Abstract
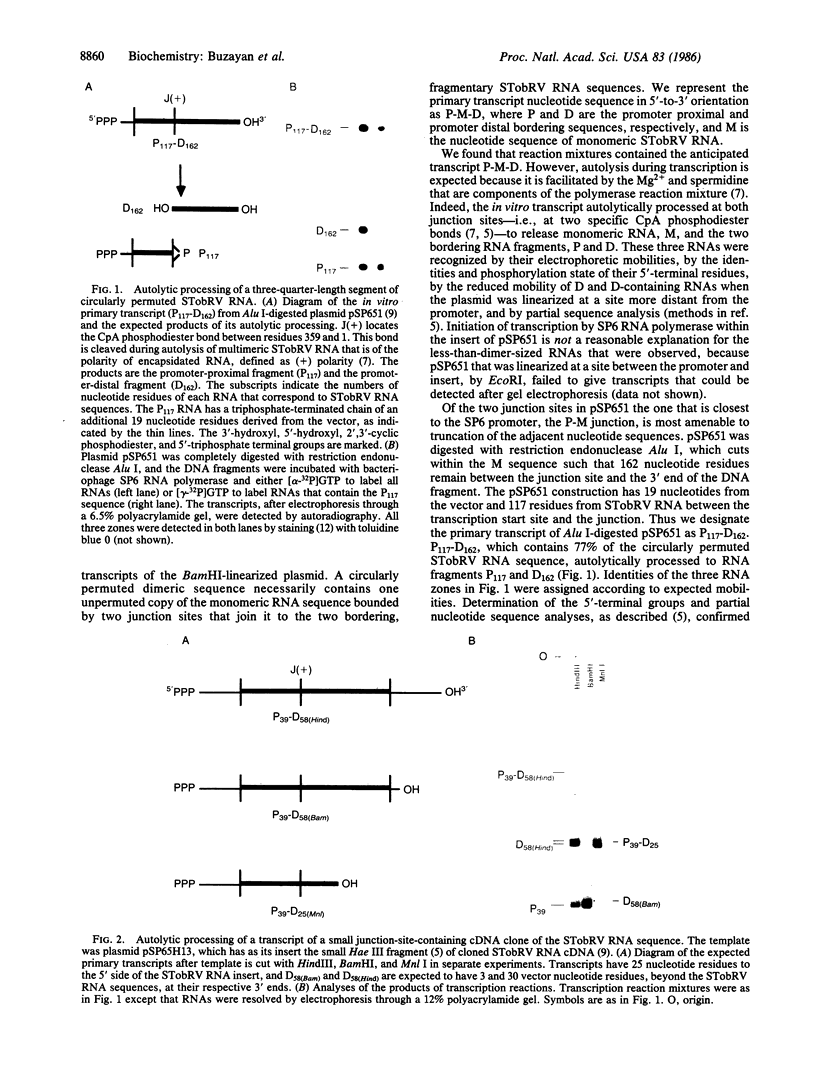
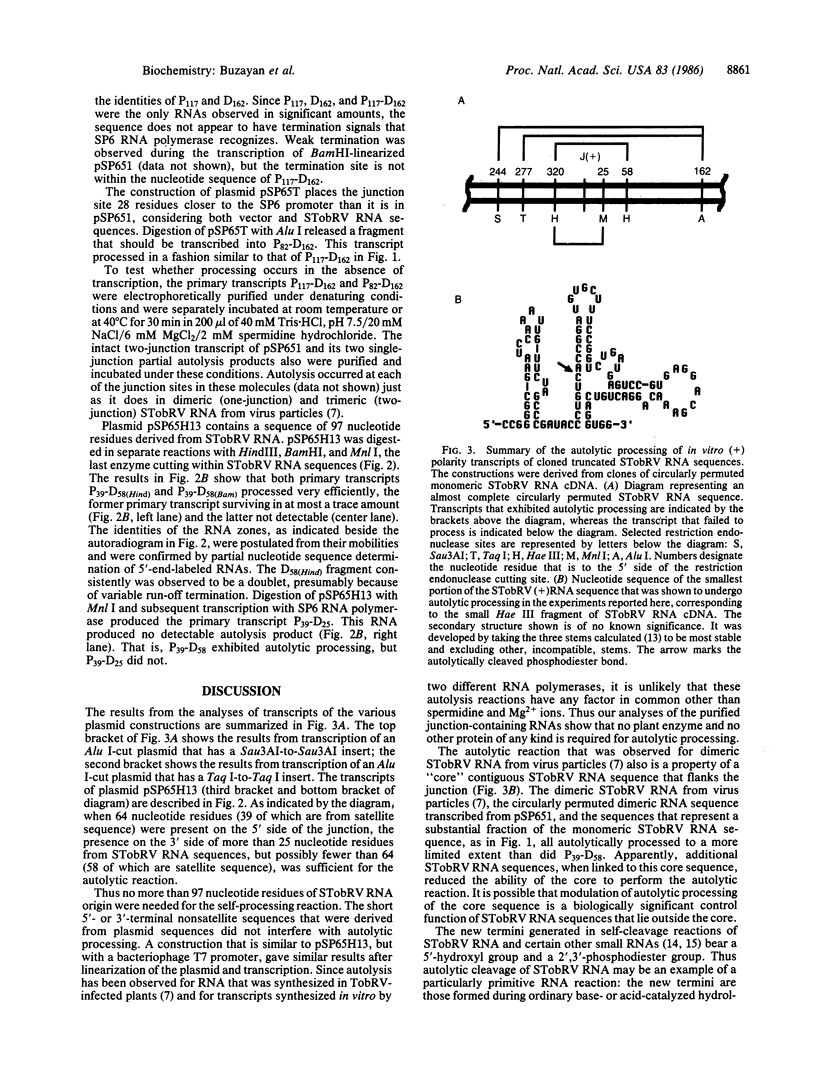
The satellite RNA of tobacco ringspot virus depends upon tobacco ringspot virus for its replication and source of coat protein. The satellite RNA reduces virus accumulation and the severity of virus-induced symptoms. Repetitive sequence, dimeric, and higher forms of the satellite RNA are known to autolytically process to form biologically active monomeric RNA of 359 nucleotide residues [Prody, G. A., Bakos, J. T., Buzayan, J. M., Schneider, I. R. & Bruening, G. (1986) Science 231, 1577-1580], with a 5′-hydroxyl and a 2′,3′-cyclic phosphodiester as the new terminal groups. We show here that transcripts of full-length and truncated DNA clones of the satellite RNA sequence also process in a nonenzymic reaction. One such transcript was an RNA that has about one-fourth of the satellite RNA sequence, representing the 3′-terminal and 5′-terminal portions of monomeric RNA joined in the junction that is cleaved in dimeric RNA. This RNA autolytically processed more efficiently than molecules with a larger proportion of the satellite RNA nucleotide sequence.
Keywords: plant virus, in vitro transcription, RNA processing efficiency
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. M., Irvine K. D., Kaneko K. J., Kerker B. J., Oettgen A. B., Tierney W. M., Williamson C. L., Zaug A. J., Cech T. R. Role of conserved sequence elements 9L and 2 in self-splicing of the Tetrahymena ribosomal RNA precursor. Cell. 1986 Apr 25;45(2):167–176. doi: 10.1016/0092-8674(86)90380-6. [DOI] [PubMed] [Google Scholar]
- Francki R. I. Plant virus satellites. Annu Rev Microbiol. 1985;39:151–174. doi: 10.1146/annurev.mi.39.100185.001055. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C., Haydock K., Allen L., Altman S. Metal ion requirements and other aspects of the reaction catalyzed by M1 RNA, the RNA subunit of ribonuclease P from Escherichia coli. Biochemistry. 1986 Apr 8;25(7):1509–1515. doi: 10.1021/bi00355a006. [DOI] [PubMed] [Google Scholar]
- Hutchins C. J., Rathjen P. D., Forster A. C., Symons R. H. Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 1986 May 12;14(9):3627–3640. doi: 10.1093/nar/14.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaper J. M., Tousignant M. E. Viral satellites: parasitic nucleic acids capable of modulating disease expression. Endeavour. 1984;8(4):194–200. doi: 10.1016/0160-9327(84)90084-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Symons R. H. Cucumber mosaic virus contains a functionally divided genome. Virology. 1973 Jun;53(2):487–492. doi: 10.1016/0042-6822(73)90232-8. [DOI] [PubMed] [Google Scholar]
- Prody G. A., Bakos J. T., Buzayan J. M., Schneider I. R., Bruening G. Autolytic processing of dimeric plant virus satellite RNA. Science. 1986 Mar 28;231(4745):1577–1580. doi: 10.1126/science.231.4745.1577. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Watson N., Gurevitz M., Ford J., Apirion D. Self cleavage of a precursor RNA from bacteriophage T4. J Mol Biol. 1984 Jan 25;172(3):301–323. doi: 10.1016/s0022-2836(84)80028-5. [DOI] [PubMed] [Google Scholar]





