Abstract
Objectives
The revised 2009 FIGO staging system for endometrial cancer included many changes over the 1988 system, particularly for stage I subgroups. We sought to describe the overall survival (OS) of women with stage I endometrial cancer and examine how the estimated stage-specific OS is altered in the 2009 system.
Methods
A prospectively maintained institutional endometrial database was analyzed. All patients underwent primary surgery between 1/93 - 6/09.
Results
Data from 1658 women were analyzed, including 1307 patients with FIGO 1988 stage I disease. The 5-year OS for the 1988 stage IA (92.4%), IB (87.3%), and IC (75.7%) significantly differed (P<0.001). When patients were restaged using the 2009 system, we identified 1411 stage I patients with 5-year OS for 2009 stage IA of 89.2%, vs. OS of 75.1% for IB (P=0.001). The adjusted concordance probabilities for the 1988 stage I group and 2009 stage I group were 0.612 ± 0.0014 and 0.536 ± 0.0111, respectively.
Conclusions
The 1988 FIGO classification of stage I endometrial cancer correctly identified 3 subgroups of patients that had significantly different OS. Specifically, 1988 FIGO stage IA and IB had distinct oncologic outcomes. The revised 2009 system eliminates the most favorable group from the new classification system, and estimates of stage-specific OS for stage IB are substantially altered by the changes made in 2009. The revised system for stage I did not improve its predictive ability over the 1988 system. These data highlight the importance of developing individualized risk-prediction models and nomograms in endometrial cancer.
Introduction
For the last 20 years, gynecologists have utilized the 1988 FIGO surgical staging system in the management of endometrial cancer patients. The 1988 system was a significant change from the prior clinical system in that it included surgical pathologic findings as an integral component of staging based on well conducted large scale clinicopathologic studies of endometrial cancer patients [1,2,3].
The revised 2009 FIGO staging system for endometrial cancer is a further attempt to refine the surgical staging system. For early-stage endometrial cancer, the 2009 system describes stage IA as no or <50% myoinvasion, essentially combining the 1988 FIGO stage IA, IB, IIA (with <50% invasion), and IIIA (based on positive wash only and <50% invasion) into the new stage IA. Moreover, the revised 2009 stage IB is composed of 1988 stage IC and stage IIA (≥50% invasion) and IIIA (only those with positive wash and ≥50% invasion). These changes represent a significant change in the classification of early-stage patients, and combines patients of previously conceived higher stage factors into the newer early-stage classification. We sought to describe the overall survival (OS) of women with stage I endometrial cancer to see if there is a difference between these groups, and to examine how the estimated stage-specific OS is altered in the 2009 system as compared to the 1988 system.
Methods
This study was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center. A prospectively maintained institutional endometrial database was analyzed. All patients underwent primary surgery between 1/1993 – 6/2009. We only included endometrioid adenocarcinoma histology. OS was calculated from the date of surgery to either the last follow-up or the date of death. OS probabilities were estimated using the Kaplan-Meier method. The log-rank test was used to obtain the P-values for univariate survival analyses. The hazard ratios were obtained by applying Cox proportional hazard model.
We compared the 1988 and 2009 staging systems using concordance probability [4]. Similar to area under the receiver operating characteristic curve, concordance probability can range from perfect concordance (1.0) to perfect discordance (0.0). A value of 0.5 indicates that for two randomly selected patients there is a 50% chance that the patient with the higher predicted probability by the staging system will have longer survival (i.e., the prediction performance of the staging system is no better than a coin flip). The bootstrap-corrected concordance probability was reported to prevent against over-fitting [5].
Results
In all, 1658 women with endometrial endometrioid cancer were analyzed. Based on the 1988 system, 1307 stage I patients – including IA (570), IB (593), and IC (144) – were identified. Comprehensive surgical staging with lymph node dissection was performed in 791 (61%) stage I cases with a median of 19 nodes (range, 1–92). Patient characteristics are summarized in Table 1
Table 1.
Demographics of the 1988 Stage I (N=1307) endometrioid adenocarcinoma patients.
| Variable | Count | Percent (%) |
|---|---|---|
| Vital Status | ||
| AWD | 30 | 2.3 |
| NED | 1186 | 90.7 |
| DOD | 48 | 3.7 |
| DOO | 43 | 3.3 |
|
| ||
| Age at Diagnosis | ||
| Median(Mean) | 61(60.77) | |
| Range | 25~92 | |
|
| ||
| Stage | ||
| IA | 570 | 43.6 |
| IB | 593 | 45.4 |
| IC | 144 | 11 |
|
| ||
| Final Grade (missing=2) | ||
| 1 | 592 | 45.4 |
| 2 | 501 | 38.4 |
| 3 | 212 | 16.2 |
|
| ||
| Depth of Invasion | ||
| <50% | 605 | 46.3 |
| >50% | 144 | 11 |
| none | 558 | 42.7 |
|
| ||
| Nodes Taken | ||
| None | 516 | 39.5 |
| Yes | 791 | 60.5 |
| ‘Total Lymph Nodes Taken>=1 | ||
| Median(Mean) | 19(19.65) | |
| Range | 1~92 | |
| ‘Total Pelvic Nodes Taken>=1 | ||
| Median(Mean) | 15(15.96) | |
| Range | 1~80 | |
| ‘Total Aortic Nodes Taken>=1 | ||
| Median(Mean) | 5(6.09) | |
| Range | 1~26 | |
|
| ||
| Height at Diagnosis (missing=117) | ||
| Median(Mean) | 160(159.73) | |
| Range | 57.5~186 | |
|
| ||
| Weight at Diagnosis (missing=71) | ||
| Median(Mean) | 76.1(81.58) | |
| Range | 37~208.6 | |
|
| ||
| LVI (missing=829) | ||
| NO | 403 | 84.3 |
| YES | 72 | 15.1 |
| NA | 3 | 0.6 |
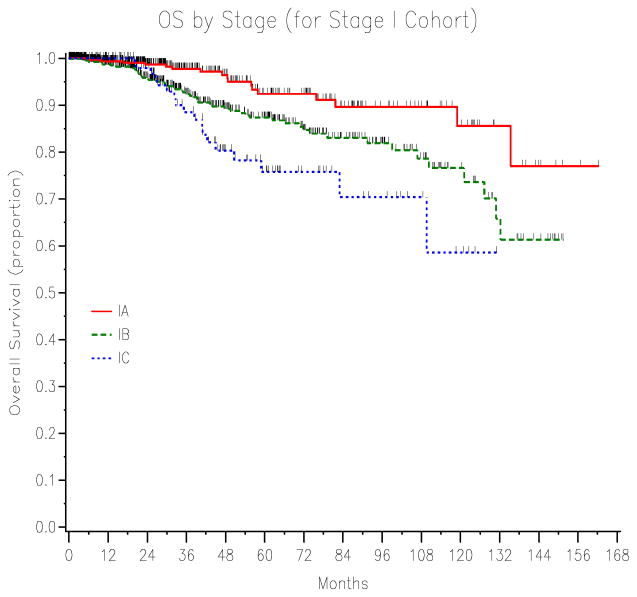
For the 1988 stage I cases, there were 91 deaths (48 of disease and 43 of other), and 1216 survivors (1186 no disease and 30 alive with disease). The median follow-up time for the survivors was 25.1 months (range, 0–162.2 months). The 5-year OS for 1988 stage IA (92.4%), IB (87.3%) and IC (75.7%) significantly differed (P<0.001) (Figure 1).
Figure 1.
The 5-year OS for 1988 FIGO stage IA (92.4%), IB (87.3%) and IC (75.7%) endometrial cancer significantly differed (P<0.001).
When patients were restaged using the 2009 system, we identified a total of 1411 stage I patients, including 1249 revised stage IA and 162 revised stage IB cases. Clinical characteristics for restaged patients are listed in Table 2.
Table 2.
Clinical Characteristics of the 2009 Stage I (N=1411) endometrioid adenocarcinoma patients.
| Variable | Count | Percent (%) |
|---|---|---|
| Vital Status | ||
| AWD | 44 | 3.1 |
| NED | 1267 | 89.8 |
| DOD | 56 | 4 |
| DOO | 44 | 3.1 |
|
| ||
| Age at Diagnosis | ||
| Median(Mean) | 60(60.66) | |
| Range | 25~92 | |
|
| ||
| 2009 Stage | ||
| IA | 1249 | 88.5 |
| IB | 162 | 11.5 |
|
| ||
| Final Grade (missing=3) | ||
| 1 | 630 | 44.7 |
| 2 | 550 | 39.1 |
| 3 | 228 | 16.2 |
|
| ||
| Depth of Invasion | ||
| <50% | 663 | 47 |
| >50% | 162 | 11.5 |
| none | 586 | 41.5 |
|
| ||
| Nodes Taken | ||
| None | 540 | 38.3 |
| Yes | 871 | 61.7 |
|
| ||
| Total Lymph Nodes Taken>=1 | ||
| Median(Mean) | 19(19.76) | |
| Range | 1~92 | |
| Total Pelvic Nodes Taken>=1 | ||
| Median(Mean) | 15(16.07) | |
| Range | 1~80 | |
| Total Aortic Nodes Taken>=1 | ||
| Median(Mean) | 5(6.14) | |
| Range | 1~26 | |
|
| ||
| Height at Diagnosis (missing=121) | ||
| Median(Mean) | 160(159.72) | |
| Range | 57.5~186 | |
|
| ||
| Weight at Diagnosis (missing=74) | ||
| Median(Mean) | 76(81.48) | |
| Range | 37~208.6 | |
|
| ||
| LVI (missing=880) | ||
| NO | 440 | 82.9 |
| YES | 87 | 16.4 |
| NA | 4 | 0.8 |
For the revised 2009 staging group, a total of 1411 patients were identified as stage I. In all, 100 deaths were documented (56 of disease and 44 of other causes), as were 1311 survivors (1267 no disease and 44 alive with disease). The median follow-up time for the 1311 survivors was 24.8 months (range, 0–162.2 months). The median survival time for the cohort was not reached.
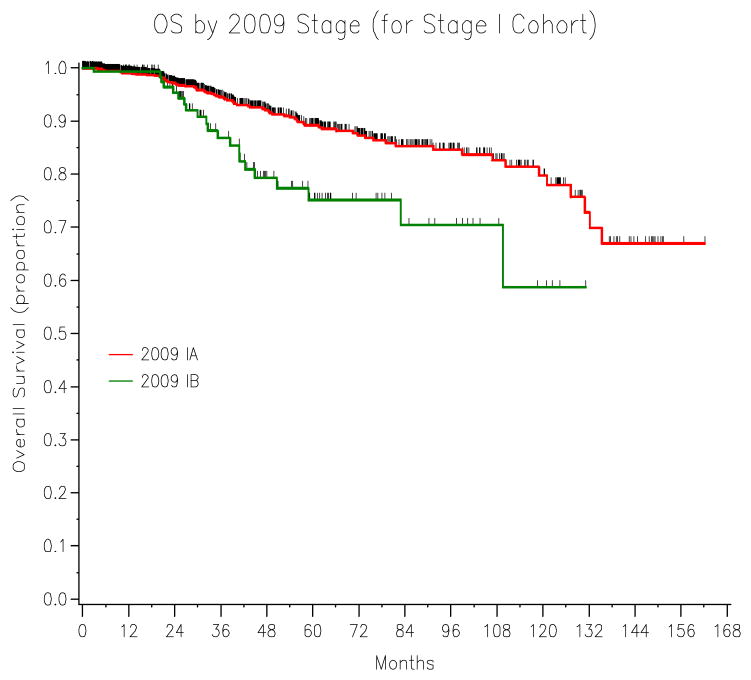
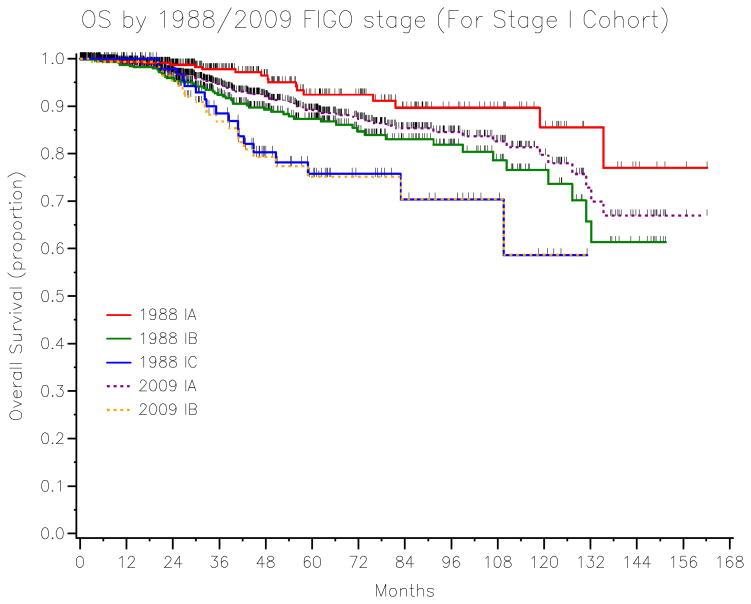
The univariate overall survival analysis stratified by stages is listed in Table 3. The 5-year OS for 2009 stage IA (89.2%) vs. IB (75.1%) (P=0.001) is shown in Figure 2. The overlaying Kaplan-Meier curves for OS stratified by 1988 vs. 2009 stage I patients is represented in Figure 3. The cross table of the 2009 stage I (N=1411) patients by the 1988 staging system is presented in Table 4.
Table 3.
The univariate overall survival
| Variable | N | %Alive | 3-Yr Survival Rate (95%CI) | 5-Yr Survival Rate (95%CI) | Hazard Ratio | p-value |
|---|---|---|---|---|---|---|
| All | 1411 | 92.90% | 93.5%(91.5~95.1%) | 87.4%(84.3~89.9%) | ||
|
| ||||||
| 1988 Stage I (N=1307) | ||||||
| IA | 570 | 96.80% | 97.7%(95.1~99%) | 92.4%(86.8~95.7%) | Ref. Level | <0.001 |
| IB | 593 | 90.70% | 92.4%(89.1~94.7%) | 87.3%(82.9~90.6%) | 2.21(1.3~3.76) | |
| IC | 144 | 87.50% | 88.4%(78.8~93.9%) | 75.7%(62.8~84.7%) | 3.54(1.84~6.82) | |
|
| ||||||
| 2009 Stage I (N=1411) | ||||||
| IA | 1249 | 93.70% | 94.5%(92.5~96%) | 89.2%(86.1~91.7%) | Ref. Level | 0.001 |
| IB | 162 | 87% | 86.8%(77.7~92.4%) | 75.1%(63.1~83.7%) | 2.19(1.35~3.56) | |
|
| ||||||
| For 1988 IIA cohort (N=40)* | ||||||
| 2009 IA | 29 | 96.60% | 93.3%(61.3~99%) | 93.3%(61.3~99%) | ||
| 2009 IB | 11 | 81.80% | 66.7%(16~91.4%) | 66.7%(16~91.4%) | ||
|
| ||||||
| For 1988 IIIA cohort (N=105)** | ||||||
| 2009 IA | 57 | 91.20% | 89.7%(64~97.4%) | 75.3%(45.3~90.3%) | ||
| 2009 IB | 7 | 85.70% | 75%(12.8~96.1%) | 75%(12.8~96.1%) | ||
| 2009>=II | 41 | 82.90% | 89.2%(73.6~95.8%) | 74.4%(52~87.5%) | ||
The results show if there is any heterogeneity among 1988 IIA cohort stratified by 2009 criteria
The results show if there is any heterogeneity among 1988 IIIA cohort stratified by 2009 criteria
Figure 2.
The 5-year OS stratified by the revised 2009 FIGO stage IA (89.2%) vs. 2009 stage IB (75.1%) endometrial cancer (P=0.001).
Figure 3.
The 5-year OS stratified by 1988 FIGO stage IA–C and 2009 FIGO stage IA–B endometrial cancer.
Table 4.
Cross table of 1988 Stage I and 2009 Stage I endometrial cancer patients
| 1988 Stage | ||||||
|---|---|---|---|---|---|---|
| IA | IB | IC | IIA | IIIA | Total | |
| 2009 Stage IA | 570 | 593 | 0 | 29 | 57 | 1249 |
|
| ||||||
| 2009 Stage IB | 0 | 0 | 144 | 11 | 7 | 162 |
|
| ||||||
| Total | 570 | 593 | 144 | 40* | 64* | 1411 |
These two groups of 104 patients are not included in the 1988 Stage I
The adjusted concordance probabilities for the 1988 stage I group and 2009 stage I group were 0.612 ± 0.0014 and 0.536 ± 0.0111, respectively. A statistical comparison and a P-value could not be provided due to the different cohorts that include some paired patients and some independent patients. However, in terms of concordance probability, the 2009 system appears inferior to the 1988 system. The concordance probabilities for the 1988 stage I and 2009 stage I patients is provided in Table 5.
Table 5.
The concordance probabilities for 1988 stage I group and 2009 stage I group
| N | Events | CPE.SE | Bootstrap-corrected CPE | |
|---|---|---|---|---|
| 1988 Stage I | 1307 | 91 | 0.0014 | 0.612 |
| 2009 Stage I | 1411 | 100 | 0.0111 | 0.536 |
Discussion
It has been reported that the main objectives of any good staging system are as follows: to aid the clinician in planning treatment; to provide indication of prognosis; to assist the physician in evaluating the results of treatment; to facilitate the exchange of information between treatment centers, and to contribute to continuing investigations into human malignancies [6]. Furthermore, a good staging system must have 3 basic characteristics: it must be valid, reliable, and practical [6]. For endometrial cancer limited to the uterus (Stage I), tumor grade and depth of myometrial penetration have been shown for more than three decades to have prognostic significance [7]. Following its adoption by the FIGO committee in 1988 as a replacement to the 1971 system, gynecologists and oncologists have utilized the 1988 FIGO staging system of endometrial cancer for more than 20 years. The significance of myoinvasion among many other surgical and pathologic factors has been highlighted in numerous previous publications [8,9]. In 1985, DiSaia et al. reported the relationship between depth of myometrial invasion and recurrence/death rates in stage I endometrial cancer. For stage I patients with no myometrial invasion the risk of death was 5% and increased to 11% with inner third invasion, 12% with middle third invasion, and 36% with deep myoinvasion [9]. However, the addition of lymph node evaluation, either formal lymphadenectomy or sampling of the pelvic and aortic lymph nodes, represented the most significant and controversial component of the 1988 system [10]. In 2009 Creasman reported in volume 26 of the FIGO annual report that the surgical stages IA G1, IB G1, IA G2 and IBG2 had similar 5-year survival rates. This report led the FIGO committee to combine some substages and resulted in the revised substaging of stage I endometrial cancer, which was adopted in 2008 and published in 2009 [11,12,13].
In the revised 2009 FIGO staging system for carcinoma of the endometrium, there are several major changes. Stage IA is now defined as “no or <50% myoinvasion,” essentially combing the 1988 FIGO stage IA, IB, IIA (with <50% invasion), and IIIA (based on positive wash only and <50% invasion) into the new stage IA. Moreover, the revised 2009 stage IB is now defined as “≥ one-half myoinvasion,” essentially combining the 1988 stage IC and stage IIA (≥50% invasion) and IIIA (only those with positive washings and ≥50% invasion). Stage II no longer has subsets A and B, and involvement of the endocervical gland of the cervix is now considered stage I. Pelvic and paraaortic lymph node involvement in the 1988 stage IIIC have been separated into stage IIIC1 (positive pelvic lymph nodes) and IIIC2 (positive paraaortic lymph nodes with or without positive pelvic lymph nodes). Lastly, positive peritoneal cytology has been excluded as factors for defining the new surgical staging [11,12,13,14].
Our study focused on the FIGO stage I changes, specifically the elimination of the most favorable subgroup, 1988 FIGO IA. When new staging systems are adopted, comparison of the old and the new systems is commonly performed. Similar studies in melanoma have examined how the estimated stage-specific survival is altered in the 2002 American Joint Committee on Cancer (AJCC) melanoma staging system compared with the 1997 AJCC staging system; researchers found that the newer system was more complex and did not improve the predictive ability over the 1997 system [15]. In a recent SEER data analysis to address similar issues with the FIGO system investigators reported that In the 2009 system, survival was 89.6% for stage IA and 77.6% for stage IB; moreover, in the 2009 system, survival for stage II was inferior to all stage I patients [16]. Our analysis indicates that the 1988 FIGO classification of stage I endometrial cancer correctly identified three subgroups of patients that had significantly different OS. Specifically, 1988 FIGO stage IA and IB had distinct oncologic outcomes. The revised 2009 system eliminates the most favorable group (1988 IA) from the new classification system, and estimates of stage-specific OS for stage IB are substantially altered by the changes made in 2009. Moreover, the revised system for stage I did not improve its predictive ability over the 1988 system as seen by the adjusted concordance probabilities for the 1988 stage I group and 2009 stage I group.
In summary, the revised 2009 FIGO system for stage I endometrial cancer simplified the staging system into two stage I subgroups, IA & IB. However, it did not improve its predictive ability over the 1988 system. These data highlight the importance of developing individualized risk-prediction models and nomograms in endometrial cancer.
References
- 1.Boronow RC, Morrow CP, Creasman WT, Disaia PJ, Silverberg SG, Miller A, Blessing JA. Surgical staging in endometrial cancer: clinical-pathologic findings of a prospective study. Obstet Gynecol. 1984;63:825–32. [PubMed] [Google Scholar]
- 2.Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer. 1987;60(8 Suppl):2035–41. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.DiSaia PJ, Creasman WT. Management of endometrial adenocarcinoma stage I with surgical staging followed by tailored adjuvant radiation therapy. Clin Obstet Gynaecol. 1986;13:751–65. [PubMed] [Google Scholar]
- 4.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92:965–70. [Google Scholar]
- 5.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Odicino F, Pecorelli S, Zigliani L, Creasman WT. History of the FIGO cancer staging system. Int J Gynaecol Obstet. 2008;101:205–10. doi: 10.1016/j.ijgo.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Homesley HD, Boronow RC, Lewis JL., Jr Treatment of adenocarcinoma of the endometrium at Memorial-James Ewing Hospitals, 1949–1965. Obstet Gynecol. 1976;47:100–5. [PubMed] [Google Scholar]
- 8.Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, Graham JE. Relationship between surgical-pathological risk factors and outcome in clinical stage I and II carcinoma of the endometrium: a Gynecologic Oncology Group study. Gynecol Oncol. 1991;40:55–65. doi: 10.1016/0090-8258(91)90086-k. [DOI] [PubMed] [Google Scholar]
- 9.DiSaia PJ, Creasman WT, Boronow RC, Blessing JA. Risk factors and recurrent patterns in Stage I endometrial cancer. Am J Obstet Gynecol. 1985;151:1009–15. doi: 10.1016/0002-9378(85)90371-0. [DOI] [PubMed] [Google Scholar]
- 10.Boronow RC. Endometrial cancer and surgical staging: a personal assessment. Philipp J Obstet Gynecol. 1998;22:71–7. [PubMed] [Google Scholar]
- 11.Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109. doi: 10.1016/j.ijgo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Mutch DN. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol Oncol. 2009;115:325–328. [Google Scholar]
- 13.Pecorelli S. Revised FIGO staging of carcinoma of the vulva, cervix, and endometrium. International J of Gynecol and Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Song YS. International Federation of Gynecology and Obstetrics (FIGO) staging system revised: what should be considered critically for gynecologic cancer? J Gynecol Oncol. 2009;20:135–6. doi: 10.3802/jgo.2009.20.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ben-Porat L, Panageas KS, Hanlon C, Patel A, Halpern A, Houghton AN, Coit D. Estimates of stage-specific survival are altered by changes in the 2002 American Joint Committee on Cancer staging system for melanoma. Cancer. 2006;106:163–71. doi: 10.1002/cncr.21594. [DOI] [PubMed] [Google Scholar]
- 16.Lewin SN, Herzog TJ, Barrena Medel NI, Deutsch I, Burke WM, Sun X, Wright JD. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet Gynecol. 2010 Nov;116(5):1141–9. doi: 10.1097/AOG.0b013e3181f39849. [DOI] [PubMed] [Google Scholar]