Abstract
Ten percent of North American patients with non–small-cell lung cancer have tumors with somatic mutations in the gene for the epidermal growth factor receptor (EGFR). Approximately 70% of patients whose lung cancers harbor somatic mutations in exons encoding the tyrosine kinase domain of EGFR experience significant tumor regressions when treated with the EGFR tyrosine kinase inhibitors (TKIs) gefitinib or erlotinib. However, the overwhelming majority of these patients inevitably acquire resistance to either drug. Currently, the clinical definition of such secondary or acquired resistance is not clear. We propose the following criteria be used to define more precisely acquired resistance to EGFR TKIs. All patients should have the following criteria: previous treatment with a single-agent EGFR TKI (eg, gefitinib or erlotinib); either or both of the following: a tumor that harbors an EGFR mutation known to be associated with drug sensitivity or objective clinical benefit from treatment with an EGFR TKI; systemic progression of disease (Response Evaluation Criteria in Solid Tumors [RECIST] or WHO) while on continuous treatment with gefitinib or erlotinib within the last 30 days; and no intervening systemic therapy between cessation of gefitinib or erlotinib and initiation of new therapy. The relatively simple definition proposed here will lead to a more uniform approach to investigating the problem of acquired resistance to EGFR TKIs in this unique patient population. These guidelines should minimize reporting of false-positive and false-negative activity in these clinical trials and would facilitate the identification of agents that truly overcome acquired resistance to gefitinib and erlotinib.
INTRODUCTION
Approximately 70% of patients whose lung cancers harbor somatic mutations in exons encoding the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) will experience significant tumor regressions when treated with the EGFR tyrosine kinase inhibitors (TKIs) gefitinib or erlotinib.1–3 However, the overwhelming majority of these patients inevitably develop acquired resistance to either drug. Currently, the clinical definition of such secondary or acquired resistance is not clear. On treatment failure, many patients with acquired resistance have been found to have second site EGFR mutations, MET amplification, or both,4–6 and this has led to a number of trials of novel agents targeting these aberrations. However, these trials have not routinely mandated genotyping of a patient's tumor on study entry and have used different inclusion/exclusion criteria, especially with respect to the duration of time a patient must be treated with an EGFR TKI before enrollment and/or the duration of time a patient should be off the EGFR TKI before starting therapy. A clear and consistent definition of acquired resistance (Table 1) will help create standard entry criteria for the studies of such patients in clinical trials. In turn, this definition should help facilitate clearer interpretation of results from such trials. Similar kinds of definitions have been published for chronic myelogenous leukemias7 and gastrointestinal stromal tumors,8 which are treated with the TKI imatinib and display a similar oncogene-addiction phenomenon.9
Table 1.
Criteria for Acquired Resistance to EGFR TKIs in Lung Cancer
|
Abbreviations: EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; RECIST, Response Evaluation Criteria in Solid Tumors.
PROPOSED CRITERIA FOR ACQUIRED RESISTANCE TO EGFR TKI
We propose that the following criteria be used to define more precisely the clinical state of acquired resistance. These criteria are based on the published literature. All patients should have the following:
Previously received treatment with a single-agent EGFR TKI (eg, gefitinib or erlotinib). (The therapeutic contribution of an EGFR TKI is difficult to assess if it was combined with other targeted or chemotherapeutic agents.)
Either or both of the following: a tumor that harbors an EGFR mutation known to be associated with drug sensitivity (ie, G719X, exon 19 deletion, L858R, L861Q) or objective clinical benefit from treatment with an EGFR TKI as defined by either documented partial or complete response (Response Evaluation Criteria in Solid Tumors [RECIST] or WHO) or significant and durable (≥ 6 months) clinical benefit (stable disease as defined by RECIST or WHO) after initiation of gefitinib or erlotinib. (Patients with only symptomatic improvement while on EGFR TKI but no corresponding evidence of radiographic stability of disease should not be routinely considered as having acquired resistance. Recovery from toxicity of prior therapies and/or biologically indolent, potentially slow-growing disease may be present in a meaningful proportion of patients.)
These criteria are based on multiple studies demonstrating that lung tumors with specific kinase domain mutations of EGFR comprise a distinct molecular subset of lung cancers with increased sensitivity to gefitinib or erlotinib.10–12 Approximately 70% of patients whose tumors harbor drug-sensitizing EGFR mutations respond radiographically to EGFR TKIs, compared with less than 5% of North American patients with non–small-cell lung cancer with wild-type EGFR.1,3 The 6-month progression-free landmark was chosen based on data showing that only 26 of 86 patients (30%; 95% CI, 21% to 41%) with a drug-sensitive EGFR mutation have duration of response or stable disease less than 6 months (Table 2). Moreover, this figure is certainly overestimated as a result of the number of patients with EGFR mutations censored for progression before 6 months (n = 21). In addition, by using a 6-month progression-free landmark, only 30 of 132 patients (23%; 95% CI, 16% to 31%) without a mutation have a progression-free period exceeding 6 months.3 Given that many patients currently do not have their tumor EGFR mutation status determined before starting an EGFR TKI, the clinical definition of time to progression longer than 6 months will identify a population of patients in whom 60 of 91 patients (66%; 95% CI, 55% to 76%) have an EGFR-sensitizing mutation and 30 of 91 patients (33%; 95% CI, 23% to 44%) whose tumors harbor wild-type EGFR. This recommendation is also supported by recent data from the landmark IPASS (Iressa Pan-Asia Study) in which approximately 70% of patients with EGFR mutations had PFS exceeding 6 months, whereas only 8% without demonstrable mutation had PFS exceeding 6 months.13 The use of a 3-month progression-free landmark is less reliable in identifying patients whose tumors are most likely to harbor an EGFR mutation and whose subsequent progression is related to development of secondary resistance. The false-positive rate of a 3-month cutoff jumps to 44% (95% CI, 36% to 52%), compared with a false-positive rate of only 23% (95% CI, 16% to 31%) with the recommended 6-month landmark. Approximately 1% of patients have EGFR mutations known to be associated with resistance or of unknown biologic behavior at present.3 Following are additional criteria for acquired resistance to EGFR TKIs:
Systemic progression of disease (RECIST or WHO) while on continuous treatment with gefitinib or erlotinib within the last 30 days.
No intervening systemic therapy between cessation of gefitinib or erlotinib and initiation of new therapy.
Table 2.
Proportion of Patients Progression Free Over Time by EGFR Genotype
| PFS Time and EGFR Status | Patients |
|
|---|---|---|
| No./Total No. | % | |
| PFS < 3 months | ||
| EGFR mutation positive | 8/86 | 9 |
| EGFR mutation negative | 74/132 | 56 |
| PFS >3 months | ||
| EGFR mutation positive | 77/86 | 90 |
| EGFR mutation negative | 58/132 | 44 |
| PFS < 6 months | ||
| EGFR mutation positive | 26/86 | 30 |
| EGFR mutation negative | 102/132 | 77 |
| PFS > 6 months | ||
| EGFR mutation positive | 60/86 | 70 |
| EGFR mutation negative | 30/132 | 23 |
Abbreviations: EGFR, epidermal growth factor receptor; PFS, progression-free survival.
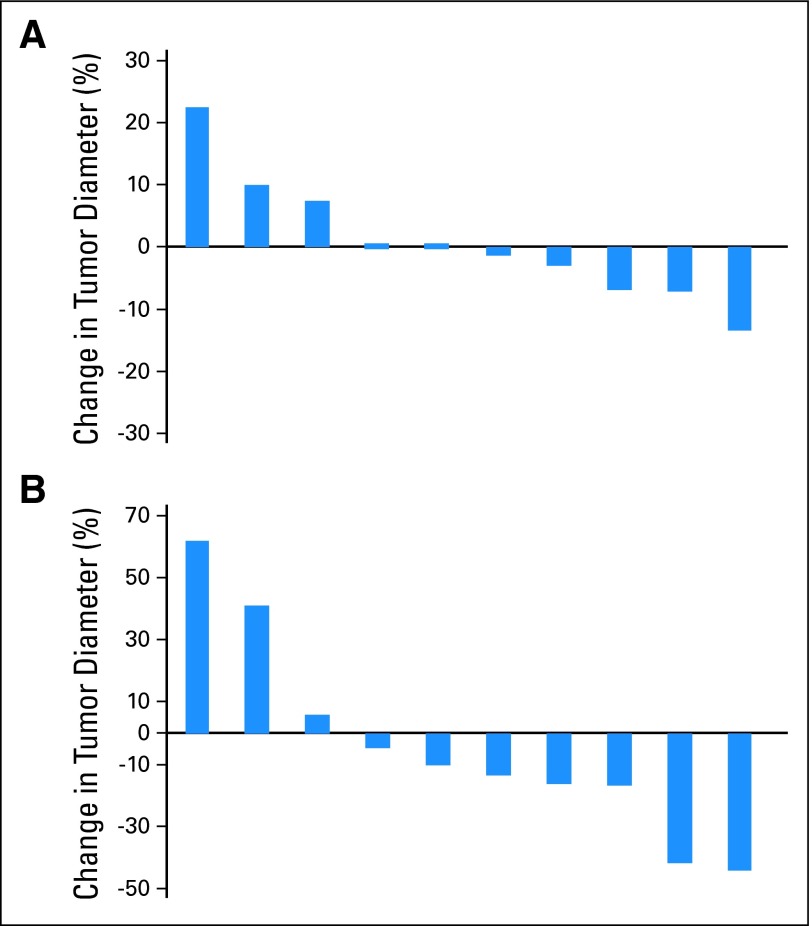
These criteria are proposed based on data showing that patients with EGFR-mutant tumors can display a disease flare within 3 weeks after stopping gefitinib or erlotinib.14 It is not known why such rapid progression occurs, but these observations suggest that continued EGFR inhibition retards cancer growth even when these cancers display slow disease progression. On reintroduction of an EGFR TKI after a drug holiday, tumor growth rates may slow, and tumors may regress in size (Fig 1A). Tumors may also display a decrease in metabolic activity as assessed by fluorodeoxyglucose avidity (Fig 1B). In clinical trials, such findings could be mistaken for therapeutic benefit. Therefore, we recommend minimizing the washout period between EGFR TKI discontinuation and initiation of the experimental agent or regimen. Whenever possible, this washout period should be no greater than 2 weeks (> five half-lives for both gefitinib and erlotinib); this criterion may not apply to patients in whom systemic toxicities attributable to EGFR TKI therapy have not adequately resolved. Patients off EGFR TKI treatment for more than 2 weeks should not be included. In addition, baseline radiographic imaging should be obtained as close as possible to commencing the experimental therapy. Optimally, the scan should be performed 1 day before or on the day of starting treatment. These measures will ensure that any responses are truly a result of the newly instituted treatment agent or regimen and not a result of re-treatment effect that might have been accomplished with gefitinib or erlotinib alone.
Fig 1.
Individual patient changes in the percentage of (A) tumor diameter by Response Evaluation Criteria in Solid Tumors (RECIST) or (B) fluorodeoxyglucose positron emission tomography maximum standardized uptake value 3 weeks after reintroduction of erlotinib or gefitinib in patients with previous response to gefitinib or erlotinib who had previously discontinued erlotinib or gefitinib.
Notably, special attention should be paid to patients whose disease progresses only in the CNS. Autopsy reports have shown that CNS metastases may remain free of mutations associated with secondary resistance, despite the development of such mutations in systemic sites of disease.15,16 This is likely a result of poor drug penetration in the CNS because sites of CNS progression in patients whose disease has been sensitive to therapy with an EGFR TKI may still have tumors that remain sensitive to treatment with the agent if adequate concentrations of drug can be delivered into the CNS.16 Therefore, patients who experience CNS-only relapse should not be considered as having systemic acquired resistance to EGFR TKI therapy. These patients could be considered for clinical trials of alternative dosing strategies of an EGFR TKI (unpublished data). These patients should be distinguished from those in which documented durable regression of brain metastases occurs followed by progression in the same lesions. Although the mechanism of progression may differ, it is reasonable to consider such patients as having acquired resistance because indeed the cause of such is unknown in many patients.
Finally, a number of trials are now being considered in which the EGFR TKI is continued despite disease progression and in which a targeted or cytotoxic agent is added to the EGFR TKI. For these trials, we recommend that patients initially receive the EGFR TKI alone for a 2- to 3-week period and that radiographic imaging be obtained before and at the end of this period. The latter imaging studies could be then used to eliminate reresponders, whereas patients with stable or progressive disease would proceed to receive the drug combination. This type of design would additionally allow evaluation of toxicity from the combination of EGFR TKI plus the second agent(s), as well as characterize any drug-drug interactions.
CONCLUSION
Use of the relatively simple, largely clinical, criteria proposed here to define this clinical situation will lead to a more uniform approach to investigating the problem of acquired resistance to EGFR TKIs in this unique patient population. These guidelines could help provide safeguards to minimize reporting of false-positive and false-negative activity signals in these clinical trials and would facilitate the identification of agents that truly overcome acquired resistance. Ideally, routine genotyping of tumors before study entry would largely overcome the uncertainties in clinical trial interpretation generated by the unique biology of EGFR-mutant lung cancers.
Footnotes
See accompanying editorial on page 191
Supported by the Memorial Sloan-Kettering Cancer Center, New York, NY, and Dana-Farber Cancer Institute, Boston, MA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: David Jackman, Genentech (C); William Pao, MolecularMD (C); Gregory J. Riely, AstraZeneca (C), Boehringer Ingelheim (C), Hoffmann-La Roche (C); Jeffrey A. Engelman, Millennium Pharmaceuticals (C), AVEO (C), Schering-Plough (C), Novartis (C), Bristol-Myers Squibb (C), Hoffmann-La Roche (C); Mark G. Kris, AstraZeneca (C), Boehringer Ingelheim (C), Pfizer (C); Pasi A. Jänne, Myriad Genetics (C), ARIAD (C), Boehringer Ingelheim (C), Janssen Pharmaceutica (C), Syndax (C), Pfizer (U), AstraZeneca (U); Thomas Lynch, Roche (C), AstraZeneca (C), Boehringer Ingelheim (C); Vincent A. Miller, Boehringer Ingelheim (C), Bristol-Myers Squibb (C), Genentech (C), Pfizer (C), Roche (C) Stock Ownership: Jeffrey A. Engelman, Gatekeeper Pharmaceuticals; Pasi A. Jänne, Gatekeeper Pharmaceuticals; Thomas Lynch, AstraZeneca, Biogen Idec, Boehringer Ingelheim, Genentech, Infinity Honoraria: David Jackman, Roche Research Funding: Jeffrey A. Engelman, Novartis; Pasi A. Jänne, Pfizer, AstraZeneca Expert Testimony: None Other Remuneration: William Pao, MolecularMD; Pasi A. Jänne, Genzyme; Thomas Lynch, Genzyme; Bruce E. Johnson, Postmarketing royalties from Dana-Farber Cancer Institute for EGFR testing
AUTHOR CONTRIBUTIONS
Conception and design: William Pao, Gregory J. Riely, Jeffrey A. Engelman, Mark G. Kris, Thomas Lynch, Vincent A. Miller
Provision of study materials or patients: David Jackman, Gregory J. Riely, Mark G. Kris, Bruce E. Johnson, Vincent A. Miller
Collection and assembly of data: David Jackman, Gregory J. Riely, Mark G. Kris, Bruce E. Johnson, Vincent A. Miller
Data analysis and interpretation: David Jackman, William Pao, Gregory J. Riely, Mark G. Kris, Pasi A. Jänne, Bruce E. Johnson, Vincent A. Miller
Manuscript writing: David Jackman, William Pao, Gregory J. Riely, Jeffrey A. Engelman, Mark G. Kris, Pasi A. Jänne, Thomas Lynch, Bruce E. Johnson, Vincent A. Miller
Final approval of manuscript: David Jackman, William Pao, Gregory J. Riely, Jeffrey A. Engelman, Mark G. Kris, Pasi A. Jänne, Thomas Lynch, Bruce E. Johnson, Vincent A. Miller
REFERENCES
- 1.Costa DB, Kobayashi S, Tenen DG, et al. Pooled analysis of the prospective trials of gefitinib monotherapy for EGFR-mutant non-small cell lung cancers. Lung Cancer. 2007;58:95–103. doi: 10.1016/j.lungcan.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller VA, Riely GJ, Zakowski MF, et al. Molecular characteristics of bronchioloalveolar carcinoma and adenocarcinoma, bronchioloalveolar carcinoma subtype, predict response to erlotinib. J Clin Oncol. 2008;26:1472–1478. doi: 10.1200/JCO.2007.13.0062. [DOI] [PubMed] [Google Scholar]
- 3.Jackman DM, Miller VA, Cioffredi LA, et al. Impact of epidermal growth factor receptor and KRAS mutations on clinical outcomes in previously untreated non-small cell lung cancer patients: Results of an online tumor registry of clinical trials. Clin Cancer Res. 2009;15:5267–5273. doi: 10.1158/1078-0432.CCR-09-0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci U S A. 2007;104:20932–20937. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 6.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 8.Van Glabbeke M, Verweij J, Casali PG, et al. Initial and late resistance to imatinib in advanced gastrointestinal stromal tumors are predicted by different prognostic factors: A European Organisation for Research and Treatment of Cancer-Italian Sarcoma Group-Australasian Gastrointestinal Trials Group study. J Clin Oncol. 2005;23:5795–5804. doi: 10.1200/JCO.2005.11.601. [DOI] [PubMed] [Google Scholar]
- 9.Weinstein IB. Cancer: Addiction to oncogenes:The Achilles heal of cancer. Science. 2002;297:63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 10.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 11.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 12.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok T, Wu Y-L, Thongprasert S, et al. Phase III, randomised, open-label, first-line study of gefitinib vs carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer (IPASS). Presented at the 33rd European Society for Medical Oncology Congress; September 12-16, 2008; Stockholm, Sweden. [Google Scholar]
- 14.Riely GJ, Kris MG, Zhao B, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res. 2007;13:5150–5155. doi: 10.1158/1078-0432.CCR-07-0560. [DOI] [PubMed] [Google Scholar]
- 15.Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res. 2006;12:6494–6501. doi: 10.1158/1078-0432.CCR-06-1570. [DOI] [PubMed] [Google Scholar]
- 16.Jackman DM, Holmes AJ, Lindeman N, et al. Response and resistance in a non–small-cell lung cancer patient with an epidermal growth factor receptor mutation and leptomeningeal metastases treated with high-dose gefitinib. J Clin Oncol. 2006;24:4517–4520. doi: 10.1200/JCO.2006.06.6126. [DOI] [PubMed] [Google Scholar]