Abstract
Checkpoint kinase Chk1 is constitutively active in many cancer cell types and new generation Chk1 inhibitors show marked antitumor activity as single agents. Here we present a hitherto unrecognized mechanism that contributes to the response of cancer cells to Chk1 targeted therapy. Inhibiting chronic Chk1 activity in cancer cells induced the tumor suppressor activity of protein phosphatase PP2A, which by dephosphorylating MYC serine 62, inhibited MYC activity and impaired cancer cell survival. Mechanistic investigations revealed that Chk1 inhibition activated PP2A by decreasing the transcription of CIP2A, a chief inhibitor of PP2A activity. Inhibition of cancer cell clonogenicity by Chk1 inhibition could be rescued in vitro either by exogenous expression of CIP2A or by blocking the CIP2A-regulated PP2A complex. Chk1-mediated CIP2A regulation was extended in tumor models dependent on either Chk1 or CIP2A. The clinical relevance of CIP2A as a Chk1 effector protein was validated in several human cancer types, including neuroblastoma where CIP2A was identified as a NMYC-independent prognostic factor. Since the Chk1-CIP2A-PP2A pathway is driven by DNA-PK activity, functioning regardless of p53 or ATM/ATR status, our results offer explanative power for understand how Chk1 inhibitors mediate single-agent anticancer efficacy. Further, they define CIP2A-PP2A status in cancer cells as a pharmacodynamic marker for their response to Chk1-targeted therapy.
Keywords: Claspin, PPP2R2A, Chk1 serine 345
Introduction
Inhibition of phosphatase activity of the protein phosphatase 2A (PP2A) complex is fundamental for transformation of immortalized human cells to malignant cancer cells(1-3). Expression of the cancerous inhibitor of PP2A (CIP2A) has been proposed to be a potential mechanism for widespread inhibition of PP2A tumor suppressor activity in human malignancies (2, 4). In line with its PP2A inhibiting activity, CIP2A promotes, among other PP2A target proteins(5-7), expression of serine 62 phosphorylated MYC and supports MYC-mediated gene transcription(4, 8). CIP2A promotes human cell transformation(4), and is required for xenograft tumor growth of several types of human cancer cells(4, 9, 10). Moreover, hypomorphic CIP2A mouse model(11) shows impaired MMTV-neu-driven mammary tumor development and progression(7). Importantly, CIP2A overexpression is seen in over 70% of patient samples in almost all cancer types studied thus far, and its expression correlates with clinical progression in a large variety of human malignancies(4, 7, 9, 10, 12).
Recent studies have shown widespread activation of several DNA damage response (DDR) proteins in human cancers(13, 14). Among the DDR proteins that are activated in cancer cells in the absence of any DNA-damaging treatments, Chk1 and DNA-PK have been recently identified to be required for tumor growth (13, 15-20). Notably, recent studies have shown that Chk1 activity is required for MYC-induced tumorigenesis(15, 16, 18). Moreover, even though Chk1 haploinsufficiency was shown to modestly promote tumorigenesis in combination with certain oncogenic lesions(17, 21, 22), two recent studies provide genetic evidence that Chk1 may benefit tumor development(17, 23). Together with studies using either RNAi-based or chemical inhibition of Chk1(13, 15-20), these results demonstrate that in the context of transformed cells, Chk1 activity promotes cell viability(13, 14, 24). However, even though induction of mitotic catastrophe explains cell-killing activity of Chk1 inhibitors when used in combination with DNA-damaging agents(13, 14, 24), mechanisms by which chronic Chk1 activity in unperturbed cancer cells promotes their viability and clonogenicity, are very poorly understood. Moreover, even though ATR kinase is a well-established upstream activator of Chk1 when DNA replication is impeded(14), or when cells are treated with DNA-damaging agents(14, 22), we do not understand the mechanisms driving chronic Chk1 activity in unperturbed human cancer cells.
In this study we demonstrate that chronic Chk1 activity in unperturbed cancer cells promotes CIP2A transcription and thereby inhibits PP2A tumor suppressor activity. Furthermore, our data shows that CIP2A downregulation and subsequent increase in PP2A activity is essential for maximal inhibition of cancer cell viability and clonogenicity in response to Chk1 inhibition.
Materials and Methods
Cell Viability assay
One day before transfection of siRNAs AGS, MKN-28 and MDA-MB231 cells were seeded in RPMI-1640 medium supplemented with 10% FCS at a density of 1 × 10 3 to 2 × 10 3 cells per well in 96-well plates. The cells were transfected with the following conditions: medium only, Lipofectamine 2000 reagent only or 20nM of indicated siRNAs (with Lipofectamine 2000 reagent) in 200 μL of RPMI-1640 supplemented with 10% FCS. Subsequently relative numbers of viable cells were measured by fluorescence at the 544 and 590 nm wavelengths in a FLUOstar OPTIMA Microplate Reader (BMG Labtech, Inc., Durham, NC), using the resazurin based CellTiter-Blue Assay (Promega Corporation) according to the manufacturer's instructions.
MCF10A MycER culture and cell cycle analysis
MCF10A MycER cells were cultured as described previously (34) in human mammary epithelial cell basal growth media MCDB 170 (US Biological) with supplements. For cell cycle analysis, the cells were seeded on glass coverslips and allowed to attach overnight. Next day, normal growth medium was replaced with medium lacking EGF and insulin and the cells were starved for 24 hours in the presence or either DMSO or Chk1 inhibitor SB218078. To activate MycER , 4-OHT (or ethanol as vehicle control) was added to the cells, which were fixed 24h later with 4% paraformaldehyde (PFA) and subjected to immunostaining with antibody against proliferation marker Ki-67 (Zymed, rabbit polyconal). The immunostained cells were analyzed by immunofluorescence microscopy and the percentages of cells expressing Ki-67 were scored from each assay.
In vitro kinase Assays
For in vitro kinase assays, MKN-28 cells were homogenized in ice-cold lysis buffer. Beads were removed by centrifugation, and DNA-PKc was immunoprecipitated from the supernatants using the DNA-PKc (G4) antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Kinase assay was then carried out for 15 min at 30°C by mixing 30ul of immunoprecipitated sample, Chk1 (100ng; c0870,Sigma) and ATP (50 μM final concentration) and samples were incubated for 15 min at 30°C. Then 30ul of 2× sample buffer was added and samples heated for 5min at 95°C and then immunoblotted for phospho-Chk1-serine345 (Cell Signaling Technology, Inc., Danvers, MA), Chk1 rabbit polyclonal (Santa Cruz Biotechnology, Santa Cruz, CA) and DNA-PKc antibodies.
Statistical Analysis
Overall survival was calculated from date of diagnosis to death. Associations between protein expression of the studied biomarkers (CIP2A, Chk-1, c-MYC and Claspin) were assessed by Fisher's exact test according to the Monte Carlo method at the 99% confidence level (IBM SPSS Statistics, version 19.0 for Mac; SPSS, Inc., an IBM Company, Chicago, IL, USA). All statistical tests were two-sided. Student's t-test was two sided.
Additional materials and methods used in the manuscript are provided in the supplementary file.
Results
Co-expression and prognostic role of CIP2A and Chk1 in human malignancies
CIP2A is involved in gastric cancer progression(12). Analysis of tissue microarray (TMA) of human gastric carcinoma specimens for Chk1 and for Claspin, the latter being a Chk1 scaffold protein that promotes Chk1 activity(14), demonstrated that Chk1 and Claspin were expressed in 162 out of 195 (83.2%) and 163 out of 199 (81.9%) specimens, respectively(Fig. 1A,B). Moreover, a statistically significant association between Chk1 and Claspin expression levels was observed(Fig. 1B). Cancer selective expression of Chk1 mRNA in gastric carcinoma versus normal samples was validated using the Oncomine™ database(Fig. S1A). Notably, CIP2A expression was significantly associated with expression of both Chk1 and Claspin(Fig. 1C,D). In addition, a significant association between Chk1 and CIP2A expression was detected at the mRNA level in human ovarian and colon cancer tissue samples(Fig. S1B and S1C). On the basis of recent identification of Chk1 as a drug target candidate in human neuroblastomas(20), we mined genomic analysis of 88 neuroblastoma samples(25) for expression of both Chk1 and CIP2A. Similar to gastric, ovarian and colon cancers(Fig. 1C,D and Fig. S1B,C), a statistically significant correlation was observed between the mRNA expression of Chk1 and that of CIP2A in neuroblastomas(Fig. 1E). Importantly, in addition to their co-expression, Kaplan-Meier analysis for both Chk1 and CIP2A demonstrated a significant decrease in overall(Fig. 1F) and relapse-free(Fig. S1D,E) survival in neuroblastoma patients with high expression of either of these genes. Importantly, CIP2A expression and relationship between Chk1 and CIP2A in neuroblastoma is independent on NMYC amplification status, a known major prognostic factor in this disease (Fig. 1G, H). These results indicate that low CIP2A expression could be used as a novel NMYC independent marker of neuroblastoma patients with favorable prognosis. Together, these results demonstrate co-expression of Chk1 and CIP2A in several human cancer types and indicate that understanding of the functional relevance of their co-expression would be of clinical importance.
Figure 1.
Co-expression and prognostic role of CIP2A and Chk1 in human malignancies. A, Paraffin block sections of gastric cancer specimens from patients (n=223) were subjected to IHC analysis using antibodies against Chk1 and Claspin (CLSPN). B, Statistical analysis of association between Chk1 and Claspin immunopositivity in gastric cancer specimens. C, Immunohistochemical analysis of Chk1, Claspin and CIP2A protein expression in gastric cancer specimens (n=223). D, Statistical analysis of association between Chk1, Claspin and CIP2A immunopositivity in gastric cancer specimens. E, Statistically significant correlation between Chk1 and CIP2A mRNA expression in human neuroblastomas. F, Kaplan-Meier curves demonstrating a significant decrease in overall survival of neuroblastoma patients with high Chk1 and CIP2A mRNA expression. G, Expression of CIP2A mRNA in MYCN amplification positive and negative neuroblastoma patients. H, Co-relation coefficient between Chk1 and CIP2A expression in MYCN amplification positive and negative neuroblastoma patients.
Chk1 activity promotes CIP2A expression in tumors
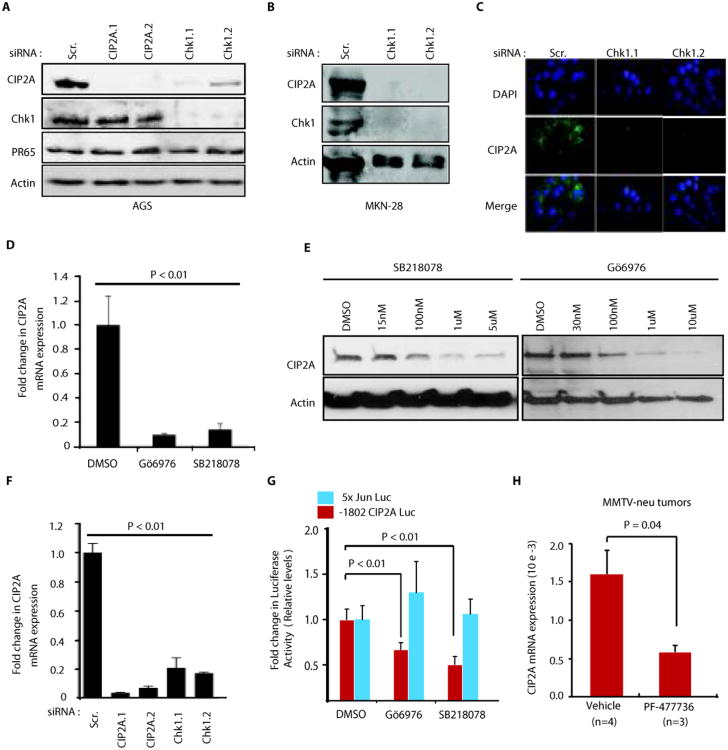
To determine whether co-expression of Chk1 and CIP2A is due to their functional effects on each other, both proteins were depleted from gastric cancer cells using two independent siRNAs. Although CIP2A depletion did not affect Chk1 expression, inhibition of Chk1 either by siRNA(Fig. 2A,B,C,F) or by small molecule inhibitors (SB218078 and GÖ6796)(Fig. 2D,E and Fig. S2A), potently decreased CIP2A mRNA and protein expression. Inhibition of Chk1 kinase activity by SB218078 and GÖ6796, or by RNAi, was confirmed by decreased phosphorylation of direct Chk1 target site serine216 on Cdc25c(Fig. S2B). Two different siRNAs specific for Claspin also inhibited CIP2A protein expression(Fig. S2C). A positive role for Chk1 in promoting CIP2A expression was observed in a large variety of cancer cell lines, independent of their p53 status(Fig. 2A,B, and Fig. S2D,E,F). CIP2A downregulation by Chk1 inhibition is unlikely to be caused by inhibition cell cycle activity, as CIP2A expression does not significantly change in synchronized versus unsynchronized AGS and MKN-28 cells(Fig S3A,B,C,D,E). Accordingly, CIP2A expression is not associated with serum-induced cell cycle progression in AGS cells(12), and synchronization of HeLa cells by serum starvation did not impact CIP2A protein expression(Fig. S3F,G,H).
Figure 2.
Chk1 activity promotes CIP2A expression both in vitro and in vivo. A, Effect of Chk1 and CIP2A siRNAs on protein expression of CIP2A, Chk1 and PR65 in AGS gastric cancer cells 72h post transfection. B, Inhibition of CIP2A protein expression by Chk1 siRNAs in MKN-28 gastric cancer cell line 72 hr post-transfection. C, Immunoflourescent stainings of AGS cells with DAPI and CIP2A antibodies 72h post transfection with scrambled (Scr), Chk1.1 or Chk1.2 siRNAs. D, Inhibition of CIP2A mRNA expression in AGS cells by two different small molecule inhibitors of Chk1 post 24h treatment. E, Effect of small molecule inhibitors of Chk1 on CIP2A protein expression in AGS cells post 24h treatment. F, Effect of scrambled (Scr.) and two different CIP2A and Chk1 siRNAs on CIP2A mRNA expression in AGS cells. G, Activity of either CIP2A (-1802bpCIP2ALuc) or AP-1 (5xJunLuc) promoter luciferase reporter in AGS cells treated either with DMSO, or with Chk1 inhibitors Gö6796 (1uM) and SB218078 (1uM) for 24h. (E, F, G) Error bars indicate +SD of three independent experiments H, Systemic treatment with Chk1 inhibitor PF-477736 for 72 hours inhibits CIP2A mRNA expression in vivo in MMTV-neu transgenic mice mammary tumors. Shown is mean + S.E.M, Student t-test was used obtain the statistical significance value. Number of tumors analyzed for each treatment is shown in parenthesis.
In concert with Chk1's role in transcription regulation(26, 27), Chk1 was found to regulate CIP2A at mRNA level(Fig. 2D,F and Fig. S4A), and Chk1 inhibition reduced luciferase activity of CIP2A promoter/luciferase reporter(28)(Fig. 2G). However, inhibition by Chk1 inhibitors did not generally inhibit transcriptional activity, because c-Jun-driven AP-1 promoter activity was not affected(Fig. 2G).
We have recently shown that Chk1 inhibitor PF-477736 significantly inhibits human neuroblastoma tumor growth by using xenograft model(20). To confirm that Chk1 activity promotes CIP2A expression also in vivo, in a model that is dependent on Chk1 activity, mice carrying neuroblastoma xenografts were treated with the Chk1 inhibitor PF-477736, and CIP2A mRNA expression was studied 48 hours after treatment. Indeed, CIP2A mRNA expression was decreased by 45% in neuroblastoma tumors in vivo by PF-477736 compared to vehicle control(Fig. S4B). Again, analysis of control genes expression from the same tumor samples indicated that Chk1 inhibition did not result in general inhibition of transcription(Fig. S4C). We recently demonstrated that CIP2A hypomorph mouse model displays reduced MMTV-neu-induced mammary tumorigenesis(7). CIP2A expression was positively regulated by Chk1 also in this CIP2A-dependent tumor model, as Chk1 inhibition by systemic PF-477736 treatment inhibited CIP2A mRNA expression in MMTV-neu mammary tumors(Fig. 2H). Together these results demonstrate inhibition of CIP2A expression by Chk1-targeted cancer therapy in vivo.
Inhibition of CIP2A expression defines cellular response to Chk1 inhibition
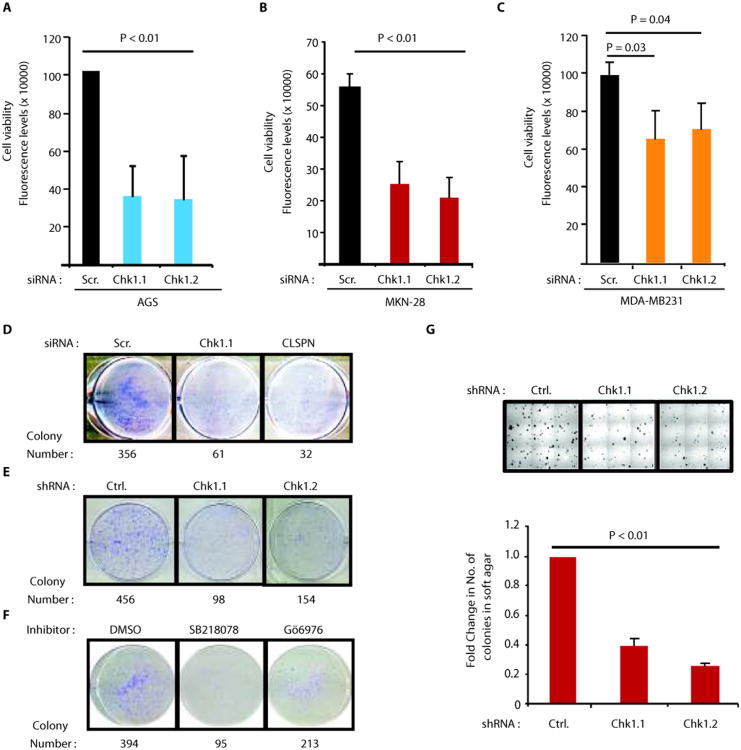
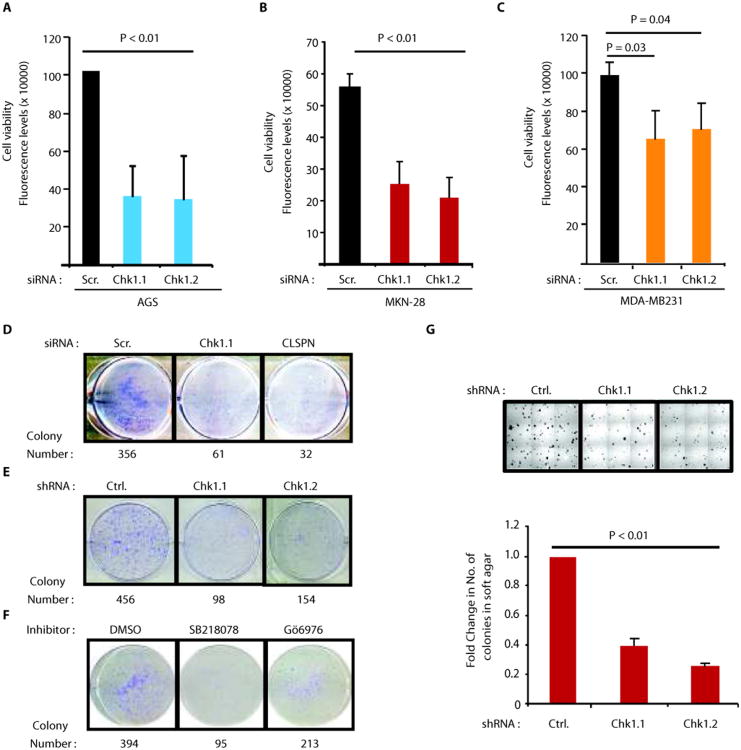
Importantly, depletion of either Chk1 or Claspin decreased the viability of AGS cells (Fig. 3A and Fig. S4E). In concert with p53 independent role for Chk1 in regulation of CIP2A expression(Figs. 2A,B and Figs. S2D,E,F), Chk1 RNAi inhibited also viability of two p53 mutant cancer cell lines, MKN-28 (gastric cancer)(Fig. 3B) and MDA-MB-231 (breast cancer)(Fig. 3C). Inhibition of both Chk1 and Claspin also inhibited colony growth of AGS cells(Fig. 3D,E). Moreover, the growth of AGS cell colonies was dependent on Chk1 kinase activity, as demonstrated by experiments using two small molecule inhibitors of Chk1(Fig. 3F). Finally, the anchorage-independent growth of gastric cancer cells was also significantly decreased by two different small hairpin RNAs specific for Chk1(Fig. 3G) and by small molecule Chk1 inhibitor SB218078(Fig. S4F,G). Therefore, Chk1 activity supports viability and clonogenicity of the same cell lines in which it promotes CIP2A expression, and in which CIP2A promotes cell viability and tumor growth(4, 9, 12)(Fig. S4D).
Figure 3.
Chk1 promotes cell viability, clonogenicity and anchorage-independent growth of CIP2A-dependent human cancer cells. A, Inhibition of cell viability by two different Chk1 siRNAs in AGS, B, MKN-28 and C, MDA-MB231 cells. Shown are mean values + S.D., of representative results from three independent experiments (student's t-test). D, Assessment of clonogenic potential of AGS cells when transfected with Scr. (control), Chk1 and Claspin(CLSPN) siRNAs E, Assessment of clonogenic potential of AGS cells stably transfected with Control and two different Chk1 shRNAs, F, Effect of two small molecule inhibitors of Chk1 (1uM) on clonogenic potential of AGS cells. Shown are representative pictures with quantitated colony numbers from that experiment. G, Effect of two different Chk1 shRNAs on anchorage-independent growth of MKN-28 cells in soft agar assay. Representative pictures from each well are shown above the bar graph. Shown is mean values + S.D., of representative results from three independent experiments (student's t-test)
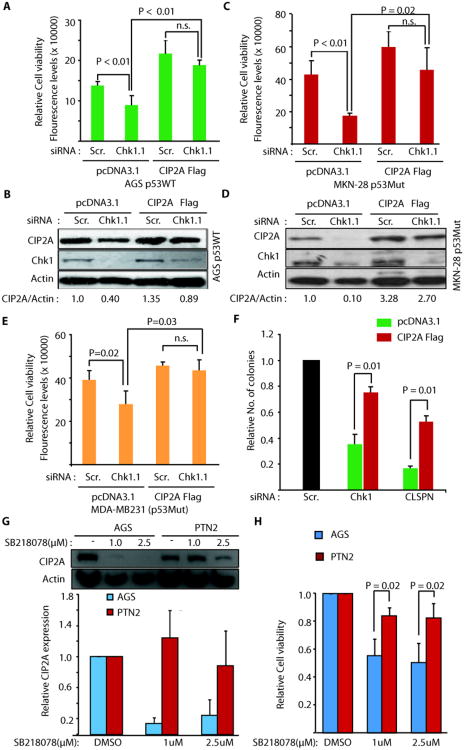
Notably, exogenous expression of CIP2A(Fig. 4B and Fig. S4H) increased AGS cell viability in scrambled siRNA transfected cells, and rescued in large both inhibition of CIP2A levels and cell viability in Chk1 siRNA treated cells(Fig. 4A,B). Statistical analysis of difference in relative cell viability between cells transfected with either control plasmid, or with CIP2AFlag, demonstrated that CIP2A protein expression levels define the response of these cells to RNAi-mediated Chk1 inhibition (Fig. 4A). These findings were again replicated in two p53 mutant cell lines(Fig. 4C,D,E and Fig. S4I). Importantly, in addition to short-term cell viability effects, exogenous CIP2A expression also significantly inhibited Chk1 and Claspin siRNA effects in a clonogenicity assay in AGS cells (Fig. 4F).
Figure 4.
Inhibition of CIP2A expression defines cellular response to Chk1 inhibition. A-E, Exogenous CIP2A expression (CIP2A Flag) prevents inhibition of CIP2A protein expression, and cell viability in Chk1 siRNA transfected AGS (A and B), MKN-28 (C and D) and MDA-MB231 (E) cells. F, Exogenous CIP2A expression (CIP2A Flag) prevents inhibition of colony growth of Chk1 and Claspin siRNA transfected AGS cells. G, Effect of small molecule inhibitor of Chk1, SB218078 (1uM for 48h) on CIP2A expression in CIP2A-dependent AGS and CIP2A-independent PNT2 cells. H, Effect of small molecule inhibitor of Chk1, SB218078 (1uM for 48h) on cell viability of AGS and PNT2 cells. Shown are mean values + S.D., from three to four independent experiments (student's t-test).
As an independent approach to demonstrate relevance of CIP2A regulation for therapeutic response to Chk1 inhibition, we assessed Chk1 inhibitor effects on CIP2A expression and viability regulation in an immortalized human prostate cell line PNT2 (29) that we have recently identified as a rare cell line, whose viability is not inhibited by CIP2A-targeted siRNA (unpublished results). Interestingly, as compared to one of the CIP2A-dependent cell lines, AGS, inhibition of Chk1 activity in PNT2 cells did not result in inhibition of CIP2A expression (Fig. 4G). Moreover, PNT2 cells were significantly more resistant to inhibition of cell viability in response to Chk1 inhibitor treatment(Fig. 4H).
Together, these results strongly indicate inhibition of CIP2A expression as a decisive mechanism whether cell viability is impaired in response to Chk1 inhibition.
Chk1 inhibition re-activates PP2A tumor suppressor activity
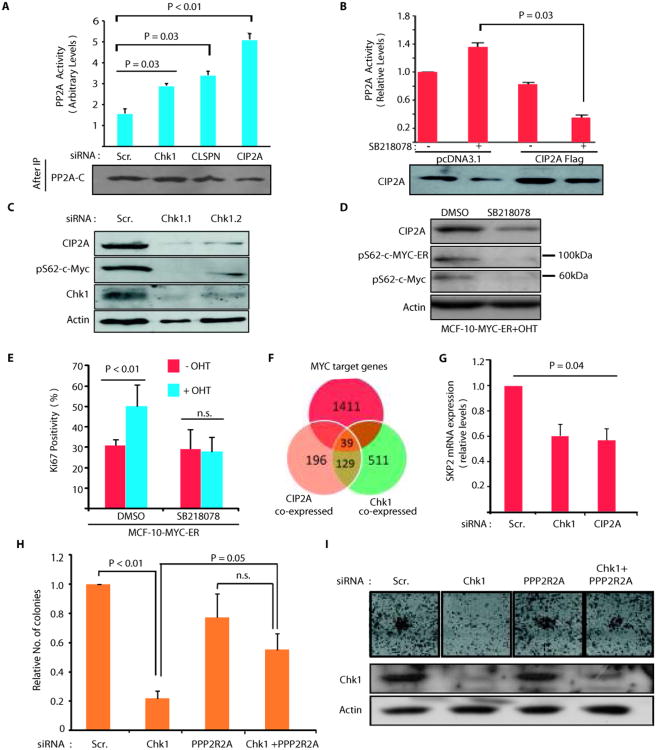
CIP2A interacts with PP2A and inhibits its tumor suppressor phosphatase activity towards several phosphoprotein targets, including MYC, AKT, DAPK and E2F1(4-7, 12). Consequently, siRNA-mediated inhibition of CIP2A, Chk1 or Claspin expression re-activated PP2A phosphatase activity in AGS cells(Fig. 5A). Importantly, in cells in which CIP2A downregulation by Chk1 inhibitor SB218078 was prevented by exogenous CIP2A expression, SB218078 treatment rather decreased than increased PP2A activity(Fig. 5B). These results further demonstrate that CIP2A expression levels define cellular response to Chk1 inhibition.
Figure 5.
Chk1 inhibition re-activates PP2A tumor suppressor activity. A, Depletion of Chk1, Claspin or CIP2A induces PP2A activity in AGS cells. B, PP2A activity in pcDNA3.1 or CIP2A Flag transfected AGS cells treated with SB218078 (1uM; 48h) as indicated. C, Chk1 depletion inhibits CIP2A protein expression and of serine62 phosphorylated MYC in AGS cells 72h post transfection. D, Western blot analysis of SB218078 effects(1 uM, 24h) on serine62 phosphorylation of MYC-ER fusion protein (appr. 100 kDa) or endogenous MYC protein (appr. 65 kDa) in tamoxifen-treated MCF-10 cells stably transfected with MYC-ER. E, Serum starved MYC-ER expressing MCF-10A cells, pretreated for 24h with either DMSO or SB218078(1 uM), were induced to MYC-mediated proliferation by tamoxifen treatment. Quantitation of Ki-67 positive cells demonstrates requirement of Chk1 activity for MYC-induced proliferation. F, Venn diagram displaying overlap of genes that significantly associate with either CIP2A (orange) or Chk1 (green) or MYC (red) expression in human neuroblastomas (n= 168), p < 10−10, hypergeometric distribution. G, Real-time PCR analysis of SKP2 mRNA expression from AGS cells transfected with CIP2A and CHk1 siRNA for 72 hours. H-I, Inhibition of CIP2A target PP2A B-subunit PPP2R2A expression by siRNA rescues Chk1 siRNA effects on the AGS cell colony growth. Shown are mean values + S.D., of representative results from three independent experiments (student's t-test).
To verify the PP2A activity assay results, and to examine functional effects of PP2A re-activation in Chk1-inhibited cells, expression of serine 62 phosphorylated MYC, the prototypic target for CIP2A-regulated PP2A activity(4, 10, 12) was studied after depletion of Chk1, Claspin and CIP2A. Transfection of Chk1, Claspin or CIP2A siRNA resulted in inhibition of expression of both CIP2A and serine 62 phosphorylated MYC(Fig. 5C and Fig. S5A). However, neither Chk1 nor CIP2A siRNA inhibited MYC mRNA expression, confirming post-transcriptional regulation of MYC(Fig. S5B). We have previously shown that CIP2A expression levels positively correlate with MYC protein expression in gastric cancer(12). Importantly, in the same tumor material, also Chk1 and nuclear MYC protein were found to be co-expressed(Table S1).
To examine whether Chk1 activity contributes to MYC-mediated proliferation, we employed conditional MYC-ER expressing MCF-10A cells, that after serum starvation are driven to MYC-induced cell cycle progression by nuclear translocation of MYC-ER fusion protein in response to tamoxifen treatment(30). Similar to endogenous MYC, serine 62 phosphorylation of MYC-ER was also inhibited by SB218078, along with CIP2A downregulation (Fig. 5D), whereas, in accordance with post-translational mechanism of MYC regulation by CIP2A, total MYC-ER expression was not affected(Fig. S5C). Intriguingly, SB218078-elicited inhibition of CIP2A expression and MYC serine 62 phosphorylation was accompanied by total loss of MYC-induced proliferation of growth factor deprived cells, as measured by the number of Ki-67 and histone 3 phosphorylation(ser-10) positive cells, 24 hours after tamoxifen application(Fig. 5E and Fig. S5D).
To further validate the assumption that Chk1 promotes MYC activity through CIP2A, we identified sets of genes that are co-expressed with CIP2A or Chk1 (absolute Spearman correlation ρ, p < 10−10, t-distribution) in the neuroblastoma database(25), and assessed whether MYC target genes would be particularly enriched among genes that associated with CIP2A and Chk1. The CIP2A and Chk1 associated expression profiles(Table S2) were highly positively correlated (ρ, p < 10−11), which further implies a functional link between them. Importantly, MYC target genes comprised almost 1/4 of the 168 genes that were associated with CIP2A and Chk1 and thus showed very significant enrichment (39 shared MYC targets, p < 10−10, hypergeometric distribution)(Fig. 5F). Among the Chk1 and CIP2A correlating MYC targets, SKP2 is an independently validated direct MYC target that mediates MYC's proliferative activity(31, 32). Significant inhibition of SKP2 gene expression was validated in AGS cells transfected with both Chk1 and CIP2A siRNAs(Fig. 5G).
We recently showed that depletion of PP2A complex B-subunit PPP2R2A reversed CIP2A RNAi effects on proliferation, and on regulation of MYC target genes(8). Therefore, to finally link CIP2A, PP2A and MYC to Chk1-regulated clonogenicity, we assessed whether depletion of PPP2R2A could reverse effects of Chk1 RNAi. Indeed, cells that were depleted of PPP2R2A(Fig. S5E) were relatively resistant to inhibition of colony growth by Chk1 siRNA(Fig. 5H,I).
Identification of DNA-PK as upstream mediator of Chk1 serine 345 phosphorylation and CIP2A expression in unperturbed cancer cells
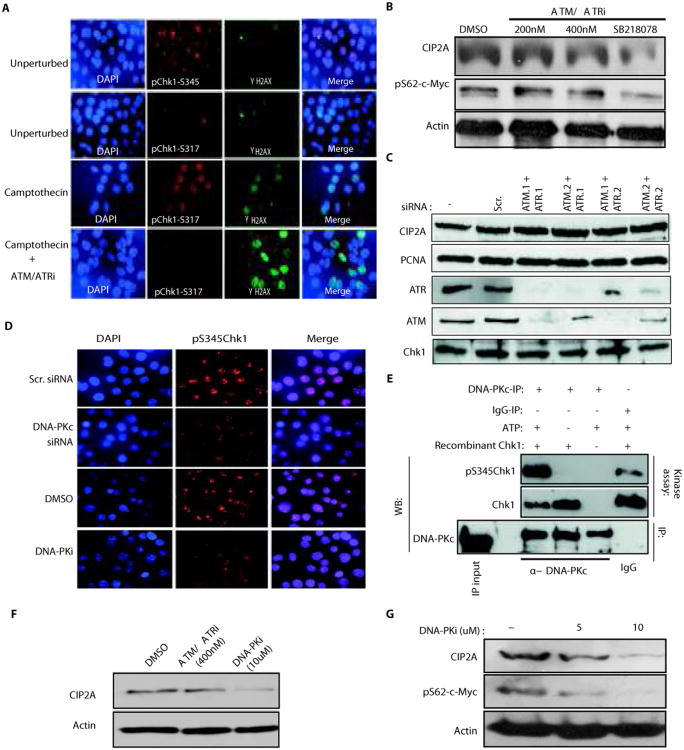
Upon acutely induced DNA-damage, Chk1 is phosphorylated on both serines 317 and 345 via ATM/ATR-mediated mechanisms(14, 22). However, the mechanisms supporting serine 345 phosphorylation in cancer cells in the absence of DNA-damaging agents are not yet understood. Importantly, a recent study demonstrated a fundamental difference between the biological consequences of phosphorylation of Chk1 on either serine 317 or 345(33). In agreement with the requirement of Chk1 serine345 phosphorylation for cell viability in unperturbed conditions(33), in exponentially growing AGS cells, Chk1 was constitutively phosphorylated on serine 345, whereas serine 317 phosphorylation was hardly detected under these conditions (Fig. 6A). Constitutive serine345 Chk1 staining was specific, as demonstrated by the loss of positive staining for phosphorylated serine 345 on Chk1 in cells transfected with two individual siRNAs targeting Chk1(Fig. S5F). When excessive DNA damage was induced by camptothecin treatment, however, ATM/ATR-dependent phosphorylation of serine 317 at Chk1 was observed (Fig. 6A). In line with previously published results(14, 22), induction of DNA damage by camptothecin also increased Chk1 serine 345 phosphorylation, and this DNA-damage induced serine345 phosphorylation of Chk1 was sensitive to ATM/ATR inhibition (Fig. S5G). However, while camptothecin-induced phosphorylation of both serines 317 and 345 of Chk1 were effectively blocked by treatment with chemical ATM/ATR inhibitor (Fig. 6A and Fig. S5G), neither Chk1 serine 345 phosphorylation (Fig. S5H), expression of CIP2A, or expression of serine 62 phosphorylated MYC (Fig. 6B), were affected by treatment with the ATM/ATR inhibitor in unperturbed cells. Furthermore, CIP2A expression was not affected by siRNA-mediated inhibition of ATM/ATR expression in either MKN-28 (Fig. 6C) or HeLa cells (Fig. S5I). Together these results confirm involvement of different regulatory mechanisms of serine 345 phosphorylation of Chk1 between unperturbed cells, and cells in which excessive DNA-damage has been induced. Furthermore, these results strongly indicate that constitutive Chk1 serine 345 phosphorylation, and Chk1-mediated regulation of CIP2A, occurs via an ATM/ATR-independent mechanism.
Figure 6.
DNA-PKc acts as an upstream mediator of Chk1 serine345 phosphorylation and CIP2A expression in unperturbed cancer cells. A, Immunoflourescent stainings of unperturbed and Camptothecin (400nM for 24h) and/or ATM-ATR inhibitor (400 nM for 24h) treated AGS cells with indicated antibodies and DAPI. γ-H2AX staining was used to demonstrate Camptothecin-induced double-stranded DNA breaks. B, Western blot analysis of CIP2A and phosphoserine62 MYC expression levels in AGS cells treated either with indicated concentrations of ATM/ATR inhibitor (ATM/ATRi) or with Chk1 inhibitor SB218078(1uM) for 48 h. C, Expression of CIP2A, PCNA, ATM, ATR and Chk1 proteins in MKN-28 cells co-transfected with two independent siRNAs targeting both ATM and ATR, 72h post-transfection. D, Immunoflourescent stainings of AGS cells with DAPI and phospho Chk1-Serine-345 antibody after either transfection of DNA-PK targeting siRNA or treatment with DNA-PK inhibitor (DNA-PKi; 10uM for 48h). E, DNA-PKc immunoprecipitated from exponentially growing MKN-28 cells was used in in-vitro kinase assay with recombinant Chk1 protein as a substrate. Chk1 protein amounts and serine345 phosphorylation was studied form kinase reaction by western blotting. F, CIP2A protein expression in AGS cells treated for 48h with ATM/ATR inhibitor(400nM) or DNA-PK inhibitor(10uM). G, CIP2A protein expression and phospho-MYC-serine62 levels in MKN-28 cells treated for 48h with DNA-PK inhibitor at indicated concentrations. Shown are representative results from two independent experiments.
Therefore, we examined whether a different DDR kinase was involved. The DNA-dependent protein kinase (DNA-PK) transduces DNA-damage responses and directly phosphorylates Chk2(34), making it a plausible candidate to mediate also Chk1 phosphorylation. Importantly, DNA-PKc inhibition, by either the specific small molecule inhibitor DMNB(DNA-PKi), or by DNA-PKc specific siRNAs (Fig.S5J), inhibited expression of serine 345 phosphorylated Chk1(Fig. 6D). Moreover, in an in vitro kinase assay, DNA-PKc immunoprecipitated from MKN-28 cells was capable of phosphorylating recombinant Chk1 on serine 345, identifying DNA-PKc as Chk1 serine 345 kinase (Fig. 6E). Inhibition of DNA-PK by either DNA-PKi or by RNAi, inhibited the expression of CIP2A (Fig. 6F and Fig. S5K) and, similar to inhibition of Chk1 (Fig. 5C) and CIP2A(4, 8, 10, 12)(Fig. S5A), DNA-PKc inhibition also inhibited the expression of serine 62 phosphorylated MYC (Fig. 6G). Together these results indicate that in unperturbed cancer cells, DNA-PKc activity supports Chk1 serine 345 phosphorylation and CIP2A expression. However, our data does not exclude other unidentified active signaling mechanisms that may contribute to Chk1 serine 345 phosphorylation and activity in unperturbed cancer cells.
Discussion
New generation small molecule inhibitors of Chk1 demonstrate single agent therapeutic activity in several preclinical cancer models(13, 15, 16, 18-20). However, most of our current knowledge related to regulation and functional role of Chk1 has been acquired from experiments in which DNA damage has been induced acutely in combination with Chk1 inhibition(14, 22, 24, 35). When Chk1 is inhibited in this context, inability to repair the damaged DNA leads to cellular crisis and massive apoptosis(14, 24). This synthetic lethal interaction between DNA damage induction and Chk1 inhibition, have served as the dogmatic model to explain non-oncogene addiction of malignant cells to Chk1 activity(14, 24, 35). However, our understanding of the mechanisms, which contribute to single agent activity of Chk1-targeted therapies in cancer cells with continuous Chk1 phosphorylation, is very limited.
In this study, we reveal hitherto unrecognized downstream mechanism by which chronic Chk1 activity promotes cancer cell viability and clonogenicity. Our results demonstrate that inhibition of Chk1 activity inhibits expression of human oncoprotein CIP2A, which in turn results in reactivation of PP2A tumor suppressor activity (Fig. 7). Importantly, results of several experiments demonstrate that CIP2A expression levels define cellular response to Chk1 inhibition in vitro. CIP2A dependency of Chk1-regulated phenotypes was evidenced both at the level of regulation of PP2A activity (Fig. 5B), and cell viability (Fig. 4A-F). As a proof-of-principle functional target for this newly identified pathway, we show that both MYC serine 62 phosphorylation, and MYC activity are regulated by Chk1 and CIP2A (Fig. 5C-G). General applicability of Chk1-dependent regulation of CIP2A expression and the functional relevance of this regulation was confirmed by using several cell lines derived from different types of human cancers irrespective of p53 status. Importantly, in addition to in vitro data, we also present significant evidence indicating that Chk1-CIP2A-MYC pathway functions in vivo in tumors. These data include demonstration of co-expression of Chk1, CIP2A and MYC in human tumors as well as prognostic role of both Chk1 and CIP2A in the same tumor type. Moreover, we show that among the genes which expression significantly associates with Chk1 and CIP2A expression in human tumors, MYC target genes are significantly over-presented. Lastly, we show that in in vivo tumors that are dependent on either Chk1 or CIP2A expression, inhibition of Chk1 activity by small molecules in clinical development results in inhibition of CIP2A transcription. In future, it would be of great interest to use genetically modified mouse models to assess the degree by which PP2A inhibition contributes to in vivo tumor response to single-agent Chk1 inhibition. It is anticipated that high CIP2A expression, or loss of PP2A B-subunit PPP2R2A observed recently in human breast cancer(37), would induce relative resistance to Chk1 inhibitors due to lack of induction of PP2A tumor suppressor activity.
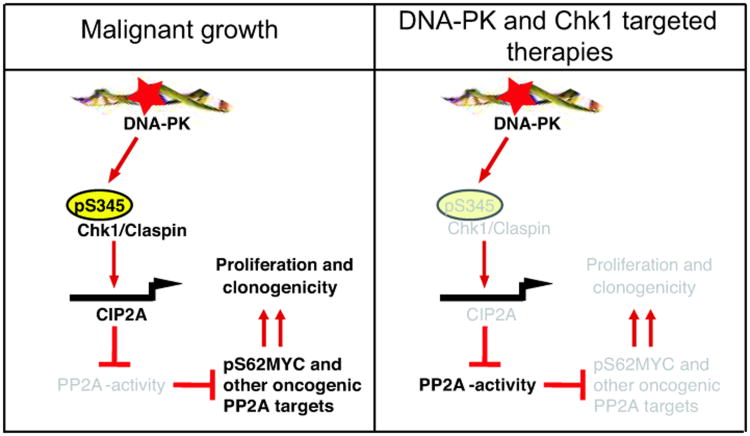
Figure 7.
In unperturbed cancer cells, DNA-PK activity promotes constitutive Chk1 serine 345 phosphorylation and CIP2A expression. (Left panel) Constitutively active Chk1 together with Claspin, promotes CIP2A gene transcriptio and protein expression. CIP2A in turn inhibits PP2A tumor suppressor activity and thereby increases activity/expression of oncogenic PP2A targets (such as MYC). (Right panel) Inhibition of the Chk1 activity by means of cancer therapy results in increase in PP2A activity, dephosphorylation of PP2A target proteins, and inhibition of cancer cell viability. Inactive molecules and functions are shown in grey.
Our results strongly indicate that constitutive serine 345 phosphorylation of Chk1 promotes CIP2A expression and cancer cell viability in unperturbed conditions. Association of Chk1 serine 345 phosphorylation with increased cell viability is strongly supported by recent study by Bunz and collaborators demonstrating that, whereas serine 317 phosphorylation of Chk1 is not relevant to cell viability or proliferation in unperturbed conditions, mutated serine 345 did not support viability(33). Moreover, a recent study showed that constitutive phosphorylation of serine 345 was observed in human neuroblastoma cell lines derived only from high-risk primary tumors(20). Consequently, only the cell lines expressing serine 345 phosphorylated Chk1 were highly sensitive to small molecule Chk1 inhibitors also used in the present study(20). Association of high Chk1 serine 345 phosphorylation with increased malignancy of cancer cells perfectly corroborates the findings that CIP2A expression associates with increased tumor grade and poor patient prognosis in most of the studied human cancer types(4, 9, 10, 12).
The upstream mechanisms promoting serine 345 phosphorylation in unperturbed cells have been elusive. Even though ATR kinase mediates serine 345 phosphorylation of Chk1 under acute DNA damage conditions(14, 22)(Fig. S5G), inhibition of ATR did not affect expression of CIP2A or serine 62 phosphorylated MYC (Fig. 6B,C and S5I). Instead, we identify another member of the phosphatidylinositol 3-kinase-related kinase family of DNA damage response kinases, DNA-PK, as a strong candidate for mediating Chk1 serine 345 phosphorylation in unperturbed conditions. We also show, that DNA-PK inhibition either by RNAi or by small molecule inhibitor, recapitulates Chk1-dependent regulation of CIP2A expression and MYC serine 62 phosphorylation(Fig. 6F,G and S5J,K). Altogether, these results provide novel insights for understanding upstream mechanisms of chronic Chk1 activity in unperturbed cancer cells.
PP2A inactivation is a universal characteristic of cancer cells(1-3). Thereby, identification of novel link between DNA damage signaling and PP2A inhibition in malignant cells fosters our general understanding of constitutively active signaling circuits in cancer cells (Fig. 7). Considering the plethora of phosphoproteins regulated by PP2A(36), we postulate that in addition to MYC serine 62 phosphorylation, other oncogenic PP2A targets will be identified in future to be regulated by Chk1-driven CIP2A expression in human malignancies. Supporting this, MYC-independent but PP2A-dependent CIP2A target mechanisms and proteins have been recently identified(5-8). Therefore, our data does not exclude that other CIP2A-regulated PP2A targets than MYC would be involved in inhibition of cell viability in Chk1-inhibited cancer cells (Fig. 7). As a matter of fact, function of CIP2A as an inhibitor of broad specificity serine/threonine phosphatase complex PP2A(36), most likely explains how expression status of only one gene, CIP2A, may define functional outcome of Chk1 inhibition in unperturbed cancer cells in vitro.
Clinically, results of this work may help in understanding of the molecular basis of poor response of cancer patients to Chk1 inhibitor monotherapy. Moreover, our results indicate that assessment of transcriptional effects of Chk1 inhibition may unveil novel biomarkers of tumor response to Chk1 inhibitors. On the other hand, based on our results, it is plausible that re-activation of PP2A, by targeting of CIP2A, or by other means, would be therapeutically beneficial in cancers that are dependent on chronic Chk1 activity.
Supplementary Material
Acknowledgments
We thank Drs. D. Bohmann, T. Mäkelä and J. Ivaska for critical reading of the manuscript. Dr. D. Gillespie is greatly thanked for his critical advice during this project. Drs. M. Annala and M. Nykter are greatly acknowledged for their help in bioinformatics analysis. Dr. E. Chan is acknowledged for CIP2A antibody and Dr. H. Van Dam for 5xJunLuc construct. Cathy Chang and Cory Painter are acknowledged for their advice regarding in vivo use of Chk1 inhibitor.
Funding: This work was supported by Academy of Finland (grants 8217676, 122546 and 137687), Foundation for Finnish Cancer Institute, Finnish Cancer Associations, Sigrid Juselius Foundation, The Finnish Funding Agency for Technology and Innovation (TEKES), and by competitive research funding from Tampere University Hospital district.
Footnotes
The Authors Disclose No Potential Conflicts of Interest
References
- 1.Hahn WC, Weinberg RA. Rules for making human tumor cells. N Engl J Med. 2002;347:1593–603. doi: 10.1056/NEJMra021902. [DOI] [PubMed] [Google Scholar]
- 2.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell. 2007;130:21–4. doi: 10.1016/j.cell.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Sablina AA, Hahn WC. SV40 small T antigen and PP2A phosphatase in cell transformation. Cancer metastasis reviews. 2008;27:137–46. doi: 10.1007/s10555-008-9116-0. [DOI] [PubMed] [Google Scholar]
- 4.Junttila MR, Puustinen P, Niemela M, Ahola R, Arnold H, Bottzauw T, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 5.Chen KF, Liu CY, Lin YC, Yu HC, Liu TH, Hou DR, et al. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene. 2010;29:6257–66. doi: 10.1038/onc.2010.357. [DOI] [PubMed] [Google Scholar]
- 6.Guenebeaud C, Goldschneider D, Castets M, Guix C, Chazot G, Delloye-Bourgeois C, et al. The dependence receptor UNC5H2/B triggers apoptosis via PP2A-mediated dephosphorylation of DAP kinase. Molecular cell. 2010;40:863–76. doi: 10.1016/j.molcel.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 7.Laine A, Sihto H, Come C, Rosenfeldt MT, Zwolinska A, Niemela M, et al. Senescence Sensitivity of Breast Cancer Cells Is Defined by Positive Feedback Loop between CIP2A and E2F1. Cancer discovery. 2013;3:182–97. doi: 10.1158/2159-8290.CD-12-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niemelä M, Kauko O, Sihto H, Mpindi JP, Nicorici D, Pernila P, et al. CIP2A signature reveals the MYC dependency of CIP2A-regulated phenotypes and its clinical association with breast cancer subtypes. Oncogene. 2012;31:4266–78. doi: 10.1038/onc.2011.599. [DOI] [PubMed] [Google Scholar]
- 9.Come C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 10.Mathiasen D, Egebjerg C, Andersen S, Rafn B, Puustinen P, Khanna A, et al. Identification of a c-Jun N-terminal kinase-2-dependent signal amplification cascade that regulates c-Myc levels in ras transformation. Oncogene. 2011;31:390–401. doi: 10.1038/onc.2011.230. [DOI] [PubMed] [Google Scholar]
- 11.Ventela S, Come C, Makela JA, Hobbs RM, Mannermaa L, Kallajoki M, et al. CIP2A promotes proliferation of spermatogonial progenitor cells and spermatogenesis in mice. PloS one. 2012;7:e33209. doi: 10.1371/journal.pone.0033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khanna A, Bockelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. Journal of the National Cancer Institute. 2009;101:793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 13.Basu B, Yap TA, Molife LR, de Bono JS. Targeting the DNA damage response in oncology: past, present and future perspectives. Current opinion in oncology. 2012;24:316–24. doi: 10.1097/CCO.0b013e32835280c6. [DOI] [PubMed] [Google Scholar]
- 14.Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Advances in cancer research. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 15.Murga M, Campaner S, Lopez-Contreras AJ, Toledo LI, Soria R, Montana MF, et al. Exploiting oncogene-induced replicative stress for the selective killing of Myc-driven tumors. Nat Struct Mol Biol. 2011;18:1331–5. doi: 10.1038/nsmb.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrao PT, Bukczynska EP, Johnstone RW, McArthur GA. Efficacy of CHK inhibitors as single agents in MYC-driven lymphoma cells. Oncogene. 2012;31:1661–72. doi: 10.1038/onc.2011.358. [DOI] [PubMed] [Google Scholar]
- 17.Tho LM, Libertini S, Rampling R, Sansom O, Gillespie DA. Chk1 is essential for chemical carcinogen-induced mouse skin tumorigenesis. Oncogene. 2012;31:1366–75. doi: 10.1038/onc.2011.326. [DOI] [PubMed] [Google Scholar]
- 18.Hoglund A, Nilsson LM, Muralidharan SV, Hasvold LA, Merta P, Rudelius M, et al. Therapeutic implications for the induced levels of Chk1 in Myc-expressing cancer cells. Clin Cancer Res. 2011;17:7067–79. doi: 10.1158/1078-0432.CCR-11-1198. [DOI] [PubMed] [Google Scholar]
- 19.Walton MI, Eve PD, Hayes A, Valenti MR, De Haven Brandon AK, Box G, et al. CCT244747 is a novel potent and selective CHK1 inhibitor with oral efficacy alone and in combination with genotoxic anticancer drugs. Clin Cancer Res. 2012;18:5650–61. doi: 10.1158/1078-0432.CCR-12-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole KA, Huggins J, Laquaglia M, Hulderman CE, Russell MR, Bosse K, et al. RNAi screen of the protein kinome identifies checkpoint kinase 1 (CHK1) as a therapeutic target in neuroblastoma. Proceedings of the National Academy of Sciences of the United States of America; 2011; pp. 3336–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fishler T, Li YY, Wang RH, Kim HS, Sengupta K, Vassilopoulos A, et al. Genetic instability and mammary tumor formation in mice carrying mammary-specific disruption of Chk1 and p53. Oncogene. 2010;29:4007–17. doi: 10.1038/onc.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Q, Guntuku S, Cui XS, Matsuoka S, Cortez D, Tamai K, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes & development. 2000;14:1448–59. [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez-Contreras AJ, Gutierrez-Martinez P, Specks J, Rodrigo-Perez S, Fernandez-Capetillo O. An extra allele of Chk1 limits oncogene-induced replicative stress and promotes transformation. The Journal of experimental medicine. 2012;209:455–61. doi: 10.1084/jem.20112147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma CX, Janetka JW, Piwnica-Worms H. Death by releasing the breaks: CHK1 inhibitors as cancer therapeutics. Trends Mol Med. 2011;17:88–96. doi: 10.1016/j.molmed.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geerts D, Koster J, Albert D, Koomoa DL, Feith DJ, Pegg AE, et al. The polyamine metabolism genes ornithine decarboxylase and antizyme 2 predict aggressive behavior in neuroblastomas with and without MYCN amplification. International journal of cancer. 2010;126:2012–24. doi: 10.1002/ijc.25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, et al. Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell. 2008;132:221–32. doi: 10.1016/j.cell.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 27.Blasius M, Forment JV, Thakkar N, Wagner SA, Choudhary C, Jackson SP. A phospho-proteomic screen identifies substrates of the checkpoint kinase Chk1. Genome biology. 2011;12:R78. doi: 10.1186/gb-2011-12-8-r78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khanna A, Okkeri J, Bilgen T, Tiirikka T, Vihinen M, Visakorpi T, et al. ETS1 mediates MEK1/2-dependent overexpression of cancerous inhibitor of protein phosphatase 2A (CIP2A) in human cancer cells. PloS one. 2011;6:e17979. doi: 10.1371/journal.pone.0017979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthon P, Cussenot O, Hopwood L, Le Duc A, Maitland NJ. Functional expression of SV40 in normal human prostatic epithelial and fibroblastic cells: differentiation pattern of non-tumorigenic cell lines. Int J Oncol. 1995;6:333–43. doi: 10.3892/ijo.6.2.333. [DOI] [PubMed] [Google Scholar]
- 30.Partanen JI, Nieminen AI, Makela TP, Klefstrom J. Suppression of oncogenic properties of c-Myc by LKB1-controlled epithelial organization. Proceedings of the National Academy of Sciences of the United States of America; 2007; pp. 14694–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim J, Lee JH, Iyer VR. Global identification of Myc target genes reveals its direct role in mitochondrial biogenesis and its E-box usage in vivo. PloS one. 2008;3:e1798. doi: 10.1371/journal.pone.0001798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bretones G, Acosta JC, Caraballo JM, Ferrandiz N, Gomez-Casares MT, Albajar M, et al. SKP2 oncogene is a direct MYC target gene and MYC down-regulates p27(KIP1) through SKP2 in human leukemia cells. The Journal of biological chemistry. 2011;286:9815–25. doi: 10.1074/jbc.M110.165977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilsker D, Petermann E, Helleday T, Bunz F. Essential function of Chk1 can be uncoupled from DNA damage checkpoint and replication control. Proceedings of the National Academy of Sciences of the United States of America; 2008; pp. 20752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Stern DF. Regulation of CHK2 by DNA-dependent protein kinase. The Journal of biological chemistry. 2005;280:12041–50. doi: 10.1074/jbc.M412445200. [DOI] [PubMed] [Google Scholar]
- 35.Lord CJ, Ashworth A. The DNA damage response and cancer therapy. Nature. 2012;481:287–94. doi: 10.1038/nature10760. [DOI] [PubMed] [Google Scholar]
- 36.Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Molecular cell. 2009;33:537–45. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 37.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346–52. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.