Abstract
Purpose
To describe the characteristics of a series of study populations of ovarian cancer patients with identical elegibility criteria in second or subsequent clinical remission (cCR) and to propose endpoint benchmarks for future consolidation studies.
Patients and Methods
The patient populations consisted of those 1) untreated - U (observed until progression), n = 35; 2) receiving imatinib - G, n = 32; 3) receiving goserelin and bicalutamide - A, n = 32; and 4) receiving vaccine - V, n = 68; total = 167. The endpoint of the combined analysis was progression-free survival in second remission (PFS 2). Patient characteristics were compared by Chi-square test, and factors predicting PFS 2 evaluated in multivariate Cox model.
Results
Groups were comparable for age, stage, grade, and debulking. Multivariate model to predict PFS 2 duration included histology, stage, optimal debulking, PFS 1 duration and the type of intervention. As a benchmark for future studies, the median PFS 2 of the combined population of G, A, U (removing V which had the most impact in prolonging PFS 2, n=68) was 11.3 months (95% CI: 10.4 – 12.5 months). The percent of patients with PFS 2 > PFS 1 was 14/90 (16%). At 12 months, 43% remain progression free.
Conclusion
Preliminary benchmarks for efficacy endpoints are suggested for future consolidation trials of patients in cCR. However, the suggested strategies will require validation in randomized trials and larger data sets.
INTRODUCTION
The median overall survival for optimally debulked ovarian cancer patients has increased to more than 5 years, but less than 30% will remain disease free following surgery and platinum-based chemotherapy.1 Many patients will be highly responsive to additional chemotherapy at recurrence, and some can reenter successively shorter complete clinical remissions with additional treatment as illustrated in our previously described disease-states model.2,3 Most studies of consolidation have been performed in the first clinical remission setting and include a variety of cytotoxic and immunologic strategies. No randomized study has provided a statistically significant improvement in overall survival.4-12 Given the longer time to treatment failure in this population, and modest group of cured patients, larger studies with longer follow-up are required. Several years ago, we hypothesized that the second or third complete remission population would represent a better group in which to evaluate consolidation strategies given its shorter duration of remission and nearly certain chance of relapse, and embarked on a series of prospective investigational consolidation trials in these patients.2,13 Others are now identifying this group as an umet medical need. The challenge in designing phase II trials for this patient group is in choosing reliable endpoints, and setting a bar for “success” that, if exceeded, would merit testing of the intervention in a randomized phase III trial.
In this study, we initially considered together 4 distinct populations of patients in second or subsequent complete clinical remission. The objective of this analysis was to describe the characteristics of the combined second or subsequent complete clinical remission population for the purpose of proposing benchmarks for future study design.
PATIENTS AND METHODS
Eligibility Criteria
Eligible patients for all studies had epithelial ovarian carcinoma arising in the ovary, fallopian tube, or peritoneum from stage II-IV at diagnosis. Primary treatment included cytoreductive surgery and platinum-based chemotherapy. Patients had relapsed following primary therapy, and returned to complete clinical remission after additional treatment. Complete clinical remission was defined as serum CA-125 ≤35 IU/ml, CT scan without evidence of disease, and normal physical examination. Other requirements included Karnofsky Performance Status ≥60%; adequate organ function defined as absolute neutrophil count ≥1.0 × 103 μL, platelets ≥100,000 cells/mm3; serum creatinine ≤1.5 times institutional upper limits of normal; and liver function tests ≤2 times institutional upper limits of normal. Patients treated on the intervention trials provided written informed consent and trials were reviewed by the Memorial Sloan-Kettering Institutional Review Board.
Patient Populations
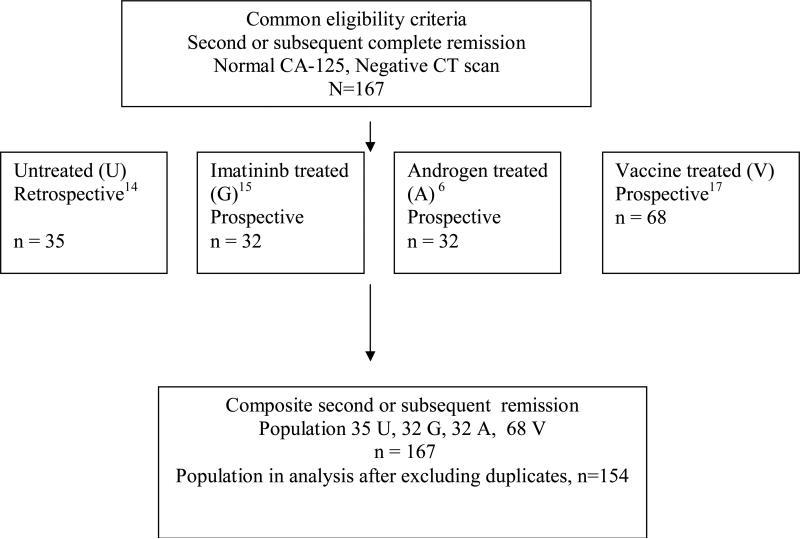
The primary endpoint was to determine the progression-free survival of the second or subsequent clinical complete remission in all patient groups utilizing common eligibility criteria. Patient flow is outlined in Figure 114-17 as follows: 1) A retrospective study of patients in second or subsequent complete clinical remission included those who were observed until progression.14 This consisted of a review of consecutive patients treated at Memorial Sloan-Kettering Cancer Center (October 1993 to December 2000) and the Royal Marden Hospital (January 1995 to April 2003). Thirty-five patients were identified who met the eligibility criteria; the duration of the second progression-free survival was 10.7 months (95% CI: 9.3-12.2 mos).
Figure 1.
Patient flow diagram from the studies to form combined population
The remaining populations were derived from separate, consecutive prospective clinical trials at MSKCC evaluating investigational approaches: 2) A phase II trial of imatinib for patients in second or subsequent complete clinical remission enrolled 35 patients between 10/2002 and 1/2005. Eligible patients received imatinib at 400mg daily orally. The duration of the second progression-free survival was 12.1 mos (95% CI: 9.4 – 15.5 mos).15 3) A phase II trial of goserelin and bicalutamide for patients in second or subsequent complete clinical remission enrolled 35 patients between 10/00 and 10/02. Eligible patients received bicalutamide 50 mg orally daily and goserelin at 3.6 mg subcutaneously every 4 weeks. The duration of the second progression-free survival was 11.8 mos (95% CI: 10.6 – 13.2 mos)16 and 4) A series of 6 phase I trials enrolling 81 patients (n = 68 in second or greater remission) evaluated monovalent or heptavalent antibody inducing vaccine strategies. Vaccines consisted of an antigen with or without conjugation to KLH mixed with an adjuvant. The clinical trials with each respective antigen were performed as follows: KSA-KLH with QS-21 (n = 20), MUC-1-KLH with QS-21 (n = 19), Le-Y-MMCCH-KLH (increased epitope ratio) with QS-21 (n = 9), Multivalent antigen – KLH with QS-21 (n=11), KH1-KLH with QS-21 (n = 9), and KSA-KLH with GPI-001 (n = 13). The duration of the second progression-free survival for the composite vaccine population was 16.1 months (95% CI: 13.6-24.2 mos).
For this analysis, 167 patients were in second or subsequent remission; with 13 patients having received more than one of the above interventions. After counting these patients once under the protocol they received in second CR, the population in the final analysis consisted of 154 patients.
Patient Assessments
For patients on prospective studies, pre-treatment evaluation included a complete medical history, physical and radiologic examination, vital signs, KPS assessment, and clinical laboratory tests including immunologic testing (vaccines only) with repeat assessment at regular intervals. CT imaging was performed every 3 months while on each study, or sooner at discretion of investigator. All patients were in complete clinical remission at the time of study enrollment on each trial.
Definitions of Progression-Free Intervals
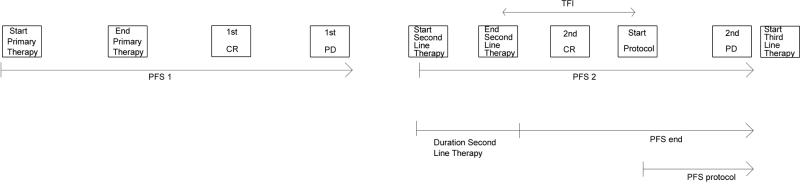
The standard convention is to define a given progression-free interval from the start of respective therapy to disease progression.3 However, consolidation therapies include the time on second-line therapy, the time that a patient is in complete response, the treatment-free interval, and the time that a patient receives the consolidation treatment. For the purpose of exploring possible endpoints, other PFS definitions were considered and reported in months as graphically depicted in Figure 2. The first PFS (pre-protocol intervention) was measured as the time interval from the start of first-line therapy to the date of first relapse (PFS 1). The second PFS was measured as the interval from the start of second-line therapy to the date of the second relapse (PFS 2). We also explored calculating PFS 2 from the end of chemotherapy to progression in order to avoid bias in prolonging PFS 2 due to unequal number of cycles on second line chemotherapy (PFS end). Finally, PFS was defined as the time from the protocol start date (i.e. start of consolidation therapy) to progression, or last follow-up for the patients who did not progress (PFS protocol). The third PFS was measured as the interval from the start of third-line therapy to the date of third relapse (PFS 3).
Figure 2.
Definitions of PFS
First PFS (pre-protocol intervention) was measured as the time interval from the start of first-line therapy to the date of first relapse (PFS 1). Second PFS was measured as the interval from the start of second-line therapy to the date of the second relapse (PFS 2). Third PFS was measured as the interval from the start of third-line therapy to the date of third relapse (PFS 3). Since there were no strict eligibility entry criteria limiting the duration of second-line chemotherapy, we also explored calculating PFS 2 from the end of chemotherapy to relapse in order to avoid bias in prolonging PFS 2 due to unequal number of cycles on second-line chemotherapy (PFS end). Finally, PFS was defined as the time from the protocol start date to progression, or last follow-up for the patients who did not progress (PFS protocol). PFS intervals are reported in months.
Statistical Analyses
The endpoint of the combined analysis of the datasets (untreated patients - U, imatinib treated - G, combined androgen blockade treated - A, and vaccine treated - V) was progression-free survival on consolidation therapy (PFS 2). Treatment failure was characterized by radiographic evidence of disease recurrence using RECIST criteria if present, or a confirmed rise in CA-125 to ≥ 70 u/ml.18 Patient characteristics across the datasets were compared by Chi-square test for categorical variables, and the non-parametric Wilcoxon test for continuous variables. Treatment-free interval (TFI) was measured as the time from the last dose of second-line chemotherapy until the start date on protocol. Estimates of PFS were obtained via the Kaplan-Meier method. Multi-variate analysis was used to determine which clinical variables were associated with longer duration of PFS 2, accounting for any potential differences among populations. Duration of PFS 2 was the outcome (dependent) variable. Covariates tested in the model included pre-treatment patient characteristics such as stage, grade, patient age, histology, as well as the duration of the progression-free interval in first-line treatment (PFS 1). The effect of the source population (i.e., U-untreated, G-imatinib, A-androgen, or V-vaccine) was analyzed as a fixed effect in a cox proportional hazard model, as well as a random effect in a frailty model evaluating Gaussian and Gamma distribution for the random effects. The fixed-effects model was more appropriate based on fit and results; it was chosen and is presented in this manuscript.19
RESULTS
Patient Characteristics
The characteristics of the combined population for patients in second or subsequent complete clinical remission (n = 154) are shown in Table 1 according to each study.
Table 1.
Combined Patient Population Characteristics – Second or subsequent complete clinical remission (n = 154)
| Protocol | U (untreated) | G (imatininb) | A (androgen) | V (vaccine) | p |
|---|---|---|---|---|---|
| No. patients 2nd CR or greater | 33 | 30 | 27 | 64 | |
| Stage | 0.17 | ||||
| Stage 1,2 | 3 (9) | 2 (7) | 1 (4) | 12 (19) | |
| Stage 3,4 | 30 (91) | 28 (93) | 26 (96) | 52 (81) | |
| Histology | 0.55 | ||||
| serous | 25 (76) | 21 (70) | 23 (85) | 41 (64) | |
| endometrioid | 6 (18) | 8 (27) | 3 (11) | 18 (28) | |
| other | 2 (6) | 1 (3) | 1 (4) | 5 (8) | |
| Grade | 0.63 | ||||
| 1 | 0/30 (0) | 1 (3) | 2 (7) | 2/61 (3) | |
| 2 | 10/30 (33) | 6 (20) | 9 (33) | 16/61 (26) | |
| 3 | 20/30 (67) | 23 (77) | 16 (59) | 43/61 (70) | |
| Optimal debulking at diagnosis | 0.47 | ||||
| Yes | 15/24 (62) | 22 (73) | 20 (74) | 49/62 (79) | |
| No | 9/24 (37) | 8 (27) | 7 (26) | 13/62 (21) | |
| Surgery at recurrence | 0.001 | ||||
| Yes | 2/33 (6) | 16 (53) | 11 (41) | 16 (25) | |
| No | 31/33 (94) | 14 (47) | 16 (59) | 48 (75) |
The median age of the population was 52 (range, 25- 72 y). The majority of patients (88%) were stage III or IV, with no stage distribution differences among the 4 groups (p = 0.17). Histology was serous or endometrioid in most patients (71%) (p = 0.55), and most (73%) were poorly differentiated (p = 0.63). Optimal debulking was common (68%) and similar in frequency among the 4 groups (p = 0.47). Secondary surgical cytoreduction was not performed in most patients (71%) at the time of relapse. Of those receiving secondary surgical cytoreduction, however, there are differences in the number of patients treated with surgery at recurrence with 53% receiving surgery in G compared with 41% with A, 25% with V, and 6% with U, respectively (p = 0.001).
Study Endpoints
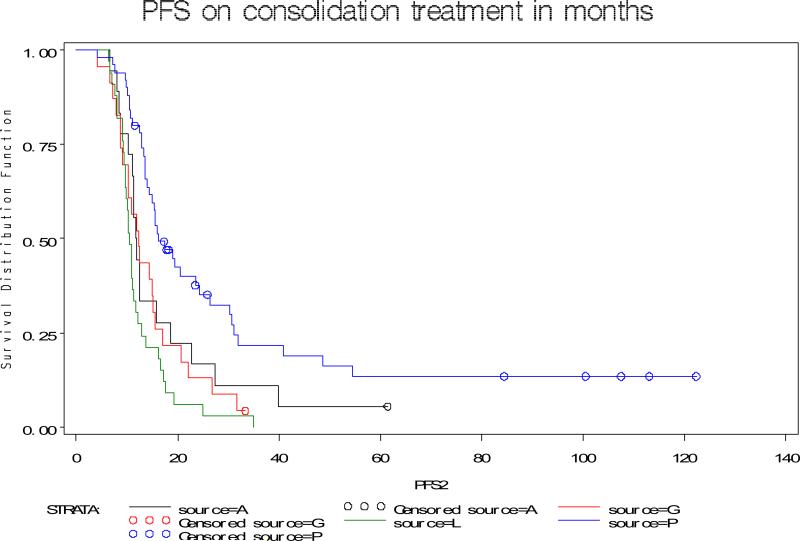
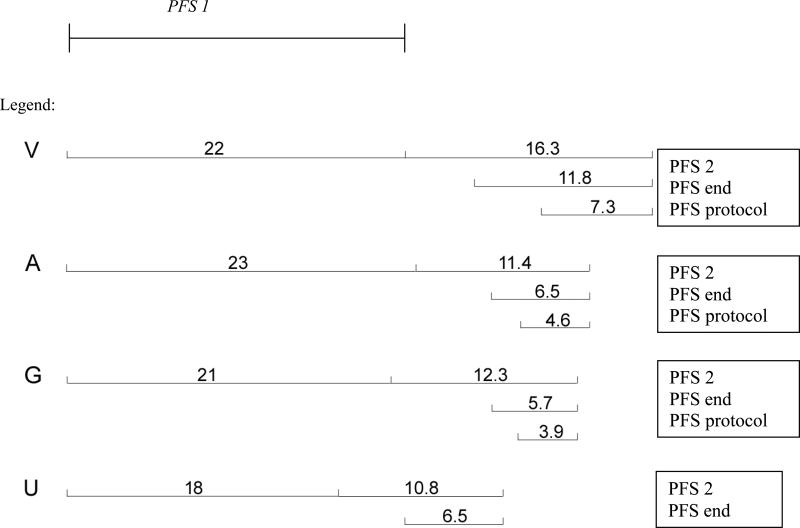
In the combined cohort of 154 patients in second or subsequent complete clinical remission, median follow-up was 33.4 mos (range, 11.6-122.4 mos). Using the standard definition of PFS, PFS 2 of each group is shown in Figure 3. It was noted in univariate analysis, that PFS 2 appears to be longer at 16 months for patients treated with vaccine compared with 11, 12, and 12 months respectively for those untreated, or treated with G and A respectively. The estimates for PFS end and PFS protocol (which starts at the protocol start date and thus is not affected by the duration of second-line therapy) are also longer for patients treated with vaccines as shown in Figure 4.
Figure 3.
Duration of PFS by study population using standard definition of PFS.
Figure 4.
Duration of PFS utilizing different definitions
Patient population: patents in second CR only. PFS intervals are defined as described in Figure 2.
The results from the multivariate analysis are shown on Table 2. This analysis includes 109 patients (96 events) in second complete remission with non-missing values, while it excludes patients in ≥ third CR in order to compare the treatment/intervention effect. Although grade and histology were not significant at the 0.05 alpha level, they were controlled for in the model. Surgery at recurrence was not significant (p = 0.8) after controlling for other important covariates. The final model included stage, histology, grade, optimal debulking at diagnosis, PFS 1 and source population (i.e., untreated, G, A or V). Patients who were low stage had a HR of 0.5 (95% CI: 0.2-1.03), and optimally debulked patients had a HR of 0.6 (95% CI: 0.3-0.99). PFS 1 was highly predictive of PFS 2 when evaluated both as a continuous and categorical covariate. Most patients had PFS 1 >12 months, so adequate cutoffs in this population seemed to be ≤12, 12-18 months, and >18. Patients with PFS 1 < 12 months were 3.5 times more likely to progress, and patients with PFS 1 between 12-18 months were 1.7 times more likely to progress compared with patients with PFS 1>18 months.
Table 2.
Multi-variate analysis (Patients in second complete remission with non-missing values)
| Covariate | HR (95% CI) | p-value | Reference Group |
|---|---|---|---|
| Duration of PFS 1 | |||
| PFS 1 < 12 mos | 3.5 (1.6-7.8) | .0024 | PFS 1 > 18 mos |
| PFS 1 12 – 18 mos | 1.7 (1.03-2.9) | PFS 1> 18 mos | |
| Source Population | |||
| Imatinib – (G) | 1.6 (0.9-3) | .0027 | Vaccine – (V) |
| Androgen – (A) | 1.6 (0.9-2.9) | Vaccine – (V) | |
| Untreated – (U) | 3.0 (1.7-5.5) | Vaccine – (V) | |
| Low stage | 0.5 (0.2-1.03) | .0595 | High stage |
| Optimal debulking | 0.6 (0.3-0.99) | .0473 | Suboptimal |
| Serous Histology | 1.2 (0.3- 4.4) | .0954 | Other Histology |
| Endometrioid Histology | 0.6 (0.16- 2.6) | ||
| Grade 3 | 1.6 (0.96-2.5) | .0726 | Grade 1-2 |
The two phase II trials (G and A) were negative as they did not meet their predefined endpoint, and have similar efficacy results as the untreated population. Owing to their longer PFS as a group in univariate and multivariate analyses, and recognizing the limitations of combining phase I trials, the vaccine population was therefore excluded from the following analysis in order to develop benchmarks from a homogeneous complete remission population (G, A, U). The PFS 2 of the second complete remission group for the three populations (untreated - U, imatinib treated - G, and combined androgen treated – A; n=90) is 11.3 months (95% CI: 10.4 – 12.5 mos). The proportion of patients with PFS 2 > PFS 1 is 14/90 [(16%) with 95% CI (8.1-23.0)] with a median difference of PFS 2 – PFS 1 of 4.1 months (range, 0.7- 38.5 mos). In selecting a fixed time point of 12 months, 43% of patients remain in remission.
DISCUSSION
The patients reviewed have been part of a consolidation program with consistent eligibility criteria and follow-up intervals.14-17 This exploratory analysis has resulted in a homogeneous combined population with respect to age, stage, histology, and debulking status. The number of patients having additional surgery at relapse was uniformly low (29%), but of these surgeries, more were performed in patients receiving imatinib (G) than in patients in the other groups. These observations are exploratory in nature, and validation in larger datasets will be required.
Previously well known variables such as stage and degree of debulking were important in terms of predicting PFS 2 but the duration of PFS 1 was most significant in our data. This is clinically intuitive in that the duration of PFS 1 may serve as a marker of the “biology” of disease. We showed a difference utilizing the categories of ≤ 12 mos, 12 to ≤ 18 mos, or > 18 months, but additional study is required in other groups to see if these or other categories should be considered in planned trials.
In order to facilitate comparison to historical studies, the standard definition of PFS 2 should be used as an endpoint (i.e., start of second-line therapy to time of relapse). However, it is necessary to restrict the number of cycles or time on secondary therapy and/or to place some restriction of TFI before the start of consolidation therapy. Our study population showed a median time on secondary therapy of 4.5 months (IQR, 3.7-5.9 mos) which corresponds to about 6 cycles of treatment. However, there were 20/154 patients (13%) with time on secondary therapy > 8 months.
Our data suggest that third-line remission (PFS 3) is 10. 3 months (95% CI: 10.1-21.5 mos), not remarkably different from PFS 2 at 11.3 months. It is unknown whether subsequent remissions could be shorter and biased against the protocol agent; for this reason, protocols should probably restrict patients to second or third remission only.
If duration of PFS 2 is used as a study endpoint, the related difficulty is in identifying a “promising” result in order to decide which strategy to take forward into a larger randomized trial. We previously used the literature to estimate the time to disease progression from platinum-containing second-line trials (since non-platinum containing regimens rarely produce complete clinical remissions) and derived an estimate of 9 (AGO study) −13 (ICON 4 study) months for PFS 2.20, 21 In designing our early studies, we decided that if a strategy moved the median PFS from 12 to 16.5 months, for example,16 we would consider it worthy of further study. One problem was that historical trials do not separately report the characteristics of the complete clinical remission population from those with stable disease or partially responding disease. We were concerned that complete clinical remissions may have an inherently more favorable outcome than partial remissions or stable disease. The median PFS 2 of our consolidation populations in this analysis (U, G, A) is in the range of 11.3 months (95% CI: 10.4-12.5 mos). This suggests that the complete clinical responders (in our populations) are not inherently different than those largely achieving a partial response (in the historical AGO and ICON4 populations) in terms of PFS 2. This important observation supports a median of 11-12 months as a reasonable estimate for PFS 2 in designing future studies. A separate problem of using a prolongation of the median PFS as sole endpoint as we did in our early trials concerns the potential for irregular timing of scans and the bias associated with interval censoring.22 While our study populations were strictly followed for PD every 3 months, some patients were symptomatic, resulting in early CT scans and CA-125 evaluations. A patient who progressed at 6 months might be missed on the second scan and thus be reported as a patient with progression at 9 months. Therefore, an exploration of other potential endpoints was warranted.
Recent data have suggested that the frequency with which second remissions exceed the first may be a useful endpoint strategy, although it has never been shown to predict overall survival.3 The proportion of patients having PFS 2 > PFS 1 in our composite population (U,G,L) remains low at 14/90 (16%) with the difference ranging from 0.7 to 38.5 months. This is higher than observed in the population evaluated by Markman et al. (which included patients with CR and PR) or in our untreated population (9%). This suggests that patients do not often have a second remission longer than the first, and a strategy that increases the proportion of patients over a predetermined amount may be worthy of further study.3 Problems with this endpoint include defining a difference in second versus first remission that is clinically meaningful and the issue of interval censoring still applies. A positive feature of including this endpoint is that it allows patients to serve as their own controls, and it may serve to reduce the potential bias introduced previously, for example, that a longer PFS 1 inherently predicts a longer PFS 2 regardless of intervention, as seen in our data.
Recent data suggest that a binary endpoint such as the proportion of patients in remission at a given time point may be least subject to bias.3, 22 In selecting a fixed time point of 12 months, 43% of our patients remained progression free in the composite population of U, G, L. This is strikingly similar to the ICON 4 dataset, in which 40-50% of patients were progression free at 12 months,20 and higher than the 35% seen in the AGO study21 or the 34% seen in our untreated population.14 Recognizing the heterogeneity of the populations, and the variability in both our estimates and those in the literature, the ranges are narrow enough that we still can provide preliminary benchmarks. Future studies in patients in second or third complete clinical remission could consider an improvement in the proportion of patients disease free at 12 months from 35% - 40% to 55%-60% as one indicator for further study.
While randomized phase III trials are required to make definitive statements regarding efficacy, this analysis provides preliminary estimates to consider when designing phase II consolidation trials in patients in a second or third complete clinical remission. It is imperative to identify benchmarks that would prompt moving from an exploratory Phase II trial to a Phase III setting. The estimates derived from these retrospective studies need further validation, which can only occur as data are collected from planned phase III prospective remission studies. To this end, it is recommended that endpoints for these trials include the proportion of patients in remission at a fixed time point (e.g., 12 months) or median PFS. The proportion of patients with a second remission longer than first can serve as a secondary endpoint.
Acknowledgments
Grant Support: NIH K23 CA893333 and PO1 CA 052477 and the Eileen Genet Fund for Cancer Research.
REFERENCES
- 1.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 2.Dizon DS, Hensley ML, Poynor EA, et al. Retrospective analysis of carboplatin and paclitaxel as initial second-line therapy for recurrent epithelial ovarian carcinoma: application toward a dynamic disease state model of ovarian cancer. J Clin Oncol. 2002;20:1238–47. doi: 10.1200/JCO.2002.20.5.1238. [DOI] [PubMed] [Google Scholar]
- 3.Markman M, Markman J, Webster K, et al. Duration of Response to Second-Line, Platinum-Based Chemotherapy for Ovarian Cancer: Implications for Patient Management and Clinical Trial Design. J Clin Oncol. 2004;22:3120–5. doi: 10.1200/JCO.2004.05.195. [DOI] [PubMed] [Google Scholar]
- 4.Hall GD, Brown JM, Coleman RE, et al. Maintenance treatment with interferon for advanced ovarian cancer: results of the Northern and Yorkshire gynaecology group randomised phase III study. Br J Cancer. 2004;91:621–6. doi: 10.1038/sj.bjc.6602037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberts DS, Hannigan EV, Liu PY, et al. Randomized trial of adjuvant intraperitoneal alpha-interferon in stage III ovarian cancer patients who have no evidence of disease after primary surgery and chemotherapy: An intergroup study. Gynecol Oncol. 2005 doi: 10.1016/j.ygyno.2005.07.117. [DOI] [PubMed] [Google Scholar]
- 6.Schilder RJ, Brady MF, Spriggs D, Shea T. Pilot evaluation of high-dose carboplatin and paclitaxel followed by high-dose melphalan supported by peripheral blood stem cells in previously untreated advanced ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. 2003;88:3–8. doi: 10.1006/gyno.2003.6882. [DOI] [PubMed] [Google Scholar]
- 7.Lambert HE, Rustin GJ, Gregory WM, Nelstrop AE. A randomized trial comparing single-agent carboplatin with carboplatin followed by radiotherapy for advanced ovarian cancer: a North Thames Ovary Group study. J Clin Oncol. 1993;11:440–8. doi: 10.1200/JCO.1993.11.3.440. [DOI] [PubMed] [Google Scholar]
- 8.Sorbe B. Consolidation treatment of advanced ovarian carcinoma with radiotherapy after induction chemotherapy. Int J Gynecol Cancer. 2003;13(Suppl 2):192–5. doi: 10.1111/j.1525-1438.2003.13359.x. [DOI] [PubMed] [Google Scholar]
- 9.Varia MA, Stehman FB, Bundy BN, et al. Intraperitoneal radioactive phosphorus (32P) versus observation after negative second-look laparotomy for stage III ovarian carcinoma: a randomized trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:2849–55. doi: 10.1200/JCO.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Nicoletto MO, Tumolo S, Falci C, et al. A Randomized Study of Epithelial Ovarian Cancer: Is Chemotherapy Useful after Complete Remission? Int J Med Sci. 2004;1:116–25. doi: 10.7150/ijms.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Placido S, Scambia G, Di Vagno G, et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO-1) randomized study. J Clin Oncol. 2004;22:2635–42. doi: 10.1200/JCO.2004.09.088. [DOI] [PubMed] [Google Scholar]
- 12.Berek JS, Taylor PT, Gordon A, et al. Randomized, placebo-controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22:3507–16. doi: 10.1200/JCO.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Sabbatini P, Spriggs DR. Consolidation for ovarian cancer in remission. J Clin Oncol. 2006;24:537–9. doi: 10.1200/JCO.2005.04.5138. [DOI] [PubMed] [Google Scholar]
- 14.Harrison ML, Gore ME, Spriggs D, et al. Duration of second or greater complete clinical remission in ovarian cancer: exploring potential endpoints for clinical trials. Gynecol Oncol. 2007;106:469–75. doi: 10.1016/j.ygyno.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juretzka M, Hensley M, Tew W, et al. A Phase of Oral Imatinib in Patients with Epithelial Ovarian, Fallopian Tube, or Pertioneal Carcinoma in Second or Greater Remission. European Journal of Gynaecological Oncology. 20082008 In press. [PubMed] [Google Scholar]
- 16.Levine D, Park K, Juretzka M, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2448–56. doi: 10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- 17.Sabbatini PJ, Ragupathi G, Hood C, et al. Pilot study of a heptavalent vaccine-keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clin Cancer Res. 2007;13:4170–7. doi: 10.1158/1078-0432.CCR-06-2949. [DOI] [PubMed] [Google Scholar]
- 18.Rustin GJ, Nelstrop AE, Tuxen MK. Defining progression of ovarian carcinoma during followup according to CA 125: a North Thames Ovary Group Study. Annals of Oncology. 1996;7:361–4. doi: 10.1093/oxfordjournals.annonc.a010602. al. e. [DOI] [PubMed] [Google Scholar]
- 19.O'Quigley J, Stare J. Proportional hazards models with frailties and random effects. Stat Med. 2002;21:3219–33. doi: 10.1002/sim.1259. [DOI] [PubMed] [Google Scholar]
- 20.Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106. doi: 10.1016/s0140-6736(03)13718-x. [DOI] [PubMed] [Google Scholar]
- 21.Pfisterer J, Plante M, Vergote I, et al. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699–707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 22.Panageas KS, Ben-Porat L, Dickler MN, Chapman PB, Schrag D. When you look matters: the effect of assessment schedule on progression-free survival. J Natl Cancer Inst. 2007;99:428–32. doi: 10.1093/jnci/djk091. [DOI] [PubMed] [Google Scholar]