Abstract
In 2005, it was estimated that more than 20 million people in the United States had diabetes. Approximately 30% of these people had undiagnosed cases. Increased risk for diabetes is primarily associated with age, ethnicity, family history of diabetes, smoking, obesity, and physical inactivity. Diabetes-related complications—including cardiovascular disease, kidney disease, neuropathy, blindness, and lower-extremity amputation—are a significant cause of increased morbidity and mortality among people with diabetes, and result in a heavy economic burden on the US health care system. With advances in treatment for diabetes and its associated complications, people with diabetes are living longer with their condition. This longer life span will contribute to further increases in the morbidity associated with diabetes, primarily in elderly people and in minority racial or ethnic groups. In 2050, the number of people in the United States with diagnosed diabetes is estimated to grow to 48.3 million. Results from randomized controlled trials provide evidence that intensive lifestyle interventions can prevent or delay the onset of diabetes in high-risk individuals. In addition, adequate and sustained control of blood sugar levels, blood pressure, and blood lipid levels can prevent or delay the onset of diabetes-related complications in people with diabetes. Effective interventions, at both the individual and population levels, are desperately needed to slow the diabetes epidemic and reduce diabetes-related complications in the United States. This report describes the current diabetes epidemic and the health and economic impact of diabetes complications on individuals and on the health care system. The report also provides suggestions by which the epidemic can be curbed.
Diabetes and its complications are a major cause of morbidity and mortality in the United States and contribute substantially to health care costs. Although we have already seen an epidemic of diabetes in the United States over the past 2 decades, we can expect a continued rise in the incidence of diabetes as the population ages, a continued increase in adult obesity rates, and an increase in the population of minority groups that are at high risk for diabetes. In addition, rising childhood obesity rates and the increasing diagnosis of type 2 (formerly “adult-onset” diabetes) among children and young adults have become an increasingly serious health crisis, which will result in more people having and managing diabetes for most of their lives.
Although 90% to 95% of the diabetes burden in the United States is due to type 2 diabetes, an understanding of the different types of diabetes and their impact on health is warranted. This article reviews the literature on the epidemiology of diabetes in the United States and provides background on the complications associated with diabetes, especially those complications most frequently seen by physical therapists.
Pathophysiology of Diabetes
Diabetes mellitus is a group of chronic metabolic conditions, all of which are characterized by elevated blood glucose levels resulting from the body's inability to produce insulin or resistance to insulin action, or both.1 This group of conditions can be subdivided into 4 clinically distinct types:
type 1, which results from autoimmune beta-cell destruction in the pancreas and is characterized by a complete lack of insulin production;
type 2, which develops when there is an abnormal increased resistance to the action of insulin and the body cannot produce enough insulin to overcome the resistance;
gestational diabetes, which is a form of glucose intolerance that affects some women during pregnancy; and
a group of other types of diabetes caused by specific genetic defects of beta-cell function or insulin action, diseases of the pancreas, or drugs or chemicals.1
Type 1 diabetes accounts for 5% to 10% of all cases of diabetes. Its risk factors include autoimmune, genetic, and environmental factors. To date, there are no known ways to prevent type 1 diabetes. Type 2 diabetes accounts for 90% to 95% of all diagnosed diabetes cases. This form of diabetes generally begins as insulin resistance and, because the body is unable to produce enough insulin to address the resistance, the pancreas may reduce the production of insulin or eventually stop producing it. Minority women, women who are obese, women with a family history of diabetes, and women who have had gestational diabetes in a previous pregnancy are at higher risk than other women for developing gestational diabetes. Strict glycemic control and management of women with gestational diabetes is necessary to prevent birth complications in the developing infant. Women who have had gestational diabetes have a 20% to 50% increased risk for developing type 2 diabetes later in life.2
Prediabetes is a precursor condition to diabetes in which a person has elevated blood glucose levels but does not meet diagnostic criteria for diabetes. People with prediabetes can have impaired fasting glucose or impaired glucose tolerance, or both. From 1988 to 1994, approximately 25% of a cross-sectional sample of US adults 40 to 74 years of age were classified as having prediabetes.3 For the year 2000, this would mean that 12 million people in the United States had prediabetes. This finding clearly indicates that there is a large population that is at risk for developing diabetes within a relatively short time frame.
Incidence and Prevalence
In 2005, an estimated 1.5 million new cases of diabetes were diagnosed.2 Although the incidence (or new cases of diabetes) describes increases in the number of people affected by the disease, the prevalence (or existing cases of diabetes) describes the overall burden of the disease in the population. Two population-based sources of data on diabetes, the National Health Interview Survey (NHIS)4 and the Behavioral Risk Factor Surveillance System (BRFSS),5 provide data on the prevalence of diabetes in the United States. Although these sources provide accurate self-reported data about diabetes for the United States, they have been limited to reporting the prevalence of diagnosed diabetes because they assess whether a person has been told by a physician or health care professional that he or she has diabetes. This limitation, then, does not allow for measurement of undiagnosed diabetes (ie, those people who have diabetes but have not yet been diagnosed by a physician).
The National Health and Nutrition Examination Surveys (NHANES) are the only nationally representative surveys that have taken blood samples in addition to survey questions and, therefore, can estimate both diagnosed and undiagnosed diabetes.6 Based on prevalence estimates from NHANES for 2005, the total prevalence of diabetes (both diagnosed and undiagnosed) was estimated at 20.8 million or 7.0% of the US population. Of these, 14.6 million were diagnosed and 6.2 million—almost 30% of all diabetes cases—were undiagnosed.
Trends Over Time
Diabetes mellitus is now approaching epidemic proportions.7 In the United States, the prevalence and incidence of diabetes have increased dramatically during the past 2 decades.7 According to data from the NHIS for the period from 1980 to 2005, the age-adjusted prevalence of diagnosed diabetes was fairly stable at about 3.0% from 1980 to 1990 and then began to increase. In 1990, the age-adjusted prevalence rate was 2.9%.8 It increased to 4.5% in 2000 and to 5.3% in 2005. The overall prevalence of diagnosed diabetes increases with age and the rate of increase over time has been largest in people over 65 years of age.8 The prevalence of self-reported diagnosed diabetes has increased over time from 1997 to 2005 in all age groups (Fig. 1).9 Data from the 1997–2005 NHIS indicate that older adults have consistently borne a greater burden of diabetes.9
Figure 1.

Prevalence of diagnosed diabetes by age in the United States. National Health Interview Survey, 1997–2005.9
Age-adjusted prevalence rates for diagnosed diabetes have consistently been higher among African Americans and Hispanics compared with whites. African-American women have the highest prevalence of diabetes compared with other racial or ethnic and gender groups. In 2005, the age-adjusted prevalence rate for diagnosed diabetes was 8.3% in African-American women compared with 8.0% in African-American men, 7.5% in Hispanic women, 7.1% in Hispanic men, 4.7% in white women, and 5.4% in white men.9 The number of individuals with diagnosed diabetes is estimated to triple by the year 2050.10 Estimates show that 3.2 million African Americans currently have diabetes.2 The number of African Americans with diabetes is projected to triple by the year 2050, but the number of whites with diabetes is estimated to only double.7
Risk Factors
Although the pathogenesis of diabetes is complex, a number of factors that increase the risk for the disease have been identified. Risk factors for type 1 diabetes include family history, race (with whites at higher risk than other racial or ethnic groups), and certain viral infections during childhood. Risk factors for type 2 diabetes are more diverse; some are modifiable, and others are not.
Nonmodifiable risk factors for type 2 diabetes include age, race or ethnicity, family history (genetic predisposition), history of gestational diabetes, and low birth weight. Diabetes incidence and prevalence increases with age. In 2005, the Centers for Disease Control and Prevention reported that the prevalence of diabetes among people aged 20 years or older was 20.6 million (9.6% of the people in that age group), and the prevalence of diabetes increased with age (10.3 million people aged 60 years or older, or 20.9% of those in that age group, had diabetes).2
African Americans are more likely to develop diabetes than whites.11 In addition, for Native Americans, the rates of diagnosed diabetes range from 5% to 50% in different tribes and population groups. Little difference exists by sex. Genetic factors also play a role, but nongenetic or lifestyle risk factors (such as diet and physical activity) appear to be the primary culprits.12
Modifiable or lifestyle risk factors include increased body mass index (BMI), physical inactivity, poor nutrition, hypertension, smoking, and alcohol use, among others.7,11 Increased BMI is consistently shown to be one of the strongest risk factors for development of diabetes.13 In addition, distribution of body fat,14 and specifically an increased waist-to-hip ratio, increase a person's risk for diabetes.15
Consistent findings from various studies show that lower levels of physical activity increase a person's risk for diabetes. A recent review of 10 prospective cohort studies investigating moderate-intensity physical activity and diabetes provides evidence that people who achieve recommended levels of even moderate activity are about 30% less likely to develop diabetes than their inactive counterparts.16
Total caloric intake, as well as specific components of diet such as refined carbohydrates and fats, have been linked to diabetes development. Moderate alcohol use may reduce the risk for developing diabetes,10 but smoking has been shown to be an independent risk factor for diabetes.17
Psychosocial factors such as depression, increased stress, lower social support, and poor mental health status also are associated with an increased risk for the development of diabetes.18–22 Recently, adverse housing conditions were found to be independently associated with the development of self-reported diabetes, although the mechanism by which housing conditions exert their risk is still unknown.23 (Also see the article in this issue by Kirkness et al24 documenting the number of risk factors for diabetes in a large sample of patients seen by physical therapists.)
Mortality
In 2002, diabetes was the sixth leading cause of death,2 with 73,249 death certificates listing diabetes as the underlying cause of death and an additional 224,092 death certificates listing diabetes as a contributing cause of death. Diabetes is likely to be underreported as a cause of death due to the many complications associated with diabetes that ultimately cause death. Overall, the risk of death among people with diabetes is almost twice that of people of similar age who do not have diabetes.2 Duration of diabetes also is an important determinant of mortality; younger age-of-onset groups (<45 years of age) have an increased risk of premature death. From death certificate data, it appears that age-adjusted death rates for African Americans and Hispanic Americans are similar to the rates of whites.25,26 An increased mortality rate in North American Native Americans with type 2 diabetes also is apparent.
There is general agreement about the distribution of causes of death in type 2 diabetes.27 Two thirds of people with diabetes die of heart disease and stroke. The risk for cardiovascular disease mortality is 2 to 4 times higher in people with diabetes than in people who do not have diabetes.
There are several risk factors that increase the risk for dying in people with diabetes. In a large intervention trial, men with diabetes were more likely to die as a result of cardiovascular disease when they had the conventional risk factors of elevated serum cholesterol, elevated systolic blood pressure, and cigarette smoking.28 In recent studies, “tight control” of elevated blood pressure in type 2 diabetes reduced deaths related to diabetes by 32% compared with less tight control.29
Complications
Diabetes can affect many different organ systems in the body and, over time, can lead to serious complications. Complications from diabetes can be classified as microvascular or macrovascular. Microvascular complications include nervous system damage (neuropathy), renal system damage (nephropathy) and eye damage (retinopathy).1 Macrovascular complications include cardiovascular disease, stroke, and peripheral vascular disease. Peripheral vascular disease may lead to bruises or injuries that do not heal, gangrene, and, ultimately, amputation.
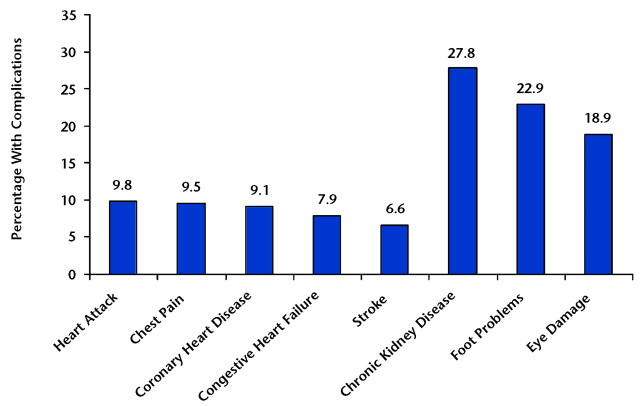
Figure 2 shows the prevalence of the most common diabetes complications among people with type 2 diabetes. Data from the 1999–2004 NHANES indicate that the prevalence of microvascular complications—chronic kidney disease (defined as microalbuminuria), foot problems (defined as foot/toe amputation, foot lesion, or numbness), and eye damage (defined as being told that diabetes had affected the eyes or had retinopathy)—are much higher than the prevalence of macrovascular complications (heart attack, chest pain, coronary heart disease, congestive heart failure, and stroke).30 Complications can be either episodic (eg, foot ulcers or infections) that can be treated and recur numerous times or progressive (eg, nephropathy), which usually begin relatively mildly, but over time result in further damage to the organ and greater loss of functionality that is generally irreversible.
Figure 2.
Prevalence of diabetes-related complications among people with diabetes. National Health and Nutrition Examination Survey, 1999–2004.29
Other complications include dental disease, reduced resistance to infections such as influenza and pneumonia, and macrosomia and other birth complications among pregnant women with diabetes. Although the types of complications are similar for type 1 and type 2 diabetes patients, the frequency or timing of occurrence can vary. The types and prevalence of the most common diabetes complications are discussed further in more detail with specific attention to differences between complications of type 1 versus type 2 diabetes.
Heart Disease and Stroke
Cardiovascular disease causes up to 65% of all deaths in people with diabetes.31 Ischemic heart disease and stroke account for the greatest proportion of morbidity associated with diabetes. In addition, as described above, mortality rates due to heart disease are 2 to 4 times higher among people with diabetes compared with those without diabetes. People with diabetes also are 2 to 4 times more likely to develop stroke than people without diabetes. More than 70% of people with diabetes have high blood pressure or are being treated with medications for hypertension. The role of hyperglycemia in cardiovascular complications among persons with diabetes is not clear.
Risk factors for cardiovascular disease among people with diabetes are similar to those for people without diabetes and include hypertension, hypercholesterolemia, and smoking. It appears, however, that the presence of even one of these risk factors leads to poorer outcomes among people with diabetes compared with those without diabetes.28 Data on trends in cardiovascular disease complications associated with diabetes are available from the 1950s to 2003 for different populations, and overall these data indicate that there have been large and significant decreases in the incidence of cardiovascular complications among people with diabetes over time.32,33 The greatest decreases appear to have occurred during the 1980s and 1990s and coincide with significant advances in medicines to control glycemic levels as well as medicines to control blood pressure and blood cholesterol levels. It appears, however, that these decreases have slowed since the late 1990s.9 (Also see the article in this issue by Cade34 for a detailed perspective on microvascular and macrovascular disease in people with diabetes.)
Peripheral Arterial Disease
Peripheral arterial disease (PAD, also referred to as peripheral vascular disease [PVD]), is caused by the narrowing of blood vessels that carry blood to the arms, legs, stomach, and kidneys. In people with diabetes, the risk for PAD is increased by age, duration of diabetes, and presence of neuropathy. Other factors associated with cardiovascular disease, such as C-reactive protein levels and homocysteine levels, also are associated with an increased risk for PAD.35 Peripheral arterial disease is characterized by 2 types of symptoms: intermittent claudication (or the intermittent pain, ache, or discomfort that may occur during exercise or walking but resolves with rest) and pain at rest (which is caused by ischemia in the limb, indicating inadequate blood flow to the affected limb).35 Peripheral arterial disease is a major risk factor for lower-extremity amputation.
Data on PAD trends come from hospital discharge data from the National Center for Health Statistics and indicate that the hospital discharge rates for PAD as the primary diagnosis have decreased steadily since 1996. The age-adjusted hospital discharge rate for PAD peaked at 7.8 per 1,000 people with diabetes in 1996 and was down to 3.3 per 1,000 people with diabetes in 2003. In addition, discharge rates for PAD were higher in men than in women and increased with increasing age.9
Retinopathy (Blindness)
Diabetic retinopathy is the most common microvascular complication among people with diabetes and results in more than 10,000 new cases of blindness per year. In addition, retinopathy is associated with prolonged hyperglycemia, it is slow to develop, and there is some evidence that it can begin to develop as early as 7 years before clinical diagnosis of type 2 diabetes.36 The age-adjusted prevalence of visual impairment decreased from 23.7 per 100 people with diabetes in 1997 to approximately 17.7 per 100 people with diabetes in 2005.9 The prevalence of visual impairment among people with diabetes increases with age. In 2005, 27% of adults with diabetes who were 75 years of age or older reported some degree of visual impairment compared with 15% of adults with diabetes who were between 18 and 44 years of age.9 Throughout the period of 1997–2005, women with diabetes were more likely than men with diabetes to have visual impairment. Prevalence rates in women with diabetes have been falling throughout this time period, whereas rates in men with diabetes have stayed fairly constant since 2001. There appears to be no difference between racial groups in the prevalence of visual impairment during the period 1997–2005. Duration of diabetes is the most significant predictor of visual impairment among people with type 2 diabetes. As much as 90% of blindness due to retinopathy among people with diabetes may be preventable if detected and treated early. Annual dilated eye examinations are recommended for all patients with diabetes.37
Nephropathy (Renal Disease)
Diabetic nephropathy is defined as persistent proteinuria (more than 500 mg of protein or 300 mg of albumin per 24 hours) in patients without urinary tract infection or other diseases causing the proteinuria. In patients with type 1 diabetes, development of clinical nephropathy is a relatively late event; however, in patients with type 2 diabetes, diabetic proteinuria may be present at diagnosis.
The incidence of diabetic nephropathy in patients with type 2 diabetes is low during the first 10 to 15 years of diabetes duration, after which it increases rapidly to a maximum at about 18 years of duration, and then declines.38–40 The actual onset of type 2 diabetes may precede its clinical diagnosis by many years,41,42 which may explain the high prevalence of nephropathy at diabetes diagnosis. In 2002, diabetes-related nephropathy accounted for 44% of new cases of end-stage renal disease (ERSD), and 153,730 people with ESRD due to diabetes had either received a kidney transplant or were on chronic dialysis treatment.2
The etiology of diabetic nephropathy is poorly understood. Several risk factors are involved, some of which are modifiable and others are not. Metabolic regulation is one of the key modifiable risk factors for development of diabetic nephropathy. In people with either type 1 or type 2 diabetes, strict metabolic control leads to a significant reduction in the risk of developing microalbinuria and the risk of progression to persistent proteinuria.43–45 The impact of strict metabolic control on prognosis is most pronounced in patients with normal levels of albumin in the urine and patients with microalbuminuria. Increasing blood pressure and hypertension also are associated with an increased risk of progression of diabetic renal disease.46 However, it is still unclear whether blood pressure at diabetic onset predicts later development of diabetic nephropathy. Other risk factors, including cigarette smoking, obesity, anemia, and genetic factors, also have been suggested.47
People with type 2 diabetes and diabetic nephropathy are at increased risk for developing many other diabetic complications. The renal-retinal syndrome has been known for years and refers to the presence of both types of diseases at the same time. People with diabetes and nephropathy also are more likely to develop coronary heart disease and stroke compared with patients with diabetes without nephropathy. People with diabetes and nephropathy also are more likely to die from macrovascular disease, as described above.
Overall, the incidence of nephropathy has declined in recent decades, due to improvements in the management of people with diabetes to promote tight control of glycemia as well as improved control of hypertension. For example, comparison of 4 cohorts of patients with type 1 diabetes whose disease was diagnosed between 1965 and 1984 showed that the cumulative incidence of diabetic nephropathy over the following 20 years were lowest in the most recently diagnosed cohorts.48,49
Peripheral Neuropathy
Diabetic peripheral neuropathy (DPN) is a common complication estimated to affect 30% to 50% of individuals with diabetes.50–53 The primary risk factor for DPN is hyperglycemia.52,54 Other independent risk factors include age, duration of disease, cigarette smoking, hypertension, elevated triglycerides, higher BMI, alcohol consumption, and taller height.53–56
Chronic sensorimotor distal symmetric polyneuropthy is the most common form of DPN.57 Polyneuropathy can lead to sensory loss, muscle weakness, and pain. The typical presentation of polyneuropathy is a gradual onset of sensory impairment, including burning and numbness in the feet. The onset is so gradual that the disease may go undetected for years. Neuropathic pain may be severe when present; however, it is reported to occur in only 11% to 32% of individuals with polyneuropathy.58–60
Diabetic peripheral neuropathy leads to a number of impairments and functional limitations. Individuals with DPN are at high risk for foot ulceration and subsequent lower-extremity amputation.61–63 In individuals with diabetes, the presence of DPN is associated with a greater number of health care visits per year and an inability to work due to physical limitations.50 Other potential complications of DPN, such as falls, are less clearly attributable to the illness; however, they can result in significant functional limitations. (Also see the articles in this issue by Mueller et al,64 Sinacore et al,65 and Hilton et al66 about neuropathic skin, bone, and muscle in people with diabetes mellitus.)
Data from the National Center for Health Statistics indicate that the hospital discharge rates for DPN have steadily increased from 1996 to 2003. The age-adjusted hospital discharge rate for DPN increased from 4.7 per 1,000 people with diabetes in 1996 to 6.8 per 1,000 people with diabetes in 2003. Discharge rates were higher in men than in women and higher for people younger than 45 years of age compared with those who were 45 years of age and older.9
Lower-Extremity Amputations
Nontraumatic lower-extremity amputations (LEAs) are a devastating complication of diabetes. As many as 15% of people with diabetes will have such amputations during their lifetime. People with diabetes are 10 to 20 times more likely to have LEAs than those without diabetes. People 65 years of age and older account for about 55% of patients with diabetes who had nontraumatic LEAs.67
The annual number of diabetes-related hospital discharges with LEA increased from about 33,000 in 1980 to 84,000 in 1997.9 Although large increases in the number of LEA discharges occurred in the early 1990s, the number of discharges for LEAs leveled off afterward. In 2003, there were about 75,000 diabetes-related hospital discharges with LEAs.9 The age-adjusted rate of hospital discharges for LEAs per 10,000 population increased after the 1983 implementation of the prospective reimbursement system by the Center for Medicare and Medicaid Services, leveled off starting in the mid-1980s, and then began increasing in the early 1990s. After reaching a peak in 1996, LEA rates decreased slightly. In 2003, the age-adjusted LEA rate was 4.4 per 1,000 people with diabetes.9
There are several risk factors for LEA, including increasing age, being male, being African American, having peripheral neuropathy, and having chronic ulcers. About 85% of all LEAs occurring in people who had diabetes for more than 30 months were preceded by a chronic, nonhealing foot ulcer.62,68 Diabetic foot ulcers are common and are estimated to affect about 15% of all individuals with diabetes during their lifetimes.69 Peripheral vascular disease contributes to about half of all amputations in people with diabetes.62 Many foot ulcers might be prevented by regular foot inspections, access to foot care, and adequate footwear.70,71 However, the majority of individuals with diabetes do not get regular inspections of their feet, adequate shoes, or proper foot care.72 (Also see the article in this issue by Mueller et al64 about physical stresses contributing to skin breakdown in people with diabetes mellitus.)
There is still controversy concerning the benefit of primary minor amputation versus primary major amputation. The advantage of primary minor amputation is that there is a lower risk for new major amputation and better rehabilitation potential.73 However, in a prospective study of 189 patients with diabetes, there was no difference in the rate of new amputations between people with index minor amputations and people with index major amputations, and those with index minor amputations had longer healing times.74 People with diabetes who had an index major amputation had a higher mortality rate, an equal rate of new amputations regardless of level, an increased rate of major amputations, and lower rehabilitation potential compared with patients with an index minor amputation.74 Disability as a result of an LEA is quite common.
Control of Risk Factors to Reduce Complications
Across all of the diabetes-related complications described above, the 3 most significant risk factors are hyperglycemia, high blood pressure, and hypercholesterolemia. It has been suggested that improvements in glycemic control, blood pressure, and cholesterol level can reduce a person's risk for complications.2 For example, in a person with diabetes, each percentage point reduction in glycosylated hemoglobin (Hb A1c) level can reduce that person's risk for microvascular complications by 40%; a 10 mm Hg decrease in blood pressure can reduce that person's risk for any diabetic complication by up to 12%; and control of serum lipids can reduce that person's risk for cardiovascular complications by 20% to 50%.2 Clearly, better control of these risk factors in people with diabetes can lead to more favorable outcomes.
Burden to the Health Care System
According to the American Diabetes Association, the estimated costs associated with diabetes in the United States in 2002 totaled $132 billion, with direct medical costs of $92 billion and indirect costs (disability, loss in work productivity and premature mortality) of $40 billion.75 Given no additional increase in the prevalence of diabetes in the United States, these expenditures would be expected to reach approximately $192 billion by 2020.75 Of the $92 billion in direct costs for 2002, $23 billion was due to health care events with a primary diagnosis of uncomplicated diabetes and an additional $25 billion was for treatment of diabetes-related cardiovascular disease.75 (Also see the perspective article in this issue by Cohn76 about the economic realities for the care of people with diabetes mellitus.)
Approximately 40% of the total cost of diabetes in the United States is due directly to inpatient care for treatment of diabetes complications.77 Several studies have estimated annual and cumulative economic costs of diabetes complications over time.78,79 These studies found that macrovascular disease (mainly cardiovascular events and stroke) accounted for as much as 85% of the costs of complications associated with diabetes and that these conditions are a significant determinant of costs at an earlier time during the course of the disease than microvascular complications.78 It is important to note, however, that relatively mild microvascular complications can become more serious over time and contribute significantly to morbidity and related costs in later years.
In addition, a key factor in the development of diabetes complications is glycemic level, both at diagnosis and an “upward drift” in glycemic level over time. People with higher initial Hb A1c levels had higher cumulative costs than people with lower levels, and people who experienced higher annual drift in Hb A1c levels had even further increased costs.78 These economic estimates suggest that improving glycemic control and other known risk factors for diabetes, particularly those for cardiovascular disease among people with diabetes, will significantly affect long-term costs.78,79 Although the evidence is strong that Hb A1c control and reduction can reduce a patient's risk for microvascular complications, the evidence is not so strong that glycemic control greatly reduces a person's risk for cardiovascular complications. Clearly, a combined effort to control blood glucose, blood pressure, and blood lipids will have the greatest effect on reducing a person's risk for diabetes-related complications and, ultimately, will have a favorable impact on the economic costs associated with diabetes.80–82 (Also see the articles in this issue by Turcotte and Fisher83 and Gulve84 on the effects of exercise in managing these risk factors in people with diabetes mellitus).
Conclusion
Early projections for the number of people with diagnosed diabetes in the United States in 2050 were calculated to be around 39 million.85,86 Since those calculations were done, however, the national incidence of diabetes has continued to increase from 2000 to 2004, and the mortality rate among people with diabetes has declined. Therefore, new projections for the diabetes burden in 2050 were published in 2006.10 The number of people with diagnosed diabetes in the United States is expected to increase from 16.2 million in 2005 to 48.3 million in 2050. These new estimates clearly depend on a stable incidence rate for diabetes over time; even incremental increases in incidence will have a significant effect on the expected number of people with diagnosed diabetes in the future. In addition, these estimates assume no advances in prevention, treatments, or control of risk factors; no increases in life expectancy; and no discovery of a cure. Changes in any of these factors could substantially alter the projections for 2050.
It is clear that there is a growing epidemic of diabetes in the United States. An increasing prevalence of diabetes risk factors will only exacerbate the problem; therefore, population-based efforts that affect the modifiable diabetes risk factors, particularly obesity and physical inactivity, are needed to reduce the burden of diabetes. In addition, among people with diabetes, the rate of complications is high. These complications not only significantly affect the morbidity and mortality associated with diabetes, but also contribute to the ever-growing costs related to diabetes. Adoption of appropriate diet and exercise behaviors and adherence to medication regimens will result in tighter glycemic control that, along with controlled blood pressure and blood lipids, will greatly reduce the burden of diabetes complications in the United States.
Footnotes
Dr Deshpande and Dr Schootman provided concept/idea/project design. All authors provided writing. Dr Harris-Hayes provided consultation (including review of manuscript before submission).
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29:S43–S48. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. National Diabetes Fact Sheet: General Information and National Estimates on Diabetes in the United States, 2005. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention; 2005. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf Accessed May 29, 2008.
- 3.Benjamin SM, Valdez R, Geiss LS, et al. Estimated number of adults with prediabetes in the US in 2000: opportunities for prevention. Diabetes Care. 2003;26:645–649. [DOI] [PubMed] [Google Scholar]
- 4.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey. Available at: http://www.cdc.gov/nchs/nhis.htm
- 5.National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System survey data. Available at: http://www.cdc.gov/brfss/
- 6.National Center for Health Statistics, Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey (NHANES). Available at: http://www.cdc.gov/nchs/nhanes.htm
- 7.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. [DOI] [PubMed] [Google Scholar]
- 8.Skyler JS, Oddo C. Diabetes trends in the USA. Diabetes Metab Res Rev. 2002;18(suppl 3):S21–S26. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Diabetes Data and Trends. Available at: http://www.cdc.gov/diabetes/statistics/newDataTrends.htm Accessed April 18, 2008
- 10.Narayan KM, Boyle JP, Geiss LS, et al. Impact of recent increase in incidence on future diabetes burden: U.S., 2005–2050. Diabetes Care. 2006;29:2114–2116. [DOI] [PubMed] [Google Scholar]
- 11.Egede LE, Dagogo-Jack S. Epidemiology of type 2 diabetes: focus on ethnic minorities. Med Clin North Am. 2005;89:949–975, viii. [DOI] [PubMed] [Google Scholar]
- 12.Schulz LO, Bennett PH, Ravussin E, et al. Effects of traditional and western environments on prevalence of type 2 diabetes in Pima Indians in Mexico and the U.S. Diabetes Care. 2006;29:1866–1871. [DOI] [PubMed] [Google Scholar]
- 13.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. [DOI] [PubMed] [Google Scholar]
- 14.Rewers M, Hamman RF. Risk factors for non-insulin-dependent diabetes. In: Harris MI, Cowie CC, Stern MP, et al, eds. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:179–220. NIH publication 95–1468.
- 15.Kaye SA, Folsom AR, Sprafka JM, et al. Increased incidence of diabetes mellitus in relation to abdominal adiposity in older women. J Clin Epidemiol. 1991;44:329–334. [DOI] [PubMed] [Google Scholar]
- 16.Jeon CY, Lokken RP, Hu FB, van Dam RM. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30:744–752. [DOI] [PubMed] [Google Scholar]
- 17.Will JC, Galuska DA, Ford ES, et al. Cigarette smoking and diabetes mellitus: evidence of a positive association from a large prospective cohort study. Int J Epidemiol. 2001;30:540–546. [DOI] [PubMed] [Google Scholar]
- 18.Strodl E, Kenardy J. Psychosocial and non-psychosocial risk factors for the new diagnosis of diabetes in elderly women. Diabetes Res Clin Pract. 2006;74:57–65. [DOI] [PubMed] [Google Scholar]
- 19.Eaton WW, Armenian H, Gallo J, et al. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care. 1996;19:1097–1102. [DOI] [PubMed] [Google Scholar]
- 20.Arroyo C, Hu FB, Ryan LM, et al. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grandinetti A, Kaholokula JK, Chang HK. Delineating the relationship between stress, depressive symptoms, and glucose intolerance. Diabetes Care. 2000;23:1443–1444. [DOI] [PubMed] [Google Scholar]
- 22.Diez Roux AV, Jacobs DR, Kiefe CI. Neighborhood characteristics and components of the insulin resistance syndrome in young adults: the coronary artery risk development in young adults (CARDIA) study. Diabetes Care. 2002;25:1976–1982. [DOI] [PubMed] [Google Scholar]
- 23.Schootman M, Andresen EM, Wolinsky FD, et al. The effect of adverse housing and neighborhood conditions on the development of diabetes mellitus among middle-aged African Americans. Am J Epidemiol. 2007;166:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirkness CS, Marcus RL, LaStayo PC, et al. Diabetes and associated risk factors in patients referred for physical therapy in a national primary care electronic medical record database. Phys Ther. 2008;88:1408–1416. [DOI] [PubMed] [Google Scholar]
- 25.Tull ES, Roseman JM. Diabetes in African Americans. In: Harris MI, Cowie CC, Stern MP, et al, eds. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:613–630. NIH publication 95-1468.
- 26.Stern MP, Patterson JK, Mitchell BD, et al. Overweight and mortality in Mexican Americans. Int J Obes. 1990;14:623–629. [PubMed] [Google Scholar]
- 27.Welborn T. Diabetes mortality. In: Ekoé JM, Zimmet P, Williams R, eds. The Epidemiology of Diabetes Mellitus: An International Perspective. Chichester, United Kingdom: John Wiley & Sons Ltd; 2001:369–382.
- 28.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 29.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 30.American Association of Clinical Endocrinologists. State of diabetes complications in America. Available at: http://www.aace.com/newsroom/press/2007/images/DiabetesComplicationsReport_FINAL.pdf Accessed April 16, 2008
- 31.Geiss LS, Herman WH, Smith PJ. Mortality in non-insulin-dependent diabetes. In: Harris MI, Cowie CC, Stern MP, et al, eds. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:233–257. NIH publication 95-1468.
- 32.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292:2495–2499. [DOI] [PubMed] [Google Scholar]
- 33.Booth GL, Kapral MK, Fung K, Tu JV. Recent trends in cardiovascular complications among men and women with and without diabetes. Diabetes Care. 2006;29:32–37. [DOI] [PubMed] [Google Scholar]
- 34.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King KD, Jones JD, Warthen J. Microvascular and macrovascular complications of diabetes mellitus. Am J Pharm Educ. 2005;69:article 87. [Google Scholar]
- 36.Harris R, Leininger L. Preventive care in rural primary care practice. Cancer. 1993;72(3 suppl):1113–1118. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Standards of medical care in diabetes—2006. Diabetes Care. 2006;29:S4–S42. [PubMed] [Google Scholar]
- 38.Andersen AR, Christiansen JS, Andersen JK, et al. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501. [DOI] [PubMed] [Google Scholar]
- 39.Kofoed-Enevoldsen A, Borch-Johnsen K, Kreiner S, et al. Declining incidence of persistent proteinuria in type I (insulin-dependent) diabetic patients in Denmark. Diabetes. 1987;36:205–209. [DOI] [PubMed] [Google Scholar]
- 40.Bojestig M, Arnqvist HJ, Hermansson G, et al. Declining incidence of nephropathy in insulin-dependent diabetes mellitus. N Engl J Med. 1994;330:15–18. [DOI] [PubMed] [Google Scholar]
- 41.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4–7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–819. [DOI] [PubMed] [Google Scholar]
- 42.Gall MA, Rossing P, Skøtt P, et al. Prevalence of micro- and macroalbuminuria, arterial hypertension, retinopathy and large vessel disease in European type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1991;34:655–661. [DOI] [PubMed] [Google Scholar]
- 43.Feldt-Rasmussen B, Mathiesen ER, Deckert T. Effect of two years of strict metabolic control on progression of incipient nephropathy in insulin-dependent diabetes. Lancet. 1986;2:1300–1304. [DOI] [PubMed] [Google Scholar]
- 44.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 45.Wang PH, Lau J, Chalmers TC. Meta-analysis of effects of intensive blood-glucose control on late complications of type I diabetes. Lancet. 1993;341:1306–1309. [DOI] [PubMed] [Google Scholar]
- 46.Mogensen CE. Long-term antihypertensive treatment inhibiting progression of diabetic nephropathy. Br Med J (Clin Res Ed). 1982;285:685–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jermendy G, Ruggenenti P. Preventing microalbuminuria in patients with type 2 diabetes. Diabetes Metab Res Rev. 2007;23:100–110. [DOI] [PubMed] [Google Scholar]
- 48.Chaturvedi N. The burden of diabetes and its complications: trends and implications for intervention. Diabetes Res Clin Pract. 2007;76(suppl 1):S3–S12. [DOI] [PubMed] [Google Scholar]
- 49.Hovind P, Tarnow L, Rossing K, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. [DOI] [PubMed] [Google Scholar]
- 50.Candrilli SD, Davis KL, Kan HJ, et al. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21:306–314. [DOI] [PubMed] [Google Scholar]
- 51.Gregg EW, Sorlie P, Paulose-Ram R, et al. Prevalence of lower-extremity disease in the US adult population ≥40 years of age with and without diabetes: 1999–2000 national health and nutrition examination survey. Diabetes Care. 2004;27:1591–1597. [DOI] [PubMed] [Google Scholar]
- 52.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973 [in French]. Diabetes Metab. 1977;3:97–107. [PubMed] [Google Scholar]
- 53.Adler AI, Boyko EJ, Ahroni JH, et al. Risk factors for diabetic peripheral sensory neuropathy: results of the Seattle Prospective Diabetic Foot Study. Diabetes Care. 1997;20:1162–1167. [DOI] [PubMed] [Google Scholar]
- 54.Shaw JE, Zimmet PZ. The epidemiology of diabetic neuropathy. Diabetes Review. 1999;7:245–253. [Google Scholar]
- 55.Perkins BA, Greene DA, Bril V. Glycemic control is related to the morphological severity of diabetic sensorimotor polyneuropathy. Diabetes Care. 2001;24:748–752. [DOI] [PubMed] [Google Scholar]
- 56.Tesfaye S, Chaturvedi N, Eaton SE, et al; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352:341–350. [DOI] [PubMed] [Google Scholar]
- 57.Boulton AJ, Vinik AI, Arezzo JC, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. [DOI] [PubMed] [Google Scholar]
- 58.Vinik AI, Park TS, Stansberry KB, Pitteneger GL. Diabetic neuropathies. Diabetologia. 2000;43:957–973. [DOI] [PubMed] [Google Scholar]
- 59.Eaton S, Tesfaye S. Clinical manifestations and measurement of somatic neuropathy. Diabetes Review. 1997;7:312–325. [Google Scholar]
- 60.Slyke MP. Painful peripheral diabetic neuropathy: therapeutic approaches. Consult Pharm. 2000;15:544–555. [Google Scholar]
- 61.Apelqvist J, Larsson J, Agardh CD. Long-term prognosis for diabetic patients with foot ulcers. J Intern Med. 1993;233:485–491. [DOI] [PubMed] [Google Scholar]
- 62.Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care. 1990;13:513–521. [DOI] [PubMed] [Google Scholar]
- 63.Gonzales ER, Oley MS. The management of lower-extremity diabetic ulcers. Manag Care Interface. 2000;13:80–87. [PubMed] [Google Scholar]
- 64.Mueller MJ, Zou D, Bohnert KL, et al. Plantar stresses on the neuropathic foot during barefoot walking. Phys Ther. 2008;88:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sinacore DR, Hastings MK, Bohnert KL, et al. Inflammatory osteolysis in diabetic neuropathic (Charcot) arthropathies of the foot. Phys Ther. 2008;88:1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hilton TN, Tuttle LJ, Bohnert KL, et al. Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function. Phys Ther. 2008;88:1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sugarman JR, Reiber GE, Baumgardner G, et al. Use of the therapeutic footwear benefit among diabetic Medicare beneficiaries in three states, 1995. Diabetes Care. 1998;21:777–781. [DOI] [PubMed] [Google Scholar]
- 68.Reiber GE, Boyko EJ, Smith DG. Lower extremity foot ulcers and amputation in diabetes. In: Harris MI, Cowie CC, Stern MP, et al, eds. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:409–428. NIH publication 95-1468.
- 69.Palumbo PJ, Melton LJ III. Peripheral vascular disease and diabetes. In: Harris MI, Cowie CC, Stern MP, et al, eds. Diabetes in America. 2nd ed. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 1995:401–408. NIH publication 95-1468.
- 70.Reiber GE, Lipsky BA, Gibbons GW. The burden of diabetic foot ulcers. Am J Surg. 1998;176(suppl 2A):5S–10S. [DOI] [PubMed] [Google Scholar]
- 71.Boulton AJ, Vileikyte L. The diabetic foot: the scope of the problem. J Fam Pract. 2000;49(11 suppl):S3–S8. [PubMed] [Google Scholar]
- 72.Uccioli L, Faglia E, Monticone G, et al. Manufactured shoes in the prevention of diabetic foot ulcers. Diabetes Care. 1995;18:1376–1378. [DOI] [PubMed] [Google Scholar]
- 73.Larsson J, Apelqvist J. Towards less amputations in diabetic patients. Acta Orthop Scand. 1995;66:181–192. [DOI] [PubMed] [Google Scholar]
- 74.Larsson J, Agardh CD, Apelqvist J, Stenström A. Long-term prognosis after healed amputation in patients with diabetes. Clin Orthop Relat Res. 1998;350:149–158. [PubMed] [Google Scholar]
- 75.Hogan P, Dall T, Nikolov P; American Diabetes Association. Economic costs of diabetes in the US in 2002. Diabetes Care. 2003;26:917–932. [DOI] [PubMed] [Google Scholar]
- 76.Cohn R. Economic realities associated with diabetes care: opportunities to expand delivery of physical therapist services to a vulnerable population. Phys Ther. 2008;88:1417–1424. [DOI] [PubMed] [Google Scholar]
- 77.Zhang P, Engelgau MM, Norris SL, et al. Application of economic analysis to diabetes and diabetes care. Ann Intern Med. 2004;140:972–977. [DOI] [PubMed] [Google Scholar]
- 78.Caro JJ, Ward AJ, O'Brien JA. Lifetime costs of complications resulting from type 2 diabetes in the U.S. Diabetes Care. 2002;25:476–481. [DOI] [PubMed] [Google Scholar]
- 79.O'Brien JA, Patrick AR, Caro J. Estimates of direct medical costs for microvascular and macrovascular complications resulting from type 2 diabetes mellitus in the United States in 2000. Clin Ther. 2003;25:1017–1038. [DOI] [PubMed] [Google Scholar]
- 80.Gilmer TP, O'Connor PJ, Rush WA, et al. Predictors of health care costs in adults with diabetes. Diabetes Care. 2005;28:59–64. [DOI] [PubMed] [Google Scholar]
- 81.Brandle M, Zhou H, Smith BR, et al. The direct medical cost of type 2 diabetes. Diabetes Care. 2003;26:2300–2304. [DOI] [PubMed] [Google Scholar]
- 82.Krein SL, Funnell MM, Piette JD. Economics of diabetes mellitus. Nurs Clin North Am. 2006;41:499–511, v–vi. [DOI] [PubMed] [Google Scholar]
- 83.Turcotte LP, Fisher JS. Skeletal muscle insulin resistance: roles of fatty acid metabolism and exercise. Phys Ther. 2008;88:1279–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gulve EA. Exercise and glycemic control in diabetes: benefits, challenges, and adjustments to pharmacotherapy. Phys Ther. 2008;88:1297–1321. [DOI] [PubMed] [Google Scholar]
- 85.Honeycutt AA, Boyle JP, Broglio KR, et al. A dynamic Markov model for forecasting diabetes prevalence in the United States through 2050. Health Care Manag Sci. 2003;6:155–164. [DOI] [PubMed] [Google Scholar]
- 86.Boyle JP, Honeycutt AA, Narayan KM, et al. Projection of diabetes burden through 2050: impact of changing demography and disease prevalence in the U.S. Diabetes Care. 2001;24:1936–1940. [DOI] [PubMed] [Google Scholar]