakers board, and advisory board relationships with Abbott, Boehringer-Ingelheim, Bristol-Myers Squibb, Sanofi-Aventis, Forest, GlaxoSmithKline, Merck, Novartis, Walgreens (formulary committee), Gileada, and Sankyo and research grants from GlaxoSmithKline, Gileada, Forest, and Sanofi. Dr Rahman reports honoraria from Boehringer-Ingelheim. Dr Kopple reports membership in the Hypertension Stakeholders Group. Dr Gabbai reports consultant relationships with Bristol-Myers Squibb and Novartis and honoraria from Bristol-Myers Squibb and Novartis.
Role of the Sponsors: Representatives of the National Institute of Diabetes and Digestive and Kidney Diseases (Drs Kusek and Agodoa) contributed to the design of the study, interpretation of the data, and review of the manuscript. The National Institute of Diabetes and Digestive and Kidney Diseases also provided administrative support.
AASK Collaborative Group Members
Case Western Reserve University: Principal Investigators: J. Wright, M. Rahman, Study Coordinators: R. Dancie, T. Moore; Emory University, Atlanta, Georgia: Principal Investigator: J. Lea, Study Coordinators: B. Wilkening, A. Chapman, D. Watkins; Harbor-UCLA Medical Center: Principal Investigator: J. Kopple, Study Coordinators: L. Miladinovich, J. Choi, P. Oleskie; Harlem Hospital Center, New York: Principal Investigator: V. Pogue, Study Coordinators: D. Dowie, H. Anderson, L. Herbert, R. Locko, H. Nurse, J. Cheng, G. Darkwa, V. Dowdy, B. Nicholas; Howard University, Washington, DC: Principal Investigators: O. Randall, T. Retta, Study Coordinators: S. Xu, M. Ketete, D. Ordor, C. Tilghman; Johns Hopkins University: Principal Investigators: E. Miller, B. Astor, Study Coordinators: C. Diggs, J. Charleston, C. Harris; Martin Luther King, Jr/Charles R. Drew Medical Center: Principal Investigators: K. Norris, H. Ward, D. Martins, N. Tareen, Study Coordinators: M. Miller, H. Howell, L. Pitts; Medical University of South Carolina, Charleston: Principal Investigator: D. Cheek, Study Coordinator: D. Brooks; Meharry Medical College, Nashville: Principal Investigators: M. Faulkner,O. Adeyele, Study Coordinators: K. Phillips, G. Sanford, C. Weaver; Morehouse School of Medicine, Atlanta: Principal Investigators: W. Cleveland, K. Chapman, Study Coordinator: W. Smith; Lenox Hill–Mount Sinai School of Medicine: Principal Investigators: R. Phillips, M. Lipkowitz, Study Coordinators: A. Gabriel, E. Condren; Ohio State University, Columbus: Principal Investigators: L. Hebert, G. Shidham, Study Coordinators: L. Hiremath, S. Justice; Rush Presbyterian–St Luke’s Medical Center, Chicago: Principal Investigators: G. Bakris, J. Lash, Study Coordinators: L. Fondren, L. Bagnuolo, J. Cohan, A. Frydrych; University of Alabama, Birmingham: Principal Investigators: S. Rostand, D. Thornley-Brown, Study Coordinator: B. Key; University of California, San Diego: Principal Investigators: F. B. Gabbai, D. T. O’Connor, F. Rao, J. Little, T. Makrogiannis, Study Coordinator: B. Thomas; University of Florida, Gainesville: Principal Investigators: C. C. Tisher, G. Bichier, Study Coordinators: C. Sarmiento, A. Diaz, C. Gordon; University of Miami: Principal Investigators: G. Contreras, J. Bourgoignie, D. Florence-Green, Study Coordinators: J. Junco, J. Vassallo; University of Michigan, Ann Arbor: Principal Investigators: K. Jamerson, A. Ojo, Study Coordinators: D. Cornish-Zirker, T. Graham, W. Bloembergen, T. Corbin; University of Southern California, Los Angeles: Principal Investigators: S. Massry, M. Smogorzewski, Study Coordinator: A. Richardson; University of Texas Southwestern Medical Center, Dallas: Principal Investigators: R. Toto, G. Peterson, R. Saxena, Study Coordinators: T. Lightfoot, S. A. Blackstone, C. Loreto; Vanderbilt University: Principal Investigators: J. Lewis, G. Schulman, Study Coordinators: M. Sika, S. McLeroy; National Institute of Diabetes and Digestive and Kidney Diseases: L. Y. Agodoa, J. P. Briggs, J. W. Kusek; Steering Committee Chair: L. Appel; Data Coordinating Center (Cleveland Clinic Foundation): J. Gassman, G. Beck, T. Greene, Study Coordinators: K. Brittain, S. Sherer, L. Tuason, C. Kendrick, S. Bi, H. Litowitz, X. Liu, X. Wang, K. Wiggins, C. A. Tatum; Central Biochemistry Laboratory: F. Van Lente, J. Waletzky, C. O’Laughlin, L. Burton; External Advisory Committee: W. McClellan, L. Adams-Campbell, K. Faber-Langendoen, E. Lee, T. Meyer, H. Taylor, P. W. Wilson.
Footnotes
Author Contributions: Dr Appel had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Appel, Wright, Greene, Kusek, Lewis, Wang, Lipkowitz, Norris, Bakris, Rahman, Contreras, Rostand, Kopple, Gabbai, Schulman, Gassman, Charleston, and Agodoa. Acquisition of data: Appel, Wright, Lewis, Lipkowitz, Norris, Bakris, Rahman, Contreras, Rostand, Kopple, Gabbai, Schulman, and Charleston. Analysis and interpretation of data: Appel, Wright, Greene, Kusek, Lewis, Wang, Lipkowitz, Norris, Bakris, Rahman, Contreras, Rostand, Kopple, Gabbai, Schulman, Gassman, Charleston, and Agodoa. Drafting of the manuscript: Appel, Wright, Greene, Kusek, Lewis, Wang, Lipkowitz, Norris, Bakris, Rahman, Contreras, Rostand, Kopple, Gabbai, Schulman, Gassman, Charleston, and Agodoa. Statistical analysis: Greene, Wang, and Gassman. Study supervision: Appel, Wright, Greene, Kusek, Lewis, Wang, Lipkowitz, Norris, Bakris, Rahman, Contreras, Rostand, Kopple, Gabbai, Schulman, Gassman, Charleston, and Agodoa.
Previous Presentation: This study was presented at Renal Week 2006: American Society of Nephrology Annual Meeting; November 17, 2006; San Diego, California.
Additional Contributions: The AASK Collaborative Group appreciates the sustained commitment of its participants and staff.
REFERENCES
- 1.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the Third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161(9):1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System, USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health; 2005. [Google Scholar]
- 3.Rostand SG, Brown G, Kirk KA, Rutsky EA, Dustan HP. Renal insufficiency in treated essential hypertension. N Engl J Med. 1989;320(11):684–688. doi: 10.1056/NEJM198903163201102. [DOI] [PubMed] [Google Scholar]
- 4.Walker WG, Neaton JD, Cutler JA, Neuwirth R, Cohen JD. MRFIT Research Group. Renal function change in hypertensive members of the Multiple Risk Factor Intervention Trial: racial and treatment effects. JAMA. 1992;268(21):3085–3091. [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 6.Kidney Disease Outcomes Quality Initiative (K/DOQI). K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(5) suppl 1:S1–S29. [PubMed] [Google Scholar]
- 7.Jafar TH, Stark PC, Schmid CH, et al. Progression of chronic kidney disease: the role of blood pressure control, proteinuria, and angiotensin-converting enzyme inhibition: a patient-level meta-analysis. Ann Intern Med. 2003;139(4):244–252. doi: 10.7326/0003-4819-139-4-200308190-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Gherardi G, Gaspari F, Benini R, Remuzzi G. Lancet. 1998;352(9136):1252–1256. doi: 10.1016/s0140-6736(98)04433-x. [DOI] [PubMed] [Google Scholar]
- 9.Agodoa LY, Appel L, Bakris GL, et al. African American Study of Kidney Disease and Hypertension (AASK) Study Group. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 10.Wright JT, Jr, Bakris G, Greene T, et al. African American Study of Kidney Disease and Hypertension Study Group. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288(19):2421–2431. doi: 10.1001/jama.288.19.2421. [published correction appears in JAMA. 2006; 295(23):2726]. [DOI] [PubMed] [Google Scholar]
- 11.Gassman JJ, Greene T, Wright JT, Jr, et al. Design and statistical aspects of the African American Study of Kidney Disease and Hypertension (AASK) J Am Soc Nephrol. 2003;14(7) suppl 2:S154–S165. doi: 10.1097/01.asn.0000070080.21680.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Appel LJ, Middleton J, Miller ER, III, et al. The rationale and design of the AASK cohort study. J Am Soc Nephrol. 2003;14(7) suppl 2:S166–S172. doi: 10.1097/01.asn.0000070081.15137.c0. [DOI] [PubMed] [Google Scholar]
- 13.Sika M, Lewis J, Douglas J, et al. AASK Group. Baseline characteristics of the African American Study of Kidney Disease and Hypertension (AASK) clinical trial and cohort study. Am J Kidney Dis. 2007;50(1):78–89. doi: 10.1053/j.ajkd.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Lewis J, Agodoa L, Cheek D, et al. Comparison of cross-sectional renal function measurements in African Americans with hypertensive nephrosclerosis and of primary formulas to estimate glomerular filtration rate. Am J Kidney Dis. 2001;38(4):744–753. doi: 10.1053/ajkd.2001.27691. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Lewis J, Appel L, et al. Validation of creatinine-based estimates of GFR when evaluating risk factors in longitudinal studies of kidney disease. J Am Soc Nephrol. 2006;17(10):2900–2909. doi: 10.1681/ASN.2005101106. [DOI] [PubMed] [Google Scholar]
- 16.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Berlin, Germany: Springer-Verlag; 1997. [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 18.Kalbfleish JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York, NY: John Wiley & Sons Inc; 1980. [Google Scholar]
- 19.Jafar TH, Schmid CH, Landa M, et al. Angiotensin-converting enzyme inhibitors and progression of nondiabetic renal disease: a meta-analysis of patient-level data. Ann Intern Med. 2001;135(2):73–87. doi: 10.7326/0003-4819-135-2-200107170-00007. [DOI] [PubMed] [Google Scholar]
- 20.Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med. 2006;354(2):131–140. doi: 10.1056/NEJMoa053107. [DOI] [PubMed] [Google Scholar]
- 21.Klahr S, Levey AS, Beck GJ, et al. Modification of Diet in Renal Disease Study Group. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330(13):877–884. doi: 10.1056/NEJM199403313301301. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70(6):1000–1007. doi: 10.1038/sj.ki.5001695. [DOI] [PubMed] [Google Scholar]
- 23.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 24.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks: a population-based study of potential explanatory factors. JAMA. 1992;268(21):3079–3084. [PubMed] [Google Scholar]
- 25.McClellan W, Tuttle E, Issa A. Racial differences in the incidence of hypertensive end-stage renal disease (ESRD) are not entirely explained by differences in the prevalence of hypertension. Am J Kidney Dis. 1988;12(4):285–290. doi: 10.1016/s0272-6386(88)80221-x. [DOI] [PubMed] [Google Scholar]
- 26.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 27.Klag MJ, Whelton PK, Randall BL, et al. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334(1):13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 28.Hemmelgarn BR, Zhang J, Manns BJ, et al. Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int. 2006;69(12):2155–2161. doi: 10.1038/sj.ki.5000270. [DOI] [PubMed] [Google Scholar]
- 29.Hyman DJ, Pavlik VN. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345(7):479–486. doi: 10.1056/NEJMoa010273. [DOI] [PubMed] [Google Scholar]
- 30.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166(8):846–852. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 31.Hostetter TH, Ibrahim HN. Aldosterone in chronic kidney and cardiac disease. J Am Soc Nephrol. 2003;14(9):2395–2401. doi: 10.1097/01.asn.0000086472.65806.73. [DOI] [PubMed] [Google Scholar]
- 32.Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination Treatment of Angiotensin-II Receptor Blocker and Angiotensin Converting-Enzyme Inhibitor in Non-diabetic Renal Disease (COOPERATE): a randomised controlled trial. Lancet. 2003;361(9352):117–124. doi: 10.1016/S0140-6736(03)12229-5. [published correction appears in Lancet. 2003;361(9364):1230]. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu H, Ohtani K, Tanaka Y, Sato N, Mori M, Shimomura Y. Long-term effect of eicosapentaenoic acid ethyl (EPA-E) on albuminuria of non–insulin dependent diabetic patients. Diabetes Res Clin Pract. 1995;28(1):35–40. doi: 10.1016/0168-8227(95)01056-j. [DOI] [PubMed] [Google Scholar]
- 34.Torres VE, Cowley BD, Jr, Branden MG, Yoshida I, Gattone VH. Long-term ammonium chloride or sodium bicarbonate treatment in two models of polycystic kidney disease. Exp Nephrol. 2001;9(3):171–180. doi: 10.1159/000052609. [DOI] [PubMed] [Google Scholar]
- 35.Tian N, Thrasher KD, Gundy PD, Hughson MD, Manning RD., Jr Antioxidant treatment prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Hypertension. 2005;45(5):934–939. doi: 10.1161/01.HYP.0000160404.08866.5a. [DOI] [PubMed] [Google Scholar]
- 36.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD., Jr Renal NF-κB activation and TNF-α up-regulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2006;291(6):R1917–R1824. doi: 10.1152/ajpregu.00153.2006. [DOI] [PubMed] [Google Scholar]