Abstract
Rational
The regenerative capacity of the heart is markedly diminished shortly after birth coinciding with overall withdrawal of cardiomyocytes from cell cycle. Consequently, the adult mammalian heart has limited capacity to regenerate after injury. The discovery of factors that can induce cardiomyocyte proliferation is therefore of high interest and has been the focus of extensive investigation over the past years.
Objective
We have recently identified C3orf58 as a novel Hypoxia and Akt induced Stem cell Factor (HASF) secreted from mesenchymal stem cells that can promote cardiac repair through cytoprotective mechanisms. Here, we tested the hypothesis that HASF can also contribute to cardiac regeneration by stimulating cardiomyocyte division and proliferation.
Methods and Results
Neonatal ventricular cardiomyocytes were stimulated in culture for seven days with purified recombinant HASF protein. Compared to control untreated cells, HASF-treated neonatal cardiomyocytes exhibited 60% increase in DNA synthesis as measured by BrdU incorporation. These results were confirmed by immunofluorescence confocal microscopy showing a 50–100% increase in the number of cardiomyocytes in the mitotic and cytokinesis phases. Importantly, in vivo cardiac overexpression of HASF in a transgenic mouse model resulted in enhanced level of DNA synthesis and cytokinesis in neonatal and adult cardiomyocytes. These proliferative effects were modulated by a PI3K-AKT-CDK7 pathway as revealed by the use of PI3K pathway specific inhibitors and silencing of the Cdk7 gene.
Conclusion
Our studies support the hypothesis that HASF induces cardiomyocyte proliferation via a PI3K-AKT-CDK7 pathway. The implications of this finding may be significant for cardiac regeneration biology and therapeutics.
Keywords: Paracrine, cardiomyocytes, proliferation, regeneration
INTRODUCTION
The heart's limited ability to regenerate poses one the most significant challenges in cardiac diseases. Although neonatal cardiomyocytes retain proliferative capacity and are capable of regeneration after injury1–4, mature cardiomyocytes show very limited capacity for proliferation. As a result, after injury and loss of cardiomyocytes, the adult heart cannot regenerate adequately thereby leading to scar formation, pathological remodeling and eventually heart failure. To address this problem, considerable efforts have been invested to develop strategies that can stimulate cardiac regeneration by discovering new molecules and/or pathways that can stimulate cardiomyocytes to enter the cell cycle and undergo division3, 4. Still, only a few such molecules have been identified. Those include the p38 MAP kinase inhibitor5; periostin,6–8; Neuregulin1,9; as well as TWEAK10–12. However, even with these stimulants, the numbers of dividing cardiomyocytes are low as it is still difficult to achieve sustainable activation of cell cycle re-entry and division.
Recently, we have identified C3orf58 as a novel paracrine factor secreted from mesenchymal stem cells, which we named Hypoxia and Akt induced Stem cell Factor (HASF) (Huang et al.; paper under review). Exogenously administered HASF protein or HASF gene overexpression protected cardiomyocytes from apoptosis and cell death via the selective activation of protein kinase C epsilon both in vitro and in vivo (Huang et al.; paper under review). HASF is a relatively uncharacterized protein that does not show any similarity to known functional motifs apart from the presence of a putative signal peptide. HASF shows broad expression in adult mouse tissues such as heart, brain, liver etc., (Online Figure I). Genetic studies have shown an association of human HASF deletion with human familial autism13. In the same study, it was suggested that HASF is a downstream target of Mef2c and that HASF is upregulated in rat hippocampal cultures after membrane depolarization with KCl13.
Here, we provide evidence that treatment of rat neonatal cardiomyocytes with HASF recombinant protein increased DNA synthesis through the cell cycle regulator CDK7 complex and promoted mitosis and cell division. These effects were shown to be dependent on the PI3K and AKT pathway. Importantly, cardiac specific over-expression of HASF in transgenic mice resulted in enhanced DNA synthesis and karyokinesis of neonatal and adult cardiomyocytes in vivo. Collectively, our data identify HASF as a novel inducer of cell cycle re-entry and proliferation of neonatal and adult mammalian cardiomyocytes with potential implications for cardiac repair and regeneration.
METHODS
Recombinant protein generation
Human HASF recombinant protein was produced using bacterial, mammalian or insect cells. In brief, the open reading frame of human HASF without the predicted N-signal sequence (1158 bp) was cloned in-frame in pMal-2C vector. The fragment was next amplified, using the forward primer (underlined with Nde I restriction site), 5’-ggcggccatatggaccggcgcttcctgcag-3’ and the reverse primer (underlined with BamH I restriction site), 5’-ggcggcggatccctacctcacgttgttacttaattgtgctagg-3’, and cloned in-frame into a pET 15b vector (EMD Biosciences) to generate 6×His tagged HASF recombinant protein. For transfection and protein production using the HEK293 mammalian cell expression system, full-length human HASF cDNA without the stop codon was amplified by PCR and cloned into Gateway Entry vector for sequencing and subsequently recombined into Gateway destination vector 40 (Invitrogen) to generate an 6×His-V5-epitope tagged HASF. In addition, hpc4-tagged HASF Recombinant Protein was produced from insect cells using Invitrogen’s Drosophila Expression System. Protein was purified from the media using Anti-Protein C Affinity Matrix from Roche (catalog number 11815024001). Protein sequence was confirmed by matrix-assisted laser desorption-ionization mass spectrometry (MALDI-MS) on an Applied Biosystems 4700 Proteomic Analyzer® time of flight (TOFTOF®) mass spectrometer (Duke core facility). Positive mode time of flight was used to identify peptides, and individual peptides were sequenced by MS/MS. All peptide fingerprint data was searched by SwissProt and Mascot search engine.
Isolation and in vitro treatment of rat ventricular neonatal cardiomyocytes
Hearts were harvested from 1–2 days old rat neonates and trypsin digested overnight (4°C), followed by collagenase Type II (Worthmington, USA) digestion at 37°C. The lysate was then filtered through a 100-µm cell strainer and the cells were pelleted for 5 min at 500 rpm. Cardiomyocytes were cultured in the following growth media: Advanced DMEM/ F12 media supplemented with 0.2% BSA, 0.1% ascorbic acid, 2mM L-glutamine and 0.5% ITS5, 8 supplemented with 5% horse serum and 20uM ArabinoseC during the first 48 hours5, 8. Cultured cells were stimulated for seven days in growth media with 10–20 nM HASF. BrdU uptake was used to measure DNA synthesis. BrdU Elisa-assay was performed following the manufacturer’s instructions (Roche, USA).
Immunofluorescent staining of neonatal cardiomyocytes in vitro
Neonatal cardiomyocytes were fixed with 4% parformaldehyde and permeabilized with 1% triton (for BrdU staining) supplemented with 2M HCl for 30 min, rinsed with PBS and blocked with 10% normal goat serum. Primary antibodies for BrdU (Abcam), cardiac troponin T cTnT (abcam), Ki67 (BD biosciences), H3P (Abcam) and Aurora B (BD Biosciences) were diluted with blocking solution and Alexa fluor secondary antibodies (Invitrogen) were used for detection. Confocal images were captured using an LSM 510 Meta DuoScan microscope (Zeiss) and processed using ZEN software, version 2009. Tiled scanned images of 4 or 6 single high power field images were captured by ZEN software and analyzed.
Western blot analysis
Neonatal cardiomyocytes were lysed with 1× lysis buffer (Cell Signaling) in the presence of protease and phosphates inhibitors (Roche, USA). The lysate was centrifuged at 12000×g for 5 min and total protein concentration was measured with BCA (Pierce, USA) assay. Proteins were separated on 10% SDS-PAGE gels (Invitrogen, USA), transferred to a membrane, blocked with 5% non-fat dry milk (Biorad, USA) and probed with the primary antibodies followed by HRP-conjugated secondary antibodies.
Fluorescence activated cell sorting
Cells were isolated as before and fixed using the BD Cytoperm/Cytofix kit according to manufacturer instructions (BD, USA). Troponin staining was performed using specific Troponin T or Troponin I antibodies (Abcam). BrdU incorporation was evaluated by using a BrdU and 7-amino actinomyosin (7-AAD) intracellular staining kit (BD Pharminogen) Cells were analyzed using BD FACS caliber (USA).
Gene expression qRT-PCR
mRNA was isolated from neonatal cardiomyocytes following Qiagen kit manufacturer instructions (Qiagen). cDNA was produced using high capacity cDNA transcription kit (applied biosciences) with Biorad iCycler. The quantitative expression of each gene was assessed using Taqman Gene Expression Assays in the StepOnePlus Real-Time PCR System (Applied Biosystems).
Knock down of Cdk7 gene expression
Isolated neonatal cardiomyocytes were cultured as described above. Pools of Accell siRNAs against Cdk7 (Dharmacon) or non-targeting siRNA were used to suppress Cdk77 gene expression in the presence or absence of HASF. siRNA transfection was performed for three days using Dharmacon’s protocol. After removal of the siRNA complex the cells were treated with HASF for four additional days.
Generation of transgenic mice- cardiac specific overexpression of HASF
For the generation of HASF-Tg mice, human HASF cDNA was placed under the control of the alpha myosin heavy chain promoter in P-Bluescript plasmid as described in Subramaniam et al., 199128. The DNA was linearized and introduced into FVB embryos and maintained on an FVB background for at least 10 generations. Several HASF transgenic lines were established described in Huang et al. (paper under review).
BrdU injection in HASF transgenic neonatal mice
1.5-days old wild type (FVB) and HASF transgenic mouse neonates received three intra-peritoneal injections of BrdU (50mg/Kg) every 12 hours and then the hearts of the 3-days old neonates were harvested. The hearts were fixed in 10% formalin for paraffin embedding. 5µm thick tissue sections were used for immunohistochemistry.
Induction of myocardial infarction in adult mice
Permanent ligation of the left anterior descending (LAD) coronary artery was performed in 8–10 weeks old male HASF transgenic line (H2) and its related wild type (FVB) mice as previously described29. Every 24 hours the mice received an intra-peritoneal injection of EdU (42 mg/Kg/day) for days 2–7 and BrdU (50 mg/Kg/day for days 9–14 of post-surgery)30. On day 14 the hearts were harvested and fixed in 4% paraformaldehyde, embedded in paraffin and the 5µm thick tissue slides were processed for immunohistochemistry fluorescent staining. Animals were handled according to the approved protocols and animal welfare regulations of the Institutional Review Board at Duke University Medical Center.
Statistical analysis
Quantitative data are given as mean ± SEM and intergroup comparisons were evaluated by analysis of variance (ANOVA). P<0.05 was considered statistically significant.
RESULTS
HASF increases proliferation and induces cell cycle re-entry in neonatal cardiomyocyte in vitro
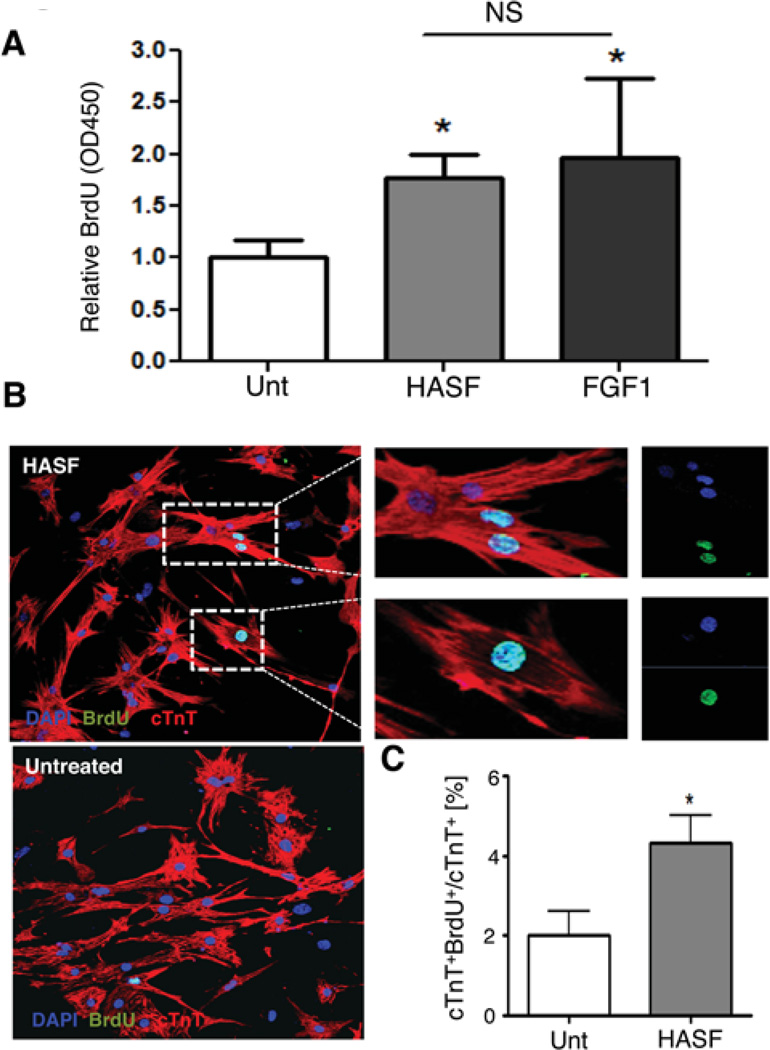
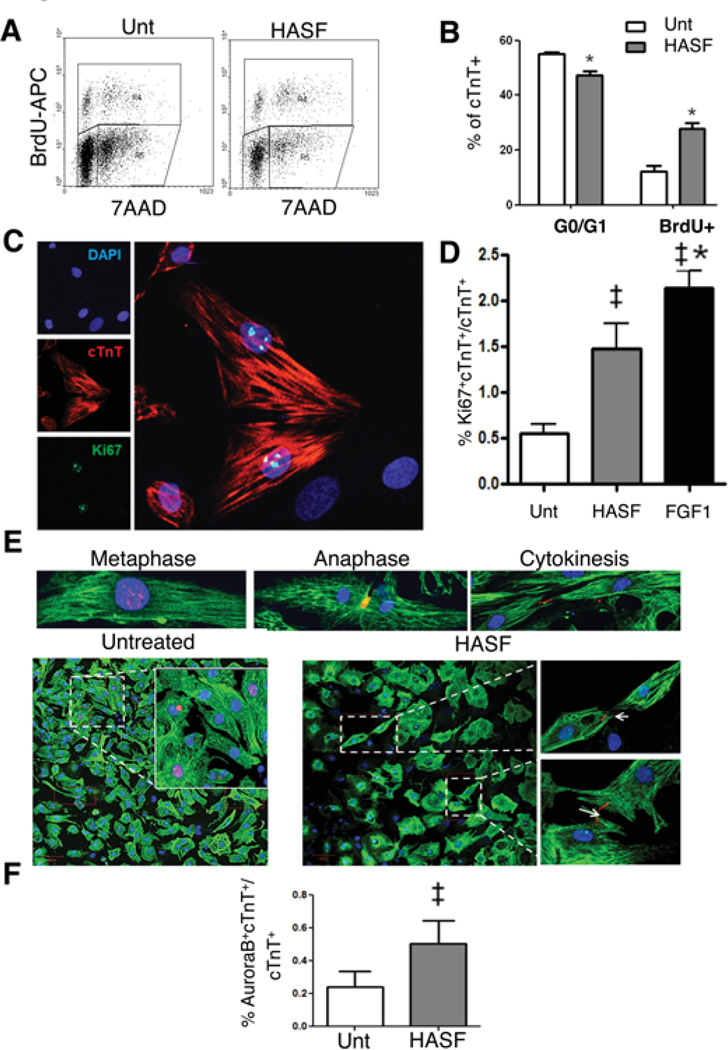
To investigate the capacity of HASF to promote DNA synthesis in cardiomyocytes we performed cell proliferation assays on ventricular cardiomyocytes isolated from 2-days old rat neonates. Isolated cardiomyocytes were allowed to attach and recover over 48 hours. Cells were then treated daily with 10–20nM HASF for 7 consecutive days and assayed for DNA synthesis by BrdU incorporation. HASF treatment increased BrdU incorporation in neonatal cardiomyocytes by 1.7 ± 0.1 fold as measured by ELISA assay (Figure 1A), similar to the levels of stimulation by FGF1 (5.8 nM), a growth factor well established to promote cardiomyocyte proliferation5, 9, 14. Consistent with this finding, immunofluorescence confocal microscopy analysis showed that HASF treatment doubled the numbers of BrdU positive neonatal cardiomyocytes (as identified by co-staining of BrdU with cardiac TroponinT) to 4.3±0.6% (Figure 1B–C). To confirm these data, we performed BrdU incorporation and FACS analysis. For this, seven days of HASF-stimulated neonatal cardiomyocytes were pulsed with 3 consecutive BrdU (30 µM) treatments every 24-hour for the last 3 days of stimulation. Next day, cells were harvested, fixed and stained for analysis. As shown in Figure 2A–B, 27.8 ± 2.03% of the HASF treated cardiomyocytes were positive for BrdU while 47.3 ± 1.39% were in the G0/G1 resting phase. In comparison, 12.2 ± 2.0% of the untreated cardiomyocytes showed to be positive for BrdU, and 55.0 ± 0.61% in the G0/G1 resting phase15, 16. These results indicate that HASF treatment increased DNA synthesis and concurrently reduced the number of cardiomyocytes in resting phase (G0/G1).
Figure 1. HASF increases DNA synthesis in neonatal cardiomyocytes in vitro.
A) BrdU uptake in neonatal cardiomyocytes as measured by ELISA. FGF1 (5.8 nM) was used as positive control. B) Representative images (20×) of BrdU+ cardiomyocytes without and with HASF treatment visualized by confocal microscopy. Magnification of insets is shown on the right. C) Quantification of BrdU+cTnT+/cTnT+ neonatal cardiomyocytes after 7 days of HASF treatment. cTnT (red), BrdU (green) and nuclear DAPI (blue), Unt; untreated. *P<0.05 vs. untreated. NS: non-significant. Representative data from 3–4 sets of experiments are shown.
Figure 2. HASF induces cell cycle re-entry and cytokinesis in neonatal cardiomyocyte in vitro.
A) Representative FACS gated plots and (B) quantitative analysis of BrdU+7AAD+ control or HASF treated neonatal cardiomyocytes. C) Representative image of Ki67+ stained cardiomyocytes. D) Quantification of Ki67+ cTnT+/cTnT+ neonatal cardiomyocytes after 7 days of HASF treatment as evaluated by confocal microscopy. 1500–2000 cells were counted from each experimental group. FGF1(5.8nM) was used as positive control, *P<0.05 vs. untreated. E) Upper panel, representative images of neonatal cardiomyocytes showing Aurora B in metaphase, anaphase and cytokinesis. Lower panel, representative images of untreated and HASF treated neonatal cardiomyocytes stained for Aurora B and visualized by confocal microscopy. Magnified inset in untreated cells shows condensed nuclear AuroraB. Magnified inset in HASF-treated shows AuroraB staining during cytokinesis (anaphase). Aurora B (red), cTnT (green) and nuclear DAPI (Blue). White arrows point to AuroraB. 4000–8000 cells were counted from each experimental group. F) Bar graph depicts quantification of Aurora B staining in dividing (cytokinesis) neonatal cardiomyocytes. *P<0.05 vs. untreated. For all images: image magnifications 40×, scale bar: 50 µm. Unt; untreated. Representative data of 3–4 sets of experiments are shown.
In order to further investigate whether HASF treated cardiomyocytes show differences in the cell cycle we also performed immunostaining and confocal microscopy imaging of the mitosis marker Ki675, 17, 18. As shown in Figure 2C–D, stimulation with HASF resulted in 1.5±0.3% Ki67 positive neonatal cardiomyocytes as compared to 0.5 ± 0.08% in untreated cardiomyocytes, corresponding to a 3–4 fold increase in cells in mitosis. During postnatal development cardiomyocytes generally undergo karyokinesis/ nuclear division without cytokinesis resulting in binucleated cells. To enquire about the potential of HASF to induce cardiomyocyte cell division, we evaluated the number of dividing cardiomyocytes using the cytokinesis marker Aurora B (Figure 2E–F). During the G2/M phase of the cell cycle Aurora B is expressed in the nucleus. In metaphase Aurora B is associated to the chromosomes while in anaphase and telophase it is localized to the mid-zone and mid-body, respectively19. This analysis revealed that seven days of HASF treatment increased the number of dividing neonatal cardiomyocytes in the anaphase and telophase (2.3 fold as compared to the untreated cardiomyocytes, P≤0.01) (Figure 2E–F). It is noteworthy to mention that only the mononucleated neonatal cardiomyocytes showed complete cytokinesis and that detection of cytokinesis in binucleated cardiomyocytes was rare. Taken together these data indicate that HASF can significantly induce mitosis and cytokinesis and enhance proliferation of neonatal cardiomyocytes in vitro.
Increased cardiomyocyte proliferation in the neonatal heart of transgenic mice with cardiac-specific HASF overexpression
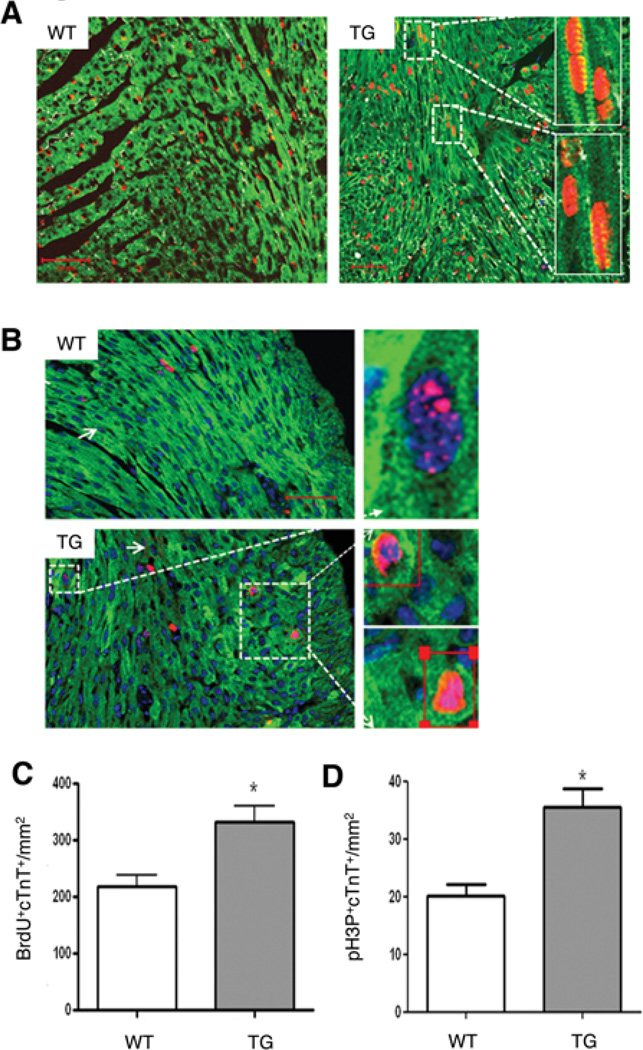
To explore if the in vitro effects of HASF could be reproduced in vivo, we measured the levels of proliferation in cardiomyocytes from neonatal mice overexpressing the human HASF gene specifically in cardiac myocytes (αMHC-HASF). These mice show normal cardiac phenotype without any evidence of cardiac hypertrophy or dysfunction (Huang et al.; paper under review and Online Figure II). For DNA synthesis, neonates received three consecutive BrdU injections every 12 hours and the harvested heart tissue was used for BrdU detection by fluorescence confocal microscopy. In total, 50–60 high power field (40×) images per heart, corresponding to 1.6–2 mm2 area of a heart tissue per animal, were captured and analyzed. This analysis revealed that in the neonatal heart, an overexpression of HASF induced 1.5–2 fold increase in cardiomyocyte DNA synthesis (Figure 3A–B).
Figure 3. HASF increases DNA synthesis and proliferation in neonatal cardiomyocyte in vivo.
A) Representative tiled images of WT and HASF transgenic (Tg) 3 day old neonatal mice heart tissue stained for BrdU (red) and cTnT (green) and laminin (white) visualized by confocal microscopy. B) Representative tiled image of 3 day old neonatal mice heart tissue stained for H3P (red), cTnT (green) and nuclei (blue) visualized by confocal microscopy. C and D) Quantification of A and B respectively. 30–60 HPF images/heart were captured and analyzed. Tiled images magnification is 40× and scale bar=50 µm with magnified insets showing nuclear BrdU (A) and H3P (B) staining. n=4, *P<0.05 vs. WT.
Moreover, to determine if HASF also regulates karyokinesis in cardiomyocytes in vivo, we assayed mitosis by immunofluorescent staining for phosphorylated histone-3 (H3P). H3P at Ser10 is an established marker for chromosome condensation during mitotic prophase in animal cells20. Immunofluorescent staining of H3P and confocal imaging analysis showed that HASF transgenic (Tg) mice had increased numbers (1.5–2 fold increase) of mitotic cardiomyocytes in the karyokinesis phase as compared to WT neonates (Figure 3C–D). Collectively, these data support that endogenous cardiac overexpression of HASF increases the proliferation of neonatal cardiomyocytes in vivo.
Cardiac overexpression of HASF results in increased adult cardiomyocyte DNA synthesis in vivo
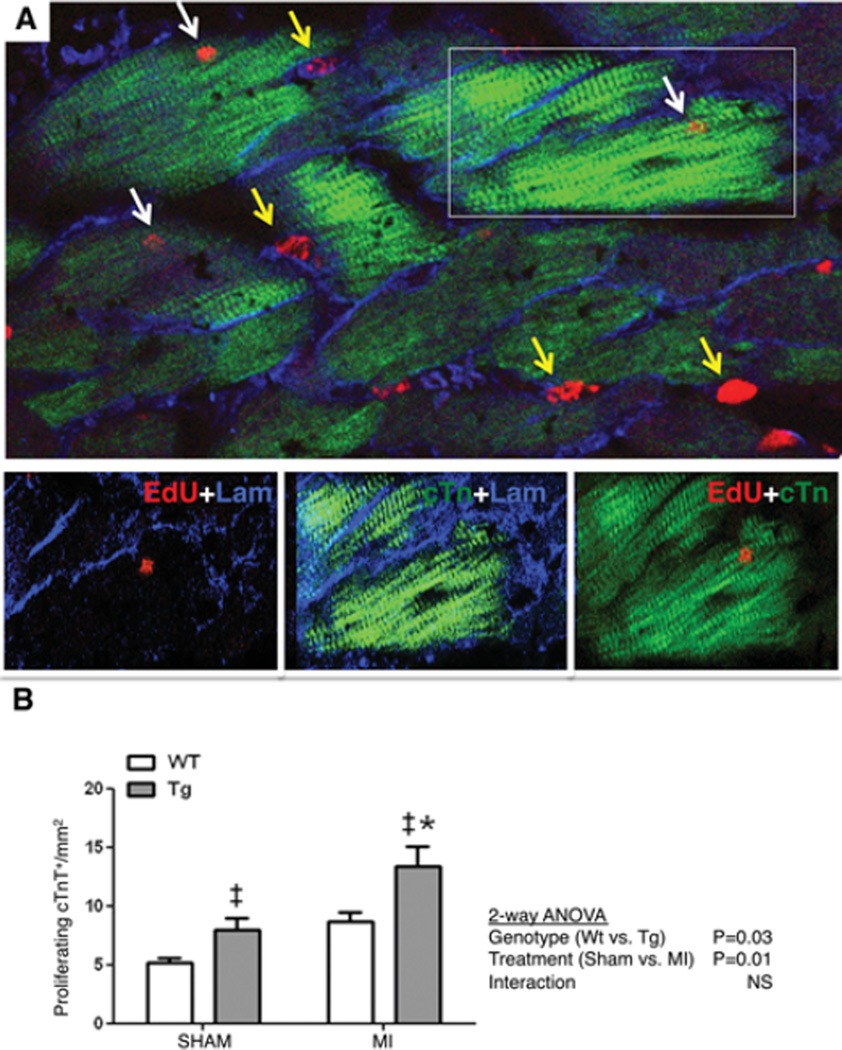
Based on the findings in the neonatal heart, we studied if HASF cardiac overexpression increased cardiomyocyte proliferation in the adult heart at baseline and in response to injury. For this, 8–10 weeks old male WT and HASF-Tg mice underwent myocardial infarction (MI) or sham surgery. Two days post-surgery the mice were administered a daily dose intra-peritoneal injection of EdU or BrdU to measure DNA synthesis and the hearts were harvested after fourteen days. For analysis, 50–60 high power field images (40×) per heart were acquired from the peri-infarct area and evaluated. For sham controls, images were acquired at mid left ventricular area below the sham ligature of the heart tissue, as previously published reports8, 9. Interestingly, in hearts from the HASF-Tg, cardiomyocyte DNA synthesis was increased at baseline (54% increase Sham, HASF-Tg vs. WT, P<0.05). Similarly, HASF overexpression showed increased DNA synthesis in adult cardiomyocytes after injury compared to WT controls (54% increase MI, HASF-Tg vs. WT, P<0.05) (Figure 4 and Online Figure III). Further analysis using 2-way ANOVA revealed that there was no significant interaction between the effect of HASF and MI suggesting that injury did not affect the proliferative properties of HASF. These data strongly suggest that HASF can regulate cardiomyocyte proliferation in vivo and that these effects are independent of injury.
Figure 4. HASF induces DNA synthesis in adult cardiomyocyte in vivo.
A) Representative image of adult cardiac tissue in the peri-infarct area 2 weeks post MI visualized by confocal microscopy (40×) showing proliferating cTnT+ cardiomyocytes (white arrows) and proliferating cTnTNeg non-cardiomyocyte (yellow arrows). An example of a proliferating cardiomyocyte (boxed area) is presented in the magnified inset at the bottom. EdU (red), cTnT (green) and laminin (blue). B) Quantification of total EdU+ and/or BrdU+ proliferating cTnT+ cardiomyocytes. MI animals N=7, sham animals N=2–3. ‡P≤0.05 for ‡Tg vs. WT for effect of treatment, and * P≤0.05 for Tg-MI vs. WT-MI as analyzed with 2-way Anova and Bonferonni post-hoc test. 48–60 HPF images/heart were analyzed. Scale bar: 50 µm.
Cardiac overexpression of HASF does not affect cardiac progenitor proliferation
It has been reported that the α-MHC promoter is active in early committed cardiac stem cells (mCPCs) raising the possibility that HASF may act at least in part by increasing proliferation of these cells. To address this possibility we have isolated c-Kit+ cardiac progenitor cells (mCPCs) from the non-cardiomyocyte fraction of HASF-Tg and WT animals. As shown in Online Figure IV (A), we did not see any expression of α-MHC on those cells. In addition, HASF-Tg and WT mCPCs showed no expression of HASF, suggesting that the α-MHC promoter is not active on these cells. Moreover, BrdU incorporation data showed that HASF overexpression in the heart did not affect the c-Kit+ progenitors in vitro or in vivo suggesting that mCPCs are not involved in the generation of the proliferating cardiomyocytes (Online Figure IV (B) and IV (C). Still, our data indicated that CD90+ (Thy1) non cardiomyocyte cells of the neonatal heart, presumably cardiac fibroblasts, have increased levels of DNA synthesis after HASF treatment or HASF cardiac overexpression (Online Figure V); The physiological importance of this observation or the mechanisms involved were not investigated, as they were beyond the focus of this study.
HASF increases cardiomyocyte proliferation via PI3K and AKT pathways
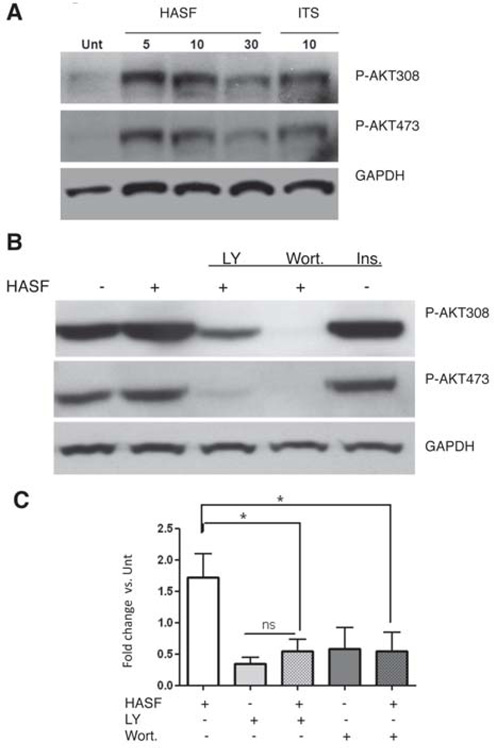
Our results so far have shown that HASF can stimulate cardiomyocyte proliferation. Next, we studied if PI3K signaling was involved in HASF mediated cardiomyocyte proliferation. To test this hypothesis, neonatal cardiomyocytes were treated with 10–20nM of HASF in a time dependent manner and Western blot analysis for the detection of AKT activation was performed. As shown in Figure 5A, HASF stimulation increased the phosphorylation level of AKT (T308, S473) in neonatal cardiomyocytes in vitro compared to untreated cells. Activation of GSK3-β or other known Akt downstream targets such mTORC1 and mTORC2 associated proteins RAPTOR and RICTOR were not observed (Online Figures VI and VII). Moreover, blocking the PI3K pathway with the pharmacological inhibitors LY294002 or wortmannin diminished proliferation and abrogated the HASF-induced DNA synthesis (Figure 5B and 5C). These results provide evidence for the direct role of PI3K-AKT pathway and its significance on mediating the HASF proliferative effects.
Figure 5. The proliferative effect of HASF in is mediated via the PI3K-AKT pathway.
A) Phosphorylation of AKT (T308 and S473) by HASF treatment at various time points in treated neonatal cardiomyocytes as analyzed by Western-blot. B) This effect is blocked by the presence of PI3K inhibitors, LY294002 (LY, 50µM) and Wortmannin (Wort. 1µM). C) BrdU uptake was measured in neonatal cardiomyocytes in the presence of PI3K inhibitors LY294002 (10µM), Wortmannin (0.2 µM) by ELISA. Insulin (10µM) was used as a positive control. *P<0.05 vs. untreated. Unt; untreated. Data are representative of 3–4 sets of experiments.
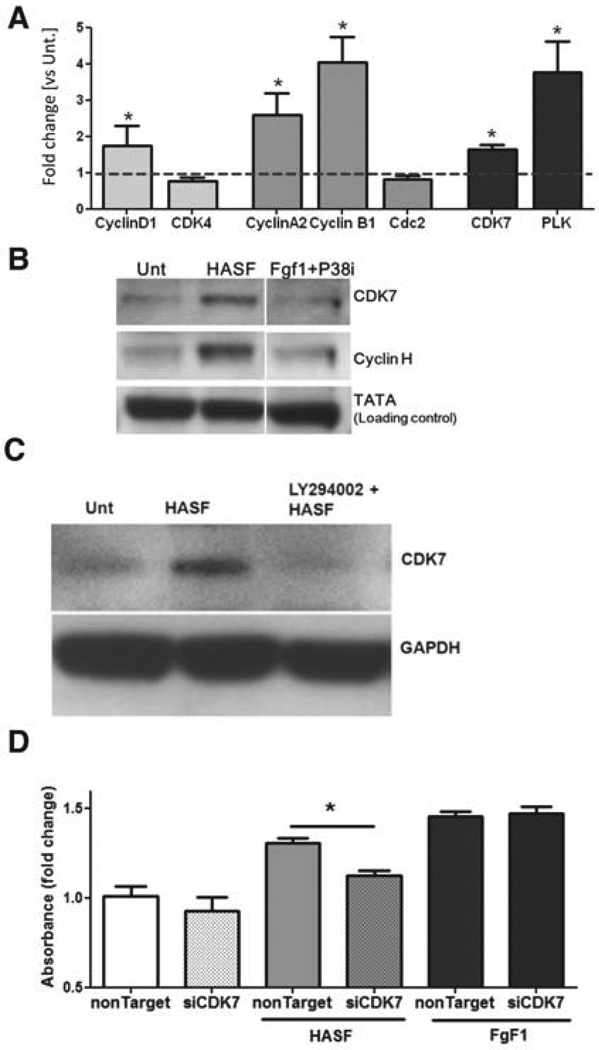
HASF augments the cell cycle regulator CDK7/cyclin H complex in cardiomyocytes via PI3K-AKT pathway
In the cell cycle machinery, DNA synthesis and ultimately cell division is largely regulated by different cell cycle dependent kinases (CDK) that can either promote cell cycle progression or transcription at different phases of the cell cycle21, 22. Based on our results above, we investigated whether HASF treatment had any effect on the major regulating CDKs/or their related cyclins. Seven days of HASF treatment in neonatal cardiomyocytes increased the gene expression levels of several G2/M phase cyclins, (e.g cylinA, cyclinB1, CDK7 and PLK1) (Figure 6A), further supporting that HASF-treated cardiomyocytes transited into S and G2/M phases leading to cell division. We next focused our analysis on the CDK7 protein and its relation to HASF. For this, we evaluated the protein expression levels of CDK7 and its binding partner cyclin H on neonatal cardiomyocyte after 7 days of HASF treatment. Western-blot analysis, as shown in Figure 6B, revealed that HASF treatment increased the protein expression of both CDK7 and cyclin H (which is part of the CDK7 activity complex), indicating increased CDK7 activity21, 22. Importantly, pre-incubation with LY294002 abolished the effect of HASF on CDK7 expression (Figure 6C). In contrast, treatment of cardiomyocytes with FGF1 growth factor in combination with p38-MAPK inhibitor (SB203580)5 (a treatment that has been shown to increase cardiomyocyte proliferation via regulation of the MAPK pathway) did not have any effect on CDK7 expression (Figure 6B). Collectively, these data demonstrate that HASF regulates CKD7 expression though the PI3K-AKT pathway.
Figure 6. HASF-mediated proliferation in neonatal cardiomyocytes is modulated by CDK7.
A) Quantification of RNA expression level of cyclin dependent kinases (CDKs) analyzed by qRT-PCR. B) Protein expression level of CDK7 and cyclinH after 7-days of HASF treatment in neonatal cardiomyocytes as shown by western-blot. The HASF effect is blocked in the presence of (C) PI3K inhibitor (LY294002, 10 µM). D) DNA synthesis assessed by Elisa for BrdU uptake in neonatal cardiomyocytes subjected to Cdk7 gene suppression with siRNA treatment. Unt: untreated, NonTarget: non targeting negative siRNA control and siCDK7: siRNA against the Cdk7 gene.
HASF induced cardiomyocytes proliferation is modulated by the cell cycle regulator CDK7
To establish the role of CDK7 on HASF-induced proliferation, we used RNA interference to specifically suppress Cdk7 gene expression. As shown in Online Figures VIII, siRNA treatment resulted in almost complete depletion of Cdk7 gene expression within one day of treatment. Neonatal cardiomyocytes were then treated for 7 days with HASF in the presence and absence of Cdk7 siRNA and BrdU uptake was measured as the marker for DNA synthesis and proliferation (Figure 6D). Transfection of cardiomyocytes with CDK7 specific siRNA significantly diminished the HASF effect on BrdU uptake proliferation. No changes were observed when the cells were transfected with control non-targeting siRNA or when treated with FGF1 instead of HASF (Figure 6D).
DISCUSSION
Numerous evidences indicate that mammalian adult cardiomyocytes harbor signaling circuits capable of resuming their proliferative potential3, 8, 9. However, in contrast to teleost fish23, 24 and urodele amphibians25 the endogenous levels of proliferation in the mammalian heart is still insufficient for significant regeneration after injury. Enabling cardiomyocyte proliferation represents an important strategy for cardiac regeneration. Here, we report the function and mechanism of C3orf58 (HASF), a novel paracrine protein, in stimulating cell cycle progression and proliferation in neonatal and adult cardiomyocytes. Our in vivo studies using a murine model with cardiac-specific overexpression of HASF showed increased levels of DNA synthesis as well as cytokinesis in the hearts of transgenic neonate and adult mice at baseline and after injury. Collectively, these results document the capability of HASF to induce cardiomyocytes proliferation. This discovery is significant, as only a few factors have been shown to possess the capacity of inducing adult cardiomyocyte proliferation. Technical limitations of EdU/ BrdU labeling and the in vivo models did not allow us to ascertain whether the proliferating cardiomyocytes in the adult myocardium of HASF-Tg mice are resulting from the maintenance of the amplifying neonatal cardiomyocytes or by induction of cell cycle re-entry of mature cardiomyocytes. Unfortunately, we did not investigate whether the response to HASF was different between different types of cardiomyocytes. These issues pose interesting questions for future studies.
Cardiac myocytes do proliferate during fetal development. However, soon after birth they undergo an additional round of DNA synthesis without cell division, which results in the majority of cells being bi-nucleated, followed by withdrawal from the cell cycle8, 9, 26. The notion that adult cardiomyocytes are incapable of proliferating and are terminally differentiated has been recently challenged9, 27. However, the factors that regulate cardiomyocyte cell cycle and division in the neonatal and adult heart are poorly understood. Our in vitro experiments on rat neonatal cardiomyocytes showed that HASF increased their cell cycle activity and increased expression of both S and G2/M phase cyclins/ and CDKs; cylinA, cyclinB1, CDK7 and PLK1. Moreover, the proliferative effect of HASF was dependent on the activation of the cell cycle regulator, CDK7. CDK7 is a member of the TFIIH complex and is known to be the only CDK capable to drive the cell cycle to complete progression as well as gene transcription. CDK7 binds to cyclin H for full activation, which binds MAT1, the third subunit, to stabilize the entire TFIIH complex21, 22. Still, the mechanism of CDK7 regulation, in particular in cardiomyocytes cell cycle, is poorly understood. Our data suggest that HASF regulates CDK7-mediated cardiomyocyte proliferation by activation of PI3K-AKT pathway. The CDK7 activation by HASF was specific to PI3K-AKT pathway and was not induced by treatment with known cardiomyocyte proliferation inducers such as FGF 1 and p38 mitogen-activated protein kinase inhibitor5. Interestingly, investigation of known Akt downstream targets associated with cardiomyocyte proliferation such as mTORC1 and mTORC2 did not show any activation suggesting alternative Akt regulated mediators might be involved in increasing CDK7 expression. Our data do not exclude the possibility that other pathways involved in proliferation are stimulated by HASF. Indeed, our previous work on HASF has shown that it can also provide cytoprotection through PKC epsilon dependent pathways (Huang et al.; paper under review). The upstream mediators of HASF stimulation of PI3K-Akt-CDK7 are still unknown; future studies to identify HASF receptor(s) and intracellular signaling involved might shed light in this question.
In conclusion, our studies provide evidence that HASF is a novel paracrine factor that induces cardiomyocyte cell cycle progression and proliferation via PI3K-AKT-CDK7 pathway. These findings may have significant implications in cardiac regeneration biology and therapeutics.
Supplementary Material
Novelty and Significance.
What Is Known?
Limited proliferative capacity of adult cardiomyocytes translates to limited capacity of the adult heart for regeneration after injury.
The identification of molecules that can stimulate cardiomyocyte proliferation, as well as understanding of the molecular mechanism governing this process, might have importance clinical implication for cardiac therapy.
What New Information Does this Article Contribute?
C3orf58, a novel secreted protein stimulates cardiomyocyte proliferation.
These effects are mediated through activation of the Akt signaling pathway and Cyclin-dependent kinase 7.
Cardiac muscle cells loose their capacity to divide and proliferate shortly after birth. As a result the adult heart shows reduced capacity to replace damaged tissue after injury such as the one that occurs after myocardial infarction. Currently, no therapeutic molecules are available that can stimulate cardiac muscle cells to proliferate. The present work identifies a novel protein, C3orf5, as a possible new modulator of cardiomyocyte proliferation and provides first insights into the molecular pathways modulating these effects.
ACKNOWLEDGMENTS
We thank Hui Mu for the original isolation of neonatal cardiomyocytes.
SOURCES OF FUNDING
Research conducted in these studies was supported by National Heart, Lung, and Blood Institute grants (RO1 HL35610, HL81744, HL72010, and HL73219 (to V.J.D.); the Edna and Fred L. Mandel Jr Foundation (to V.J.D. and M.M.); M.M. is also supported by an American Heart Association National Scientist Development Award (10SDG4280011).
Nonstandard Abbreviations and Acronyms
- αMHC
alpha myosin heavy chain
- AKT
protein kinase B (PKB)
- CDK7
cycle dependent kinase 7
- cTnT
cardiac troponin T
- FGF
fibroblast growth factor
- GSK3-β
glycogen synthase kinase 3 beta
- HASF
hypoxia and Akt induced stem cell factor
- MI
myocardial infarction
- mTORC
mammalian target of rapamycin complex
- mCPCs
mouse cardiac progenitor cells
- PKC
protein kinase C
- PI3K
phosphoinositide 3-kinase
- p38-MAPK
38-mitogen-activated protein kinase
- PLK1
polo-like kinase 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None.
REFERENCES
- 1.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331:1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mummery CL. Cardiology: Solace for the broken-hearted? Nature. 2005;433:585–587. doi: 10.1038/433585a. [DOI] [PubMed] [Google Scholar]
- 4.Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186–273. [PubMed] [Google Scholar]
- 5.Engel FB, Schebesta M, Duong MT, Lu G, Ren S, Madwed JB, Jiang H, Wang Y, Keating MT. P38 map kinase inhibition enables proliferation of adult mammalian cardiomyocytes. Genes & development. 2005;19:1175–1187. doi: 10.1101/gad.1306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvin J, Zhu S, Norris R, Markwald R. Periostin family of proteins: Therapeutic targets for heart disease. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1205–1212. doi: 10.1002/ar.a.20237. [DOI] [PubMed] [Google Scholar]
- 7.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through rho/pi 3-kinase. Developmental biology. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 9.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/erbb4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 10.Girgenrath M, Weng S, Kostek CA, Browning B, Wang M, Brown SA, Winkles JA, Michaelson JS, Allaire N, Schneider P, Scott ML, Hsu YM, Yagita H, Flavell RA, Miller JB, Burkly LC, Zheng TS. Tweak, via its receptor fn14, is a novel regulator of mesenchymal progenitor cells and skeletal muscle regeneration. The EMBO journal. 2006;25:5826–5839. doi: 10.1038/sj.emboj.7601441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao HX, Campbell SR, Burkly LC, Jakubowski A, Jarchum I, Banas B, Saleem MA, Mathieson PW, Berman JW, Michaelson JS, Putterman C. Tnf-like weak inducer of apoptosis (tweak) induces inflammatory and proliferative effects in human kidney cells. Cytokine. 2009;46:24–35. doi: 10.1016/j.cyto.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Novoyatleva T, Diehl F, van Amerongen MJ, Patra C, Ferrazzi F, Bellazzi R, Engel FB. Tweak is a positive regulator of cardiomyocyte proliferation. Cardiovascular research. 2010;85:681–690. doi: 10.1093/cvr/cvp360. [DOI] [PubMed] [Google Scholar]
- 13.Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, Mukaddes NM, Balkhy S, Gascon G, Hashmi A, Al-Saad S, Ware J, Joseph RM, Greenblatt R, Gleason D, Ertelt JA, Apse KA, Bodell A, Partlow JN, Barry B, Yao H, Markianos K, Ferland RJ, Greenberg ME, Walsh CA. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Engel FB, Hsieh PC, Lee RT, Keating MT. Fgf1/p38 map kinase inhibitor therapy induces cardiomyocyte mitosis, reduces scarring, and rescues function after myocardial infarction. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15546–15551. doi: 10.1073/pnas.0607382103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collesi C, Zentilin L, Sinagra G, Giacca M. Notch1 signaling stimulates proliferation of immature cardiomyocytes. The Journal of cell biology. 2008;183:117–128. doi: 10.1083/jcb.200806091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campa VM, Gutierrez-Lanza R, Cerignoli F, Diaz-Trelles R, Nelson B, Tsuji T, Barcova M, Jiang W, Mercola M. Notch activates cell cycle reentry and progression in quiescent cardiomyocytes. The Journal of cell biology. 2008;183:129–141. doi: 10.1083/jcb.200806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gude N, Muraski J, Rubio M, Kajstura J, Schaefer E, Anversa P, Sussman MA. Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circulation research. 2006;99:381–388. doi: 10.1161/01.RES.0000236754.21499.1c. [DOI] [PubMed] [Google Scholar]
- 18.Brown DC, Gatter KC. Ki67 protein: The immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 19.Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. Journal of molecular and cellular cardiology. 2006;41:601–612. doi: 10.1016/j.yjmcc.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhry HW, Dashoush NH, Tang H, Zhang L, Wang X, Wu EX, Wolgemuth DJ. Cyclin a2 mediates cardiomyocyte mitosis in the postmitotic myocardium. The Journal of biological chemistry. 2004;279:35858–35866. doi: 10.1074/jbc.M404975200. [DOI] [PubMed] [Google Scholar]
- 21.Sano M, Izumi Y, Helenius K, Asakura M, Rossi DJ, Xie M, Taffet G, Hu L, Pautler RG, Wilson CR, Boudina S, Abel ED, Taegtmeyer H, Scaglia F, Graham BH, Kralli A, Shimizu N, Tanaka H, Makela TP, Schneider MD. Menage-a-trois 1 is critical for the transcriptional function of ppargamma coactivator 1. Cell Metab. 2007;5:129–142. doi: 10.1016/j.cmet.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Larochelle S, Merrick KA, Terret ME, Wohlbold L, Barboza NM, Zhang C, Shokat KM, Jallepalli PV, Fisher RP. Requirements for cdk7 in the assembly of cdk1/cyclin b and activation of cdk2 revealed by chemical genetics in human cells. Mol Cell. 2007;25:839–850. doi: 10.1016/j.molcel.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poss KD, Keating MT, Nechiporuk A. Tales of regeneration in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists. 2003;226:202–210. doi: 10.1002/dvdy.10220. [DOI] [PubMed] [Google Scholar]
- 24.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 25.Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nature reviews. Molecular cell biology. 2002;3:566–574. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- 26.Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circulation research. 2002;90:1044–1054. doi: 10.1161/01.res.0000020201.44772.67. [DOI] [PubMed] [Google Scholar]
- 27.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Subramaniam A, Jones WK, Gulick J, Wert S, Neumann J, Robbins J. Tissue-specific regulation of the alpha-myosin heavy chain gene promoter in transgenic mice. The Journal of biological chemistry. 1991;266:24613–24620. [PubMed] [Google Scholar]
- 29.Ip JE, Wu Y, Huang J, Zhang L, Pratt RE, Dzau VJ. Mesenchymal stem cells use integrin beta1 not cxc chemokine receptor 4 for myocardial migration and engraftment. Mol Biol Cell. 2007;18:2873–2882. doi: 10.1091/mbc.E07-02-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circulation research. 2010;106:891–901. doi: 10.1161/CIRCRESAHA.109.208629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.