Abstract
Objective
In familial hypobetalipoproteinemia (FHBL), fatty liver is a characteristic feature, and there are several reports of associated cirrhosis and hepatocarcinoma. We investigated a large kindred in which low-density lipoprotein (LDL) cholesterol, fatty liver and hepatocarcinoma displayed an autosomal dominant pattern of inheritance.
Approach and Results
The proband was a 25 year-old female with low plasma cholesterol and hepatic steatosis. Low plasma levels of total cholesterol and fatty liver were observed in 10 more family members; 1 member was affected by liver cirrhosis and four more subjects died of either hepatocarcinoma or carcinoma on cirrhosis. To identify the causal mutation in this family, we performed exome sequencing in two participants with hypocholesterolemia and fatty liver. Approximately 22,400 single nucleotide variants were identified in each sample. After variant filtering, 300 novel shared variants remained. A nonsense variant, p.K2240X due to an A>T mutation in exon 26 of APOB (c.6718A>T) was identified and this variant was confirmed by Sanger sequencing. The gentotypic analysis of 16 family members in total showed that this mutation segregated with the low cholesterol trait. In addition, genotyping of the PNPLA3 p.I148M did not show significant frequency differences between carriers and non-carriers of the c.6718A>T APOB gene mutation.
Conclusions
We used exome sequencing to discover a novel nonsense mutation in exon 26 of APOB (p.K2240X) responsible for low cholesterol and fatty liver in a large kindred.
This mutation may also be responsible for cirrhosis and liver cancer in this family.
Keywords: Exome sequencing, FHBL, fatty liver, Hepatocarcinoma
Introduction
HBL (hypobetalipoproteinemia) represents a heterogeneous group of disorders characterized by reduced plasma levels of total cholesterol (TC), low-density lipoprotein-cholesterol (LDL-C) and apolipoprotein B (apoB) below the 5th percentile of the distribution in the population [1–2]. Familial hypobetalipoproteinemia (FHBL – OMIM 107730) is the most frequent monogenic form of HBL. It may be due to loss-of-function mutations in APOB or, less frequently, in PCSK9 genes [1–5].
The best-characterized form of FHBL occurs with dominant inheritance (approximately 50% of FHBL) and has been linked to heterozygous pathogenic mutations in the apolipoprotein B (APOB) gene [1]. Most APOB gene mutations lead to the formation of truncated apolipoprotein B (apoB) protein of various sizes which, to a variable extent, lose the capacity to form plasma lipoproteins in liver and/or intestine and to export lipids from these organs [1–2]. Missense non-truncating mutations of the APOB gene can be also cause of FHBL [6–7] and are associated with a decreased secretion of the mutant apoBs because of an increased binding to MTP [6–8].
As a consequence of impaired hepatic export of lipoproteins (VLDL), subjects with FHBL due either to truncating or non-truncating mutations of the APOB gene are prone to hepatic steatosis (9–10). In these subjects the presence of fatty liver has been documented by abdominal ultrasound examination, magnetic resonance, or liver biopsy [9, 11–14]. Anecdotal reports have documented an association between fatty liver and steatohepatitis, liver cirrhosis and hepatocarcinoma in FHBL patients [15–17].
We studied a large family where we observed an autosomal dominant pattern of low plasma cholesterol cosegregating with fatty liver and hepatocarcinoma. We hypothesized the presence in this family of a genetic susceptibility for cancer which cosegregates with a causal mutation of FHBL. In order to identify the causal mutation in this family, we performed exome sequencing, an approach that allows the identification of all the coding variants present in affected family members.
Material and Methods
Materials and Methods are available in the online-only Supplement.
Results
Phenotype
DNA samples were available from 16 members across two generations. Lipid profiles, including apoB levels and clinical characteristics of the family are presented in Table 1. The proband (subject IV-5) showed low levels of TC, TG and LDL-C; low LDL-C levels were found in 9 more subjects of the family with a dominant transmission mode of inheritance (Table 1 and Figure 1). Moreover 7 out of 10 subjects with low cholesterol levels showed fatty liver as determined by liver ultrasonography (Table 1)
Table 1.
Clinical characteristics, plasma lipids and apolipoprotein B.
| Subject | Age (years) |
TC (mg/dL) |
TG (mg/dL) |
HDL-C (mg/dL) |
ApoB (mg/L) |
LDL-C (mg/dl) |
BMI (Kg/m2) |
Fatty Liver |
Type of severe liver disease |
APOB genotype |
Other Clinical Data |
|---|---|---|---|---|---|---|---|---|---|---|---|
| II:1 | † | NA | NA | NA | NA | NA | NA | NA | Hepatocarcinoma on cirrhosis | NA | |
| II:5 | † | NA | NA | NA | NA | NA | NA | NA | Hepatocarcinoma on cirrhosis | NA | |
| III:1 | 63 | 103 | 64 | 48 | 43 | 42,2 | 28 | Yes | Not present | K2240X | |
| III:3 | † | NA | NA | NA | NA | NA | NA | NA | Hepatocarcinoma | NA | |
| III:5 | 60 | 234 | 78 | 45 | NA | 173,4 | 27 | NA | Not present | WT | |
| III:7 | 58 | 136 | 129 | 42 | 55 | 68,2 | 28 | Yes | Not present | K2240X | |
| III:9 | 50 | 140 | 40 | 76 | NA | 56 | 24.7 | NA | Not present | K2240X | |
| III:11 | † | NA | NA | NA | NA | NA | NA | Yes | Hepatocarcinoma | NA | Referred Hypocholesterolemia |
| III:13 | 50 | 121 | 94 | 52 | 37 | 50,2 | 22.5 | Yes | Not present | K2240X | |
| III:14 | 59 | 222 | 115 | 57 | NA | 142 | 26.4 | NA | Not present | WT | |
| III:15 | 66 | 220 | 91 | 66 | NA | 135,8 | 28.6 | Yes | Not present | WT | Type 2 diabetes |
| III:16 | 60 | 110 | 39 | 63 | 34 | 39,2 | 24 | Yes | Liver cirrhosis | K2240X | |
| IV:1 | 30 | 107 | 58 | 43 | 53 | 52,4 | 25 | No | Not present | K2240X | |
| IV:2 | 33 | 165 | 145 | 76 | 66 | 60 | 27.6 | No | Not present | WT | |
| IV:3 | 42 | 128 | 31 | 90 | 24 | 31,8 | 26.3 | No | Not present | K2240X | |
| IV:4 | 31 | 174 | 69 | 58 | 23 | 102,2 | NA | No | Not present | WT | |
| IV:5 | 25 | 86 | 44 | 48 | 26 | 29,2 | 22 | Yes | Not present | K2240X | “Non Organic” Seizures |
| IV:6 | 24 | 103 | 41 | 42 | 39 | 52,8 | 24.2 | Yes | Not present | K2240X | Hodgkin Lymphoma |
| IV:7 | 39 | 118 | 57 | 65 | 31 | 41,6 | 24.1 | Yes | Not present | K2240X | “Non Organic” Seizures |
| IV:8 | 37 | 141 | 63 | 62 | 57 | 66,4 | 21.1 | No | Not present | WT |
Abbreviations and symbols: †: deceased; TC: Total Cholesterol; TG: Triglycerides; HDL-C: HDL-Cholesterol; ApoB: Apolipoprotein B; BMI: Body Mass Index; WT: wilde-type; NA: not available
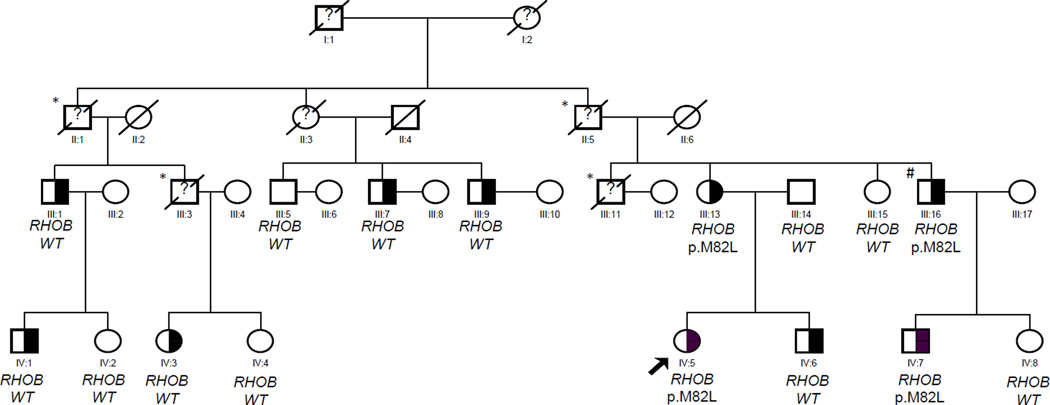
Figure 1.
Pedigree of the Family with Familial Hypobetalipoproteinemia. The proband is indicated by an arrow. Half filled symbol: affected subjects carriers of the mutation K2240X of the APOB gene; Empty symbol: unaffected subjects.
RHOB: ras homolog gene family, member B precursor; WT: wild-type
The ? indicate that the cholesterol phenotype is unknown
Subjects marked by an * died of hepatocarcinoma.
Subject#: liver cirrhosis
Exome Sequencing
The two samples that underwent exome sequencing each yielded 8.25 gigabases of sequence, with an average depth of coverage of approximately 250 reads per targeted base. Approximately 22,400 single nucleotide variants were identified in each sample, of which 15,237 passed the previously described filters and were shared by both samples. After removing variants also identified in pilot 1 of the 1000 Genomes project, 1,509 shared variants remained. After removing variants identified in three unrelated samples ascertained due to a non-LDL-C-related lipoprotein phenotype (hyperalphalipoproteinemia), 300 novel shared variants remained. Of these, 112 were synonymous, 177 were missense, 4 were nonsense, and 7 were at splice sites. Only one nonsense variant was within +/− 300 kilobases of lead SNPs in genomic regions associated with LDL-C in a recent large-scale genome-wide association study [18]: p.K2240X due to an A>T mutation in exon 26 of APOB (c.6718A>T), which encodes apolipoprotein B.
The search for shared variants in the 2cM region encompassing the c.6718A>T mutation of the APOB gene also revealed an A>C mutation in exon 1 of the RHOB (ras homolog gene family, member B precursor) gene (c.244A>C – p.M82L).
Sanger sequencing
The presence of the nonsense mutation in exon 26 (c.6718A>T - p.K2240X) was confirmed in 3 independent PCR amplifications and direct sequencing in the proband (subject IV-5). The proband’s mother (subject III-13) was found to carry the same mutation in the heterozygous state. Sanger sequencing of the same APOB gene region of exon 26 encompassing the mutation performed in the other 14 available family members confirmed that the nonsense mutation segregated with the low cholesterol trait (Figure 1).
There were 16 individuals in the family who had both plasma lipids phenotype and DNA available for genotyping. Of these 16 individuals, 10 were affected on clinical grounds (TC below the 5th percentile). All ten affecteds carried the APOB nonsense mutation. Of the six individuals who were unaffected (TC > 5th percentile), none carried the APOB nonsense mutation
The direct sequencing of the region of exon 1 of RHOB gene encompassing the c.244A>C variant showed that beside subjects III-13 and IV-15, two other family members were heterozygous carriers of the variant p.M82L (II-16 and IV-7) (Figure 1).
To predict the effect of this amino acid change on protein function we performed in silico analyses by using different algorithms: PolyPhen (www.bork.embl-heidelberg.de/PolyPhen/), SIFT (http://sift.jcvi.org/) and Mutationtaster (http://www.mutationtaster.org/). The PolyPhen and SIFT algorithms gave comparable results, indicating that the p.M82L (Polyphen score: 0.002, SIFT score: 0.11) amino acid substitution had a “benign effect”, whereas the Mutationtaster software predicted the p.M82L missense mutation to be damaging (Score: 15).
Association of PNPLA3 SNP rs738409 (I148M) with Hepatic Steatosis
As shown in Table 2, there were no significant differences in either PNPLA3 rs738409 minor allele (G) frequency allele or genotype frequencies between carriers and non carriers of the c.6718A>T APOB gene mutation.
Table 2.
PNPLA3 SNP rs738409 (I148M) in carriers and non-carriers of the c.6718A>T APOB gene mutation
| Subjects | n | PNPLA3 genotype | PNPLA3 allele frequency |
P | |||
|---|---|---|---|---|---|---|---|
| C/C n (%) |
C/G n (%) |
G/G n (%) |
C | G | |||
| c.6718A>T APOB gene mutation carriers | 10 | 2 (20) | 8 (80) | 0 (0) | 0.6 | 0.4 | NS |
| c.6718A>T APOB gene mutation carriers with fatty liver | 7 | 1 (14) | 6 (86) | 0 (0) | 0.57 | 0.43 | NS |
| Non carriers of c.6718A>T APOB gene mutation | 6 | 2 (33) | 3 (50) | 1 (17) | 0.58 | 0.42 | NS |
NS: not significant
Discussion
In the present study, we describe a large family where low plasma cholesterol, fatty liver, and hepatocarcinoma cosegregate in an autosomal dominant pattern. Using whole exome sequencing, we discovered that a novel nonsense mutation in exon 26 of the APOB gene (c.6718A>T, p.K2240X) segregates with low lipids and the liver phenotypes.
A large number of APOB gene mutations truncating ApoB have been reported to be the cause of FHBL, and novel mutations are continually being identified in FHBL subjects [19]. FHBL heterozygotes are generally asymptomatic but most of them develop fatty liver [19]. In fact, individuals heterozygous for inactivating mutations in APOB show impaired VLDL particle metabolism and have a threefold increase in hepatic TG relative to healthy individuals [10].
Earlier in vivo turnover studies have shown that effectiveness of lipid secretion from the liver depends on apoB length [20] implying that a variable amount of lipids might accumulate in the hepatocytes.of FHBL carriers of different truncated apoBs. It was also suggested that fatty liver always develops in FHBL carriers of short and medium-size truncated apoBs (<apoB-48), while other additional environmental factors are needed in carriers of longer apoB forms [21].
However, more recent data have shown that there is no evidence that the size of apoB truncation could be associated with a different degree of liver impairment. For instance in the patients cohort studied by Sankatsing et al. [22], hepatic steatosis was not more severe in patients carrying short truncated apoBs not secreted into the plasma compared with carriers of longer truncations.
Even if fatty liver in FHBL has been considered “per se” a benign condition a potential evolution to more severe forms of liver diseases such as steatohepatitis, cirrhosis or liver carcinoma appears to be a relevant clinical issue. To date, only a few case reports on the association between FHBL and severe liver diseases have been published.
Lonardo et al, described a case of hepatocarcinoma without cirrhosis in a subject with FHBL due to a truncated form of Apo B [17]. The liver histology in this patient revealed a moderate degree of steatosis and fibrosis outside the hepatocarcinoma lesion and the authors speculated that environmental factors (such as alcohol and smoking) could trigger the evolution of fatty liver due to FHBL. More recently, Bonnefont-Rousselot et al., have described a patient with FHBL and liver cirrhosis due to a truncated form of ApoB [16]. The liver biopsy revealed typical hepatic cirrhosis with irregular nodules and macrovacuolar steatosis. In this case, classical causes of fatty liver and cirrhosis were excluded by a comprehensive clinical, biological, and histological work-up.
To our knowledge, our observation is the first description of the co-occurrence of FHBL, fatty liver, cirrhosis, and liver cancer. In particular, participants II:1, II:5, III:3 and III:11 died of hepatocarcinoma. Furthermore, in participant III:11, the histology of the liver tumor revealed a rare finding of fibrolamellar hepatocellular carcinoma. In fact, fibrolamellar carcinoma is a rare primary malignant liver tumor with distinctive histology that usually affects adolescents and young adults with a nearly even sex distribution and most patients have no identifiable liver disease secondary to chronic infection with HBV or HCV [for review see 23].
An interesting question deals with the identification of factors that could elicit a progression of fatty liver due to FHBL to cirrhosis and liver cancer. Although environmental and lifestyle influences are well known and prevalent potential contributors of progression of fatty liver, other molecular processes may contribute to this condition. Recently, variants in genes affecting lipid metabolism, oxidative stress, insulin resistance, and immune regulation could act as predisposing factors to the development of hepatic steatosis and the development of progressive liver injury [for reviews see 24–25]. Among these, one genetic variant that has consistently been associated in many independent studies with non-alcoholic fatty liver disease is a missense mutation [Ile148→Met148 (p.I148M)] in patatin-like phospholipase domain–containing (PNPLA) 3 gene PNPLA3 [29].
Moreover this PNPLA3 variant is not only associated with hepatic steatosis but also to non-alcoholic steatohepatitis and cirrhosis and these data provides strong molecular evidence of the importance of genetic factor s on the progression of fatty liver to more severe forms of hepatic diseases. [26–28]. Among the APOB mutation carriers individuals with the PNPLA3 p.I148M variant in this kindred did not show a higher susceptibility for fatty liver, suggesting that in this family the PNPLA3 gene does not act as a predisposing factor to the development of hepatic steatosis and the development of progressive liver injury.
In an attempt to identify other genetic determinants that could contribute to the progression of liver disease in this family, we searched for shared variants in the 2cM region encompassing the c.6718A>T mutation of the APOB gene. This analysis revealed an A>C mutation in exon 1 of the RHOB gene (c.244A>C – p.M82L).
Ras-homologous (Rho) small GTPases, are involved in the regulation of a variety of cellular processes and recent studies further confirmed the role of the Rho proteins in cancer by showing their involvement in cell transformation, invasion, metastasis and angiogenesis [29].
In particular RhoB has a tumour-suppressive role, including inhibition of cell proliferation and induction of apoptosis in several human cancer cells, and inhibition of tumour growth in a nude mouse xenograft model [30]. Furthermore, RhoB is inducible by genotoxic stress, such as UV light, some growth factors (TGFbeta) and chemotherapeutic drugs (cisplatin and 5-FU) [31].
The results of genotyping of the p.M82L RhoB variant showed no cosegregation whit the APOB gene mutation found to be responsible for the FHBL phenotype. However it is interesting to note that the four carriers of the p.M82L variant are also carriers of the c.6718A>T mutation of the APOB gene and are all affected by fatty liver; in addition one of them (subject III:16) developed cirrhosis.
The clinical and genetic findings from this large kindred suggest that the complex relationship between APOB mutations responsible of FHBL and the clinical and pathological sequelae of fatty liver accumulation requires further mechanistic studies.
Supplementary Material
Significance.
Familial hypobetalipoproteinemia (FHBL) is a genetic disorder characterized by <th percentile plasma levels of total cholesterol, LDL-cholesterol, and apolipoprotein B (apoB). Most of FHBL cases are due to mutations in APOB gene leading to defective hepatic secretion of apoB-containing lipoproteins (VLDL). This results in an impaired export of triglycerides causing fatty liver accumulation and hepatic steatosis. Few case reports have documented the association of FHBL with steatohepatitis, liver cirrhosis and hepatocarcinoma. Here we describe a large kindred in which a novel mutation of APOB gene (p.K2240X), identified by exome sequencing, cosegregates with hypobetalipoproteinemia, fatty liver and hepatocarcinoma in an autosomal dominant pattern. We also found a variant in a tumor suppressor gene (RHOB), but no cosegregation was found with the lipid and hepatic phenotypes. In addition, genotyping of the PNPLA3 p.I148M does not show frequency differences between carriers and non-carriers of the APOB gene mutation.
Acknowledgements
We are indebted to the patients and their family for their cooperation in this study.
Sources of Funding
This work was supported by contract grants from the University of Palermo (60% to M.R.A. and A.B.C.), Grant 2009-RLKXPF “PRIN 2009” from the Italian Ministry of Education, University and Research (to M.R.A.), the “Fondazione Cassa di Risparmio di Modena” (to P.T.); J.P. was supported by the Sarnoff Cardiovascular Research Foundation. S.K. is supported by a Howard Goodman Fellowship and a Research Scholar Award from the Massachusetts General Hospital, the Donovan Family Foundation, and R01 HL-107816 from the U.S. National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
None
References
- 1.Tarugi P, Averna M, Di Leo E, Cefalù AB, Noto D, Magnolo L, Cattin L, Bertolini S, Calandra S. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007;195:e19–e27. doi: 10.1016/j.atherosclerosis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 2.Schonfeld G. Familial hypobetalipoproteinemia: a review. J. Lipid Res. 2003;44:878–883. doi: 10.1194/jlr.R300002-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat. Genet. 2005;37:161–165. doi: 10.1038/ng1509. [DOI] [PubMed] [Google Scholar]
- 4.Fasano T, Cefalù AB, Di Leo E, Noto D, Pollaccia D, Bocchi L, Valenti V, Bonardi R, Guardamagna O, Averna M, Tarugi P. A novel loss of function mutation of PCSK9 gene in white subjects with low-plasma low-density lipoprotein cholesterol. Arterioscler Thromb Vasc Biol. 2007;27:677–681. doi: 10.1161/01.ATV.0000255311.26383.2f. [DOI] [PubMed] [Google Scholar]
- 5.Miyake Y, Kimura R, Kokubo Y, Okayama A, Tomoike H, Yamamura T, Miyata T. Genetic variants in PCSK9 in the Japanese population: rare genetic variants in PCSK9 might collectively contribute to plasma LDL cholesterol levels in the general population. Atherosclerosis. 2008;196:29–36. doi: 10.1016/j.atherosclerosis.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Burnett JR, Shan J, Miskie BA, Whitfield AJ, Yuan J, Tran K, McKnight CJ, Hegele RA, Yao Z. A novel nontruncating APOB gene mutation, R463W, causes familial hypobetalipoproteinemia. J. Biol. Chem. 2003;278:13442–13452. doi: 10.1074/jbc.M300235200. [DOI] [PubMed] [Google Scholar]
- 7.Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, Zhao Y, Barrett PH, Hegele RA, van Bockxmeer FM, Zhang H, Vance DE, McKnight CJ, Yao Z. Missense mutations in APOB within the βα1 domain of human ApoB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J. Biol Chem. 2007;282:24270–24283. doi: 10.1074/jbc.M702442200. [DOI] [PubMed] [Google Scholar]
- 8.Zhong S, Magnolo AL, Sundaram M, et al. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J Biol Chem. 2010;285(9):6453–6464. doi: 10.1074/jbc.M109.060467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarugi P, Lonardo A, Ballarini G, Grisendi A, Pulvirenti M, Bagni A, Calandra S. Fatty liver in heterozygous hypobetalipoproteinemia caused by a novel truncated form of apolipoprotein B. Gastroenterology. 1996;111:1125–1133. doi: 10.1016/s0016-5085(96)70082-3. [DOI] [PubMed] [Google Scholar]
- 10.Schonfeld G, Patterson BW, Yablonskiy DA, Tanoli TS, Averna M, Elias N, Yue P, Ackerman J. Fatty liver in familial hypobetalipoproteinemia: triglyceride assembly into VLDL particles is affected by the extent of hepatic steatosis. J Lipid Res. 2003;44:470–478. doi: 10.1194/jlr.M200342-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Tarugi P, Lonardo A, Gabelli C, Sala F, Ballarini G, Cortella I, Previato L, Bertolini S, Cordera R, Calandra S. Phenotypic expression of familial hypobetalipoproteinemia in three kindreds with mutations of apolipoprotein B gene. J Lipid Res. 2001;42:1552–1561. [PubMed] [Google Scholar]
- 12.Yue P, Tanoli T, Wilhelm O, Patterson B, Yablonskiy D, Schonfeld G. Absence of fatty liver in familial hypobetalipoproteinemia linked to chromosome 3p21. Metabolism. 2005;54:682–688. doi: 10.1016/j.metabol.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Tanoli T, Yue P, Yablonskiy D, Schonfeld G. Fatty liver in familial hypobetalipoproteinemia: roles of the APOB defects, intra-abdominal adipose tissue, and insulin sensitivity. J Lipid Res. 2004;45:941–947. doi: 10.1194/jlr.M300508-JLR200. [DOI] [PubMed] [Google Scholar]
- 14.Sundaram M, Yao Z. Recent progress in understanding protein and lipid factors affecting hepatic VLDL assembly and secretion. Nutr Metab. 2010;7:35–51. doi: 10.1186/1743-7075-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarugi P, Lonardo A. Heterozygous familial hypobetalipoproteinemia associated with fatty liver. Am J Gastroenterol. 1997;92:1400–1402. [PubMed] [Google Scholar]
- 16.Bonnefont-Rousselot D, Condat B, Sassolas A, Chebel S, Bittar R, Federspiel MC, Cazals-Hatem D, Bruckert E. Cryptogenic cirrhosis in a patient with familial hypocholesterolemia due to a new truncated form of apolipoprotein B. Eur J Gastroenterol Hepatol. 2009;21(1):04–108. doi: 10.1097/MEG.0b013e3282ffd9f8. [DOI] [PubMed] [Google Scholar]
- 17.Lonardo A, Tarugi P, Ballarini G, Bagni A. Familial heterozygous hypobetalipoproteinemia, extrahepatic primary malignancy and hepatocellular carcinoma. Dig Dis Sci. 1998;43:2489–2492. doi: 10.1023/a:1026646618643. [DOI] [PubMed] [Google Scholar]
- 18.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tarugi P, Averna M. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem. 2011;54:81–107. [PubMed] [Google Scholar]
- 20.Parhofer KG, Barrett PH, Aguilar-Salinas CA, Schonfeld G. Positive linear correlation between the length of truncated apolipoprotein B and its secretion rate: in vivo studies in human apoB-89, apoB-75, apoB-54.8, and apoB-31 heterozygotes. J Lipid Res. 1996;37(4):844–852. [PubMed] [Google Scholar]
- 21.Tarugi P, Lonardo A, Gabelli C, Sala F, Ballarini G, Cortella I, Previato L, Bertolini S, Cordera R, Calandra S. Phenotypic expression of familial hypobetalipoproteinemia in three kindreds with mutations of apolipoprotein B gene. J Lipid Res. 2001;42(10):1552–1561. [PubMed] [Google Scholar]
- 22.Sankatsing RR, Fouchier SW, de Haan S, Hutten BA, de Groot E, Kastelein JJ, Stroes ES. Hepatic and cardiovascular consequences of familial hypobetalipoproteinemia. Arterioscler Thromb Vasc Biol. 2005;25(9):1979–1984. doi: 10.1161/01.ATV.0000176191.64314.07. [DOI] [PubMed] [Google Scholar]
- 23.Ward SC, Waxman S. Fibrolamellar Carcinoma: A Review with Focus on Genetics and Comparison to Other Malignant Primary Liver Tumors. Semin Liver Dis. 2011;31:61–70. doi: 10.1055/s-0031-1272835. [DOI] [PubMed] [Google Scholar]
- 24.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hooper AJ, Adams LA, Burnett JR. Genetic determinants of hepatic steatosis in man. J Lipid Res. 2011;52(4):593–617. doi: 10.1194/jlr.R008896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeo S, Huang-Doran I, Baroni MG, Kotronen A. Unravelling the pathogenesis of fatty liver disease: patatin-like phospholipase domain-containing 3 protein. Curr Opin Lipidol. 2010;21(3):247–252. doi: 10.1097/mol.0b013e328338ca61. [DOI] [PubMed] [Google Scholar]
- 27.Sookoian S, Castaño GO, Burgueño AL, Gianotti TF, Rosselli MS, Pirola CJ. A nonsynonymous gene variant in the adiponutrin gene is associated with nonalcoholic fatty liver disease severity. J Lipid Res. 2009;50(10):2111–2116. doi: 10.1194/jlr.P900013-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian C, Stokowski RP, Kershenobich D, Ballinger DG, Hinds DA. Variant in PNPLA3 is associated with alcoholic liver disease. Nat Genet. 2010;42(1):21–23. doi: 10.1038/ng.488. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson R, Pedersen ED, Wang Z, Brakebusch C. Rho GTPase function in tumorigenesis. Biochim Biophys Acta. 2009;1796(2):91–98. doi: 10.1016/j.bbcan.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Couderc B, Pradines A, Rafii A, Golzio M, Deviers A, Allal C, Berg D, Penary M, Teissie J, Favre G. In vivo restoration of RhoB expression leads to ovarian tumor regression. Cancer Gene Ther. 2008;15:456–464. doi: 10.1038/cgt.2008.12. [DOI] [PubMed] [Google Scholar]
- 31.Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81:682–687. doi: 10.1002/(sici)1097-0215(19990531)81:5<682::aid-ijc2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.