Abstract
Objectives
Traditionally we have relied mainly on final FIGO stage to estimate overall oncologic outcome in endometrial cancer patients. However, it is well known that other patient factors may play equally important roles in outcome. Our objective was to develop a clinically useful nomogram in the hope of providing a more individualized and accurate estimation of overall survival (OS) following primary therapy.
Methods
Using a prospectively maintained endometrial cancer database, 1735 patients treated between 1993–2008 were analyzed. Clinical characteristics commonly known to predict OS were collected. For each patient, points were assigned to each of these 5 variables and a total score was calculated. The corresponding 3- and 5-year OS probabilities were then determined from the nomogram.
Results
The median age was 62 years (range, 25–96). Final grade included: G1 (471), G2 (622), G3 (634), missing (8). Stage included: IA (501), IB (590), IC (141), IIA (36), IIB (75), IIIA (116), IIIB (6), IIIC (135), IVA (7), and IVB (128). Histology included: adenocarcinoma (1376), carcinosarcoma (100), clear cell (62), serous (197). Median follow-up for survivors was 29.2 months (0–162.2 months). Concordance probability estimator for the nomogram is 0.746 ± 0.011.
Conclusion
Using a large endometrial cancer database we developed a nomogram based on 5 easily available clinical characteristics to predict OS with a high concordance probability. This nomogram incorporates other important individualized patient variables beyond FIGO stage to more accurately predict outcome. This new tool may be useful to clinicians in assessing patients risk when deciding on adjuvant treatment and follow-up.
Keywords: Nomogram, endometrial cancer, staging, lymph nodes
INTRODUCTION
With the increasing emphasis on individualized cancer care, predicting individualized postoperative outcomes based on readily available clinical and pathological information may add value to medical decision-making by providing an accurate prediction of survival, and thus helping to guide treatment selection. Traditionally, in endometrial cancer we have relied mainly on the final FIGO stage to estimate overall oncologic outcome. However, it is well known that other patient factors such as age, histology, final grade, and adequacy or accuracy of staging may play equally important roles in overall outcome.
A nomogram is a chart representing numerical relationships or a graphic calculation tool [1]. Nomograms have been used for more than a decade in many solid tumors and pelvic malignancies [2,3], but nomogram development and utilization in gynecologic oncology remains in its beginnings stages [4,5]. Specifically, nomograms to predict outcome after primary treatment of endometrial cancer are lacking [6]. Our objective was to develop a clinically useful nomogram based on final 1988 FIGO stage and other important, readily available, clinical data which are commonly known in all women treated for newly diagnosed endometrial cancer. We hope that this nomogram will provide a more individualized and accurate estimation of overall survival following primary therapy.
METHODS
Patient Cohort
Using a prospectively maintained endometrial cancer database, we analyzed 1735 patients treated between1993–2008. Clinical characteristics commonly known to predict overall survival were collected; these included age, 1988 FIGO stage, histology (3 categories: adenocarcinoma, serous/clear cell, and carcinosarcoma), final FIGO grade, and number of negative regional nodes (a surrogate variable for adequate surgical staging). We previously published that the removal of 10 or more regional nodes assigns patients more accurately to their surgical FIGO stage [7]. Therefore, in using this nomogram, patients who had more regional nodes removed and examined are likely to be more accurately assigned to the correct FIGO stage; moreover, in this nomogram the node-positive cases, as expected, will be assigned stage IIIC. For each patient, points are assigned to each of these 5 clinical variables and a total score is calculated and the corresponding 3 and 5-year OS probability are then determined from the nomogram.
Model Building
The clinical endpoint was Overall Survival (OS). OS was defined as the time from surgery to death or date of last follow-up for patients who were alive and censored. OS probabilities were estimated using the Kaplan-Meier method. Multivariate analysis was conducted using the Cox Proportional Hazards Model. To permit nonlinear relationships, continuous variables were modeled with restricted cubic splines [8]. Categorical variables were grouped based on clinical reasoning and decisions regarding grouping were made before modeling. The final regression model was chosen based on the clinical and statistical significance of the predictors. The 3-year predicted survival probability was calculated for each patient using the Cox regression model underlying the nomogram. The concordance probability [9], which is a measure of the nomogram’s ability to discriminate between patients, was calculated for these predictions. The concordance probability is the chance that given two randomly selected patients, the patient who survives longer has a longer predicted survival probability based on the nomogram. Similar to the area under the receiver operating characteristic curve, concordance probability can range from perfect concordance (1.0) to perfect discordance (0.0). A value of 0.5 indicates that for two randomly selected patients there is a 50% chance that the patient with the higher predicted probability will have a high survival (i.e., the prediction performance of the nomogram is no better than a coin flip).
Model Validation
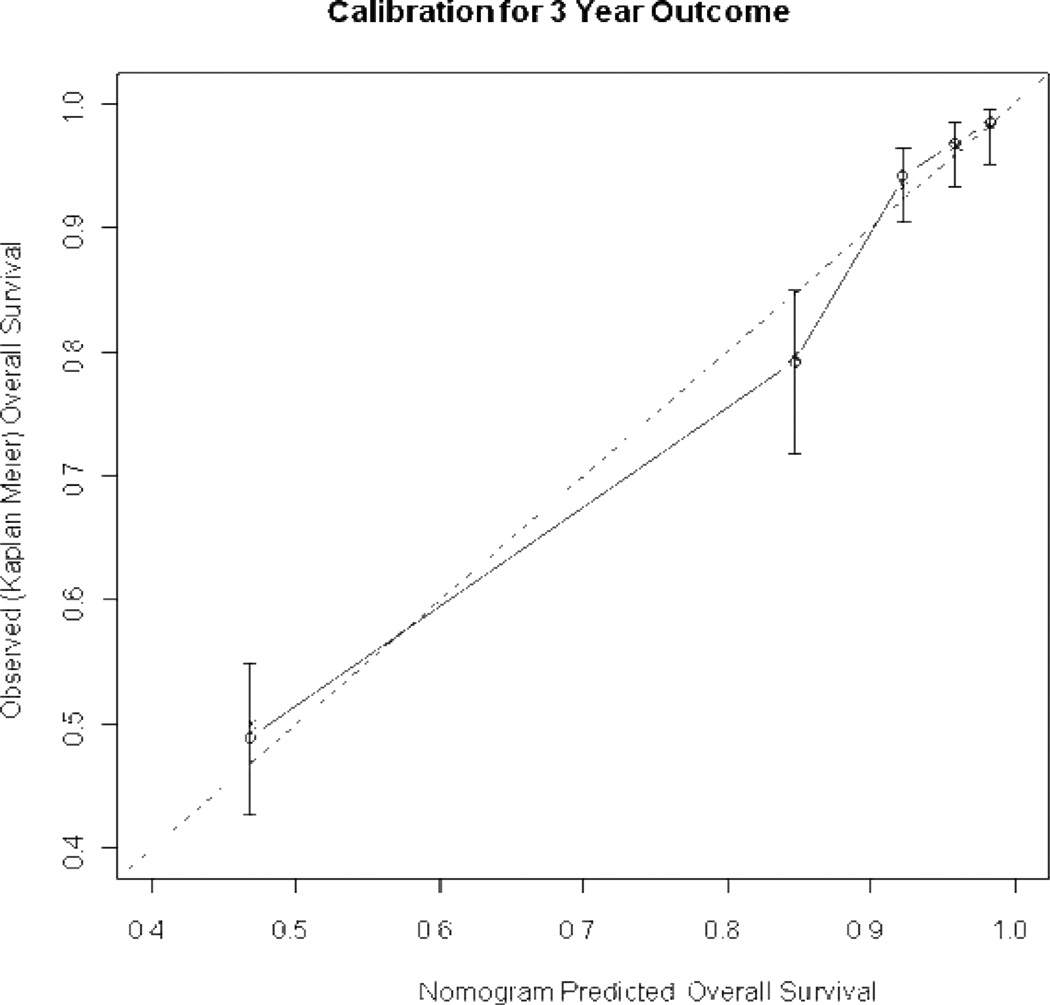
The predictive model was validated using 200 bootstrap samples to prevent against over-fitting. Specifically, a model was built on a bootstrap sample (the training set) and then evaluated on the original data (the test set) without modification. Two indices were calculated based on the training and test data sets. The difference between the two indices was the optimism of the fit. The process was repeated 200 times. The final optimism estimate was calculated as the average of the 200 differences. The difference between the original concordance probability based on all the data and the optimism estimate is the unbiased measure of the concordance probability [10], which addresses the nomogram’s ability to discriminate among patients. The calibration of the nomogram was assessed by plotting the nomogram 3-year predicted survival probability against the patient observed or actual probability, again using 200 bootstrap re-samples to reduce the overfit bias, which would overstate the accuracy of the nomogram.
Statistical analyses were performed using SAS and SPlus software S-Plus (Version 2000 Professional, Redmond, Washington) with the Design and Hmisc libraries [8] and library PHCPE in R 2.5 [9].
RESULTS
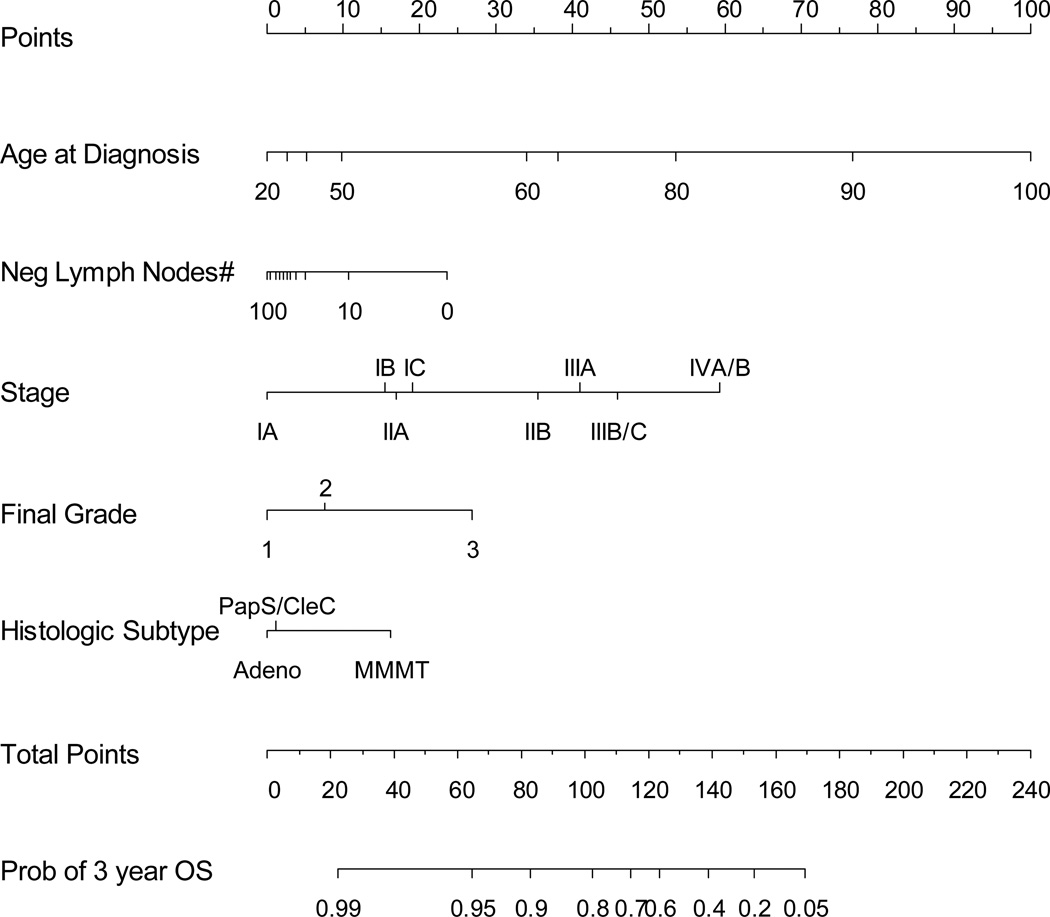
The median age for all patients was 62 years (range 25–96). Final FIGO grade included: G1 (471), G2 (622), G3 (634), missing (8).The 1988 FIGO Stage included: IA (501), IB (590), IC (141), IIA (36), IIB (75), IIIA (116), IIIB (6), IIIC (135), IVA (7), and IVB (128). Histology included: adenocarcinoma (1376), carcinosarcoma (100), clear cell (62), serous (197). Regional lymph nodes were removed and analyzed in 1063 (61%) cases with a median of 18 nodes (range, 1–92). The median follow-up for survivors was 29.2 months (0–162.2 months). The nomogram selected age, 1988 FIGO stage, final FIGO grade, number of lymph nodes and histologic subtype. The concordance probability estimator for the nomogram was 0.746 ± 0.011 (Figure 1). Figure 2 shows the nomogram. For example: a 60 year old (34 points) with stage IB (15 points), G3 (27 points), adenocarcinoma (0 points), with 10 negative nodes (10 points) has a total of 86 points, which corresponds to a 3-year OS probability of 88% and a 5-year OS probability of 82%.
Figure 1.
Nomogram using 5 easily available clinical characteristics to predict 3-year overall survival (OS) with bootstrap corrected concordance probability of 0.746.
Figure 2.
Calibration plot of endometrial cancer nomogram. X-axis shows the nomogram predicted probability. Patients were grouped by quartiles of predicted risk. Y-axis is actual 3-year probability of survival as estimated by Kaplan-Meier method. Solid line represents actual nomogram. Dotted line represents ideal agreement between actual and predicted probabilities of 3-year survival. Vertical bars represent 95% CI. Dots correspond to apparent predictive accuracy. X marks the bootstrap corrected estimates.
DISCUSSION
A nomogram is a graphic prediction tool that incorporates clinical risk factors included in a staging system as well as other clinical and pathologic factors known to have an impact on outcome. Endometrial cancer is surgically staged and many of the known risk factors for OS – such as depth of myoinvasion, cervical invasion, adnexal metastasis, and lymph node metastasis – are captured by the 1988 FIGO system. However, other important risk factors that may affect OS are not included in the FIGO system. These factors likely include age at diagnosis, FIGO grade, histologic subtype, and the adequacy of surgical staging. Assigning patients to the correct FIGO stage requires surgical staging with node dissection and although the therapeutic role of lymphadenectomy is debated, the value of lymphadenectomy in correctly assigning patients to the final surgical stage is much less controversial [11]. In our nomogram we used the total number of negative nodes as a surrogate to adequacy of surgical staging. This is based in part on our previous experience that the removal of 10 or more nodes assigns patients more accurately to the correct surgical stage [7].
Prognostic nomograms attempt to combine important clinical factors to quantify risk as precisely as possible in order to predict outcomes. Clinical nomograms have been developed as predictive tools for outcomes in malignancies such as prostate cancer, sarcoma, and gastric carcinoma [2,3,12,13]. However, in endometrial cancer such nomograms are lacking. Our current nomogram has been internally validated but a validation with an external dataset would be favored. The 5 clinical variables required by this nomogram are readily available to any treating oncologist and may help in better predicting overall outcome.
There are several limitations to any model based on data accumulated over many years from a single institution. These include heterogeneity of post-resection adjuvant treatment and the fact that not all patients in this cohort had comprehensive surgical staging (a common limitation among many endometrial cancer studies). In addition, our model did not include patient race which in some studies has been suggested as an important prognostic factor [14]. The large majority of patients included in this study are Caucasian and unfortunately our study population is limited in its ability to determine the effect, if any, race may have on OS. Future nomograms may consider including other clinical or biological tumor-related variables as our understanding of endometrial cancer increases.
In conclusion, we developed and internally validated a 5-variable nomogram for predicting overall survival after primary treatment for endometrial carcinoma. This nomogram predicted individual patient's survival with a high concordance index of 0.746. Incorporating other important clinical variables beyond FIGO stage is important to get a more accurate prediction of patients’ individualized outcome. External validation of this nomogram with an independent eternal dataset would be useful. This nomogram could be easily computerized such that when the 5 predictive factors are entered, the OS probability could be calculated automatically. The result of this calculation may help the clinician with better patient counseling on overall outcome and provide more individualized planning of postoperative management.
Footnotes
Conflict of Interest/Disclosure Statement
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Fu AZ, Cantor SB, Kattan MW. Use of Nomograms for Personalized Decision-Analytic Recommendations. Med Decis Making. 2009 Jul 31; doi: 10.1177/0272989X09342278. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Kattan MW, Stapleton AM, Wheeler TM, Scardino PT. Evaluation of a nomogram used to predict the pathologic stage of clinically localized prostate carcinoma. Cancer. 1997;79(3):528–537. [PubMed] [Google Scholar]
- 3.Kattan MW, Scardino PT. Prediction of progression: nomograms of clinical utility. Clin Prostate Cancer. 2002;1(2):90–96. doi: 10.3816/cgc.2002.n.010. [DOI] [PubMed] [Google Scholar]
- 4.Chi DS, Palayekar MJ, Sonoda Y, et al. Nomogram for survival after primary surgery for bulky stage IIIC ovarian carcinoma. Gynecol Oncol. 2008;108(1):191–194. doi: 10.1016/j.ygyno.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Kim MK, Kim JW, Lee JM, et al. Validation of a nomogram for predicting outcome of vulvar cancer patients, primarily treated by surgery, in Korean population: multicenter retrospective study through Korean Gynecologic Oncology Group (KGOG-1010) J Gynecol Oncol. 2008;19(3):191–194. doi: 10.3802/jgo.2008.19.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CM, Szabo A, Shrieve DC, et al. Descriptive nomograms of adjuvant radiotherapy use and patterns of care analysis for stage I and II endometrial adenocarcinoma: A surveillance, epidemiology, and end results population study. Cancer. 2007;110(9):2092–2100. doi: 10.1002/cncr.22997. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Rustum NR, Iasonos A, Zhou Q, et al. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am J Obstet Gynecol. 2008;198(4):457.e1–457.e5. doi: 10.1016/j.ajog.2008.01.010. discussion 457.e5–6. [DOI] [PubMed] [Google Scholar]
- 8.Harrell FE., Jr . Regression Modeling Strategies with Applications to Linear Models, Logistic Regression, and Survival Analysis. New York: Springer Verlag; 2001. [Google Scholar]
- 9.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–970. [Google Scholar]
- 10.Harrell FE, Jr, Lee KL, Mark DB. Mutlivariable prognostic models: issues in developing models evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Benedetti Panici P, Basile S, Maneschi F, et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: randomized clinical trial. J Natl Cancer Inst. 2008;100(23):1707–1716. doi: 10.1093/jnci/djn397. [DOI] [PubMed] [Google Scholar]
- 12.Kattan MW, Vickers AJ, Yu C, et al. Preoperative and postoperative nomograms incorporating surgeon experience for clinically localized prostate cancer. Cancer. 2009;115(5):1005–1010. doi: 10.1002/cncr.24083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briganti A, Gallina A, Suardi N, et al. A nomogram is more accurate than a regression tree in predicting lymph node invasion in prostate cancer. BJU Int. 2008;101(5):556–560. doi: 10.1111/j.1464-410X.2007.07321.x. [DOI] [PubMed] [Google Scholar]
- 14.Wright JD, Fiorelli J, Schiff PB, et al. Racial disparities for uterine corpus tumors: changes in clinical characteristics and treatment over time. Cancer. 2009;115(6):1276–1285. doi: 10.1002/cncr.24160. [DOI] [PubMed] [Google Scholar]