Abstract
Objective
To analyze whether serum CA-125 response to cytoreductive surgery before initiation of postoperative chemotherapy is associated with progression-free survival (PFS) in patients with stage IIIC ovarian carcinoma.
Methods
We included consecutive patients with paired pre- and postoperative CA-125 measurements who underwent primary cytoreductive surgery followed by platinum-based chemotherapy between 1989 and 2006. The association of perioperative CA-125 changes with PFS was investigated using a time-to-event analysis. A Cox proportional hazards model was fit using clinical, surgical, and postoperative treatment characteristics.
Results
The study included 307 evaluable patients. Overall, perioperative serum CA-125 changes were associated with PFS. The risk of disease progression increased incrementally as the magnitude of the serum CA-125 response to surgery decreased (trend test; P=0.003). This association was pronounced in optimally but not observed in suboptimally debulked patients. After optimal cytoreduction, a perioperative increase of serum CA-125 levels was strongly associated with an increased risk of relapse compared to patients who experienced a decline of 80% or more (adjusted HR=4.2; 95%CI: 2.04-8.66; P=0.0001).
Conclusion
Perioperative serum CA-125 changes are strongly associated with the risk of relapse in patients with optimally resected stage IIIC disease. The results of this study provide meaningful support for additional translational research correlating perioperative serum CA-125 responses of patients with molecular tumor characteristics. This may be useful for patient counseling and risk stratification during subsequent clinical trials as well as for the development of novel prognostic models.
Keywords: Perioperative serum CA-125 changes, CA-125, advanced epithelial ovarian cancer, ovarian cancer, surgery
Introduction
Ovarian cancer is the second most common gynecologic malignancy in the United States, with an estimated 21,650 new cases diagnosed in 2008, and the most lethal, with an estimated 15,520 deaths during the same year [1]. The high mortality rate can be explained by the fact that the majority of patients (70%) present with advanced stage III or IV disease.
In 1981, Bast and colleagues reported the identification of a monoclonal antibody (OC125) developed from mice immunized with an ovarian carcinoma cell line [2]. Subsequently, a radioimmunoassay was developed for the target antigen CA-125, and women with ovarian cancer were shown to have elevated CA-125 serum levels in 82% of cases, compared to 1% in healthy controls [3]. Furthermore, the authors noticed that rising or falling levels of serum CA-125 correlated with progression or regression of disease, suggesting the important role of monitoring treatment response in patients with epithelial ovarian cancer.
The clinical utility of serum CA-125 measurements to monitor treatment response to chemotherapy and predict recurrence is now well established [4,5]. Different prognostic scenarios derived from CA-125 variations have been investigated. Several multivariate analyses have demonstrated that CA-125 half-life and nadir concentrations during induction chemotherapy [6-9] are independent prognostic factors of recurrence and survival even within normal range (≤35 U/ml). Others have shown that a reduction in serum CA-125 concentration over the initial 2 cycles of platinum-based chemotherapy is a powerful independent predictor of survival for patients with suboptimally cytoreduced stage III or IV ovarian cancer [10]. Finally, baseline CA-125 serum levels before initiation of first-line chemotherapy (but after cytoreductive surgery) and maintenance chemotherapy have been shown to predict the risk of relapse and survival [11-13]. Although the negative impact of elevated serum levels of CA-125 is well known, the pathophysiology is not. CA-125 may represent a simple surrogate for tumor volume, resistance to chemotherapy, or a separate “virulence” factor, labeling tumors with more aggressive phenotypes.
While the prognostic significance of elevated CA-125 levels before, during, and after “chemical” cytoreduction is clear, there is limited knowledge regarding the prognostic relevance of perioperative CA-125 dynamics in response to surgical cytoreduction before initiation of postoperative chemotherapy. Due to the complex kinetics of CA-125, mainly influenced by factors such as surgical trauma, and the remaining tumor burden of CA-125 secreting cells, less attention has been paid to perioperative CA-125 changes. Some authors have reported that the perioperative decline in CA-125 levels correlated with the size of residual disease after cytoreductive surgery [14]; others observed that the prediction of residual disease by CA-125 decline lacked accuracy [15,16]. Therefore, the perioperative variations in serum CA-125 were considered of limited clinical relevance in the management of patients with ovarian cancer. To our knowledge, there have been no studies to investigate the prognostic relevance of CA-125 serum changes in response to surgery in patients with advanced ovarian cancer. The evaluation of perioperative serum CA-125 variations in response to surgical cytoreduction would allow us to control for residual disease remaining after surgery and separate tumor volume aspects of CA-125 from its potential additional role as a biologic factor.
Based on this background, we sought to determine whether changes in preoperative to pre-chemotherapy serum CA-125 levels in response to surgery are associated with progression-free survival (PFS) in patients with advanced-stage IIIC ovarian carcinoma.
Methods
We evaluated all patients with FIGO stage IIIC ovarian cancer undergoing primary cytoreductive surgery followed by intravenous or intraperitoneal platinum-based combination chemotherapy at our institution from January 1989 through December 2006. Patients were prospectively entered and continuously followed using a computerized database. Patients who underwent primary cytoreductive surgery at outside institutions, those who were treated with neoadjuvant chemotherapy, and those with non-epithelial histologic subtypes or borderline cancers were not included. Patients included in the analysis had to have elevated CA-125 serum levels (>35 U/ml) before undergoing cytoreductive surgery and must have had CA-125 levels documented both preoperatively and before initiation of postoperative chemotherapy. If a patient had more than one available CA-125 blood sample before or after surgery, we chose the sample taken closest to the surgery date for the preoperative measure and the sample taken closest to the date of first cycle of postoperative chemotherapy for the postoperative measure (all within 1 week prior to the initiation of postoperative chemotherapy).
Optimal surgical outcome was defined as no residual tumor lesion measuring greater than 1 cm in single, largest dimension at the completion of surgery. Progression of disease was determined based on the definitions outlined by the Gynecologic Cancer Intergroup [5,17]—a patient may be declared to have progression on the basis of either the objective Response Evaluation Criteria in Solid Tumors (RECIST) or CA-125 criteria. If both events were documented, the earlier event was chosen to represent the date of progression. When determined by CT scan, progression was noted as the detection of one or more new lesions or increased size of known, existing lesions. When determined by CA-125 value, progression was noted if the CA-125 value was greater than or equal to two times the nadir value (in patients with abnormal CA-125 values) or if it was two times or greater than the upper normal limit (35 U/ml) (in patients whose CA-125 normalized during treatment).
Statistical Methodology
The effect of CA-125 decline on PFS was investigated using a time-to-event analysis. PFS was defined as the time interval from the date of the first postoperative chemotherapy cycle to the progression of disease, death or last available follow-up, whichever occurred first.
Perioperative changes in CA-125 serum levels were computed as percent decline from the preoperative value to a value measured prior to the start of adjuvant chemotherapy. The decline of serum CA-125 levels was calculated both as a continuous variable and as a categorical variable. The patient population was categorized into 4 different groups based on changes from preoperative to postoperative CA-125 serum levels: group 1, ≥80% decline; group 2, 50-79.99% decline; group 3, 0-49.99% decline; or group 4, any increase in value. The 4 categories were chosen based on both initial log-rank tests on this dataset and clinically validated and accepted CA-125 response and/or progression criteria of patients during and after first-line chemotherapy [4,5,18,19]. We acknowledge that per definition, CA-125 response and progression criteria were developed to measure tumor response to chemotherapy and not to surgery. Therefore, the perioperative CA-125 response categories in this analysis are exploratory in nature.
Kaplan-Meier estimates of PFS stratified by the level of perioperative CA-125 response were calculated and compared using the log-rank test, both in the entire sample and separately in the subgroups categorized by the extent of cytoreduction.
A multivariate Cox proportional hazards model was fit to investigate the effect of perioperative CA-125 response on PFS. Serum CA-125 response was investigated both as a continuous variable and divided into the 4 categories as mentioned above. The following potential confounders were included in the model: age at the time of surgery, ascites volume, cytoreductive outcome (optimal versus suboptimal), chemotherapy regimen (taxane versus no taxane), and number of chemotherapy cycles (>6 versus ≤6 cycles). Because the decline was related to the absolute preoperative CA-125 level, the preoperative value was included in the model. Ascites volume and preoperative CA-125 level were included in the model on a logarithmic scale, while the number of chemotherapy cycles was treated as a time-dependent covariate. To account for potential clustering of progression times within patients who were operated by the same attending surgeon, we corrected the variance of our estimates using robust sandwich variance estimators [20]. Because a small subset of patients (n=28, 8%) who underwent optimal cytoreduction were treated with upfront intraperitoneal chemotherapy, a separate analysis was performed in patients who were exclusively treated with postoperative intravenous platinum and taxane-based chemotherapy. This was performed to avoid a potential primary intraperitoneal treatment interaction with the effect of perioperative CA-125 changes on PFS (the results of this analysis mirrored those of the whole data set and are not presented).
Perioperative serum CA-125 response was compared among groups determined by surgery outcome and by preoperative CA-125 levels using the non-parametric Wilcoxon and Kruskal-Wallis tests. All associations were regarded significant if the P value was <0.05. All P values are two-sided. The statistical analyses were performed using SAS version 9.1 (SAS Institute, Cary, North Carolina).
Results
We identified 535 patients with FIGO stage IIIC epithelial ovarian cancer who underwent primary cytoreductive surgery at our institution during the study period. Two hundred and seventeen patients were excluded from the analysis because of missing preoperative and/or postoperative CA-125 values. Eleven patients were excluded because of normal preoperative serum CA-125 levels (≤35 U/ml), leaving 307 evaluable patients for analysis. Patient characteristics are listed in Table 1. The median time from surgery to initiation of postoperative chemotherapy was 25 days (5th – 95th percentile range, 6-47 days). The median time from surgery to postoperative serum CA-125 sample was 21 days (5th – 95th percentile range, 5-46 days). All postoperative blood samples were drawn within 1 week of initiation of first-line chemotherapy.
Table 1.
Patient characteristics
| Variable | Number of Patients (%) n=307 |
|---|---|
| Age, years | |
| Median (range) | 60 (25-88) |
| Histology | |
| Serous | 245 (80%) |
| Others | 62 (20%) |
| Tumor grade | |
| High | 264 (86%) |
| Other | 43 (14%) |
| Preoperative CA-125, U/ml; Median (range) | |
| All patients (n=307) | 840 (40-38100) |
| Optimally debulked (n=209) | 613 (40-29100) |
| Suboptimally debulked (n=98) | 1258 (69-38100) |
| Ascites volume, ml | |
| Median (range) | 900 (0-12000) |
| Residual tumor size | |
| Microscopic optimal | 59 (19%) |
| Macroscopic optimal | 150 (49%) |
| Suboptimal (>1.0 cm) | 98 (32%) |
| Postoperative CA-125, U/ml; Median (range) | |
| All patients (n=307) | 225 (9-29600) |
| Optimally debulked (n=209) | 161(9-8600) |
| Suboptimally debulked (n=98) | 443 (25-29600) |
| Postoperative chemotherapy | |
| IV platinum plus taxane | 251 (82%) |
| IV platinum plus other | 31 (10%) |
| IP platinum plus taxane | 24 (8%) |
| Number of cycles | |
| Median (range) | 6 (1-12) |
| Second-look surgery | |
| Yes | 150 (49%) |
| No | 152 (50%) |
| Missing | 5 (2%) |
| Consolidation chemotherapy | |
| Yes | 143 (47%) |
| No | 161 (52%) |
| Missing | 3 (1%) |
| Perioperative CA-125 response | |
| Steep (≥80%) | 98 (32%) |
| Moderate-High (50-79.99%) | 110 (36%) |
| Moderate-Low (0-49.99%) | 64 (21%) |
| Increase | 35 (11%) |
IV, intravenous; IP, intraperitoneal
The majority of patients (n=272, 89%) experienced a decline in serum CA-125 levels after cytoreductive surgery. Thirty-five patients (11%) experienced an increase. The 209 patients (68%) who underwent optimal cytoreduction had a median perioperative CA-125 decline of 73% (range, 324% increase to 99% decline) compared to a median decline of 55% (range, 112% increase to 99% decline) for the 98 patients who underwent suboptimal cytoreduction (P<0.001). The median pre- and postoperative CA-125 serum values for both optimally and suboptimally cytoreduced patients are listed in Table 1. Among optimally debulked patients there was no difference in CA-125 response categories of patients who underwent a microscopic optimal resection versus macroscopic optimal resection (Table 2).
Table 2.
Association between CA-125 response to surgery and residual disease among optimally cytoreduced patients (microscopic optimal versus macroscopic optimal)
| CA-125 response category | Microscopic n=59 | Macroscopic n=150 | P value |
|---|---|---|---|
| Steep decline (≥80%) | 24 (41%) | 56 (38%) | 0.98 |
| Moderate-low decline (50-79.99%) | 20 (34%) | 53 (35%) | |
| Moderate-high decline (0-49.99%) | 9 (15%) | 24 (16%) | |
| Increase | 6 (10%) | 17 (11%) |
Figure 1 shows the association between preoperative serum CA-125 levels (quartile increments) and postoperative decline. Patients with high preoperative CA-125 serum levels experienced a steeper decline of serum CA-125 after cytoreductive surgery than patients with lower preoperative CA-125 levels (P<0.001).
Figure 1.
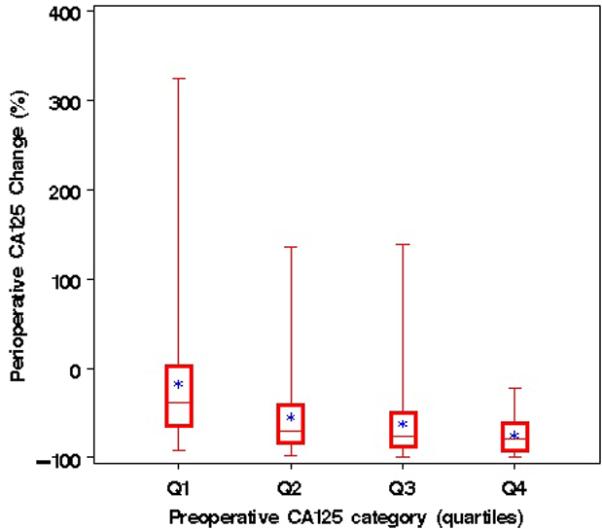
Perioperative CA-125 response (%) by quartiles of preoperative CA-125. Perioperative changes in serum CA-125 levels stratified by quartiles of absolute preoperative CA-125 serum levels (Q1: 35-293 U/ml; Q2: 293-840 U/ml; Q3: 841-2050 U/ml; Q4: >2050 U/ml).
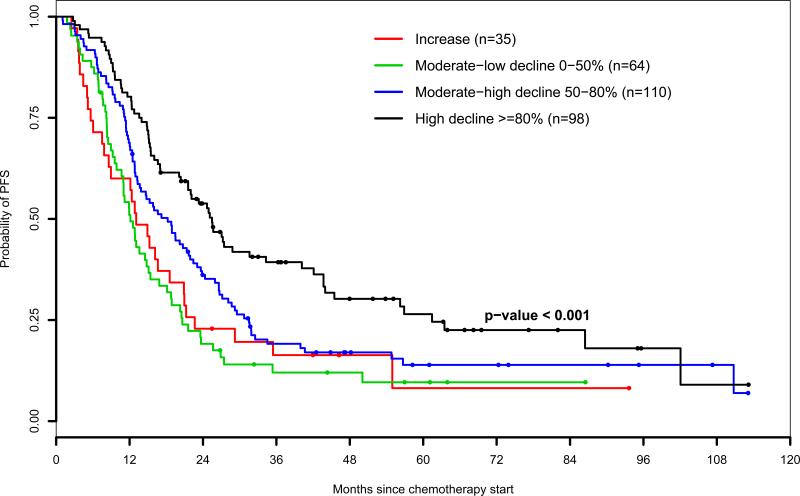
The median follow-up time for patients without progression or death was 48 months (range, 7-181 months). On a univariate analysis with CA-125 response to surgery stratified into 4 categories, Kaplan-Meier estimates revealed decreased PFS with less pronounced perioperative decline in serum CA-125 levels (P<0.001; Figure 2A). The association of CA-125 changes with progression of disease was analyzed in a multivariate analysis using the Cox model. By using the perioperative serum CA-125 decline of 80% or more as the referent, the risk of disease progression increased incrementally as the magnitude of pre- to postoperative serum CA-125 response decreased (Table 3). The highest risk was observed in patients who experienced a postoperative increase in serum CA-125 levels (adjusted hazard ratio [HR]= 2.49; 95% CI: 1.37-4.54; P=0.003). Serum CA-125 changes remained significantly associated with risk of relapse as a continuous variable (P<0.001). The absolute preoperative CA-125 serum level was not associated with PFS (P=0.29)
Figure 2A.
Progression-free survival by level of perioperative CA-125 response (all patients)
Table 3.
Multivariate Cox proportional hazards model (adjusted for surgeon effect) to estimate the association between different perioperative CA-125 response categories and progression-free survival (PFS)
| Parameter | Hazard Ratio (95% CI) | P |
|---|---|---|
| Taxane, yes versus no* | 0.70 (0.48-1.03) | 0.06 |
| Chemotherapy cycles, ≥6 versus <6* | 1.36 (0.87-2.12) | 0.15 |
| Age (5-year increments) | 1.004 (0.95-1.07) | 0.80 |
| Ascites volume** | 1.09 (1.03-1.14) | <0.001 |
| Histology (serous versus non serous) | 1.03 (0.79-1.39) | 0.91 |
| Preoperative CA-125** | 1.08 (0.94-1.23) | 0.29 |
| Residual disease | ||
| Suboptimal versus optimal | 1.47 (1.06-2.04) | 0.02 |
| Perioperative CA-125 response*** | 0.003 | |
| Increase versus ≥80% decline | 2.49 (1.37-4.52) | 0.003 |
| 0-49.99% decline versus ≥80% decline | 2.20 (1.40-3.47) | <0.001 |
| 50-79.99% decline versus ≥80% decline | 1.50 (1.08-2.08) | 0.01 |
95% CI, Hazard Ratio Confidence Interval
time-dependent covariate
Logarithmic scale
Trend test
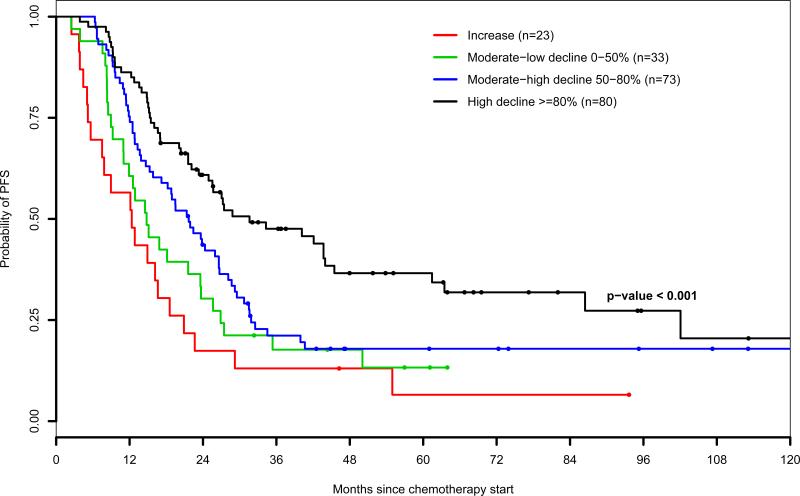
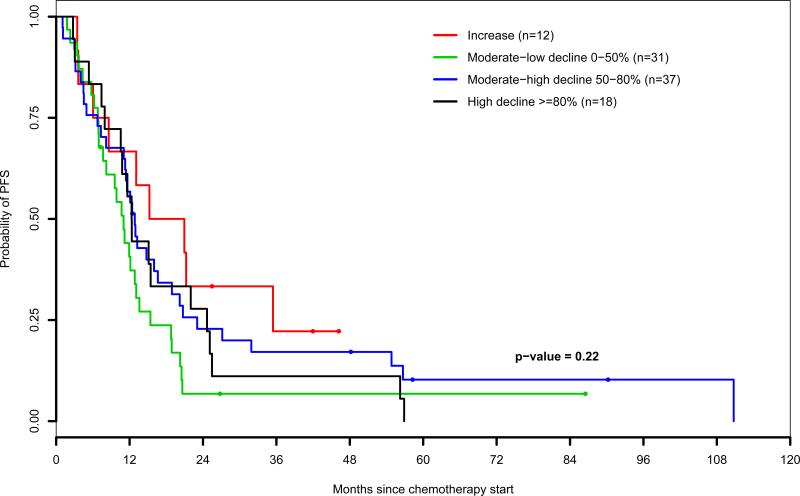
Figures 2B and 2C display Kaplan-Meier estimates of PFS stratified by perioperative CA-125 response categories of patients who underwent optimal and suboptimal cytoreduction, respectively. An interaction model was performed to estimate the effect of perioperative serum CA-125 changes on the risk of progression, at different levels of residual disease volume remaining after surgery (Table 4). Perioperative serum CA-125 dynamics were strongly associated with the risk of relapse in optimally debulked but not in suboptimally debulked patients both as continuous and categorical variables. After optimal cytoreduction, a perioperative increase of serum CA-125 levels was associated with a 4.2-fold increased risk of relapse compared to patients who experienced a decline of 80% or more (adjusted HR=4.2; 95% CI: 2.04-8.66; P=0.0001).
Figure 2B.
Progression-free survival by level of perioperative CA-125 response (patients who underwent optimal cytoreduction)
Figure 2C.
Progression-free survival by level of perioperative CA-125 response (patients who underwent suboptimal cytoreduction)
Table 4.
Multivariate Cox proportional hazards model to estimate the association between different perioperative CA-125 response categories and progression-free survival (PFS)
| Parameter | Hazard Ratio (95% CI) | P |
|---|---|---|
| Optimal Cytoreduction* | ||
| Increase versus ≥80% decline | 4.20 (2.04-8.66) | 0.0001 |
| 0-49.99% decline versus ≥80% decline | 2.61 (1.51-4.52) | 0.0006 |
| 50-79.99% decline versus ≥80% decline | 1.91 (1.31-2.79) | 0.0008 |
| Suboptimal cytoreduction* | ||
| Increase versus ≥80% decline | 0.91 (0.37-2.24) | 0.83 |
| 0-49.99% decline versus ≥80% decline | 1.31 (0.69-2.49) | 0.41 |
| 50-79.99% decline versus ≥80% decline | 0.78 (0.43-1.41) | 0.41 |
95% CI, Hazard Ratio Confidence Interval
Multivariate Cox proportional hazards model adjusted for taxane as part of chemotherapy treatment, number of chemotherapy cycles, ascites volume, preoperative CA-125 level, age, and surgeon effect.
Discussion
In the current study, we analyzed the effects of perioperative serum CA-125 dynamics in response to cytoreductive surgery in a relatively large number of patients with FIGO stage IIIC ovarian cancer and paired preoperative and postoperative CA-125 values. In general, the majority of patients experienced a decline of serum CA-125 levels after cytoreductive surgery. The magnitude of the decline varied depending on the absolute baseline CA-125 level before surgery and the amount of residual disease left after surgery. Patients with minimal residual disease had a higher decline of serum levels than patients who were left with suboptimal residual disease. This is not surprising if we assume that serum CA-125 levels reflect the burden of disease. However, when analyzing patients who were left with minimal residual disease after the surgical procedure, the serum CA-125 response to surgery was also variable, ranging from a steep decline to an increase in serum levels. Furthermore, no difference was seen in perioperative CA-125 dynamics between patients who underwent complete gross tumor resection and those who were left with minimal visible residual disease of 1 cm or less, suggesting that factors other than tumor burden may additionally contribute to individual perioperative serum CA-125 responses.
The results of this analysis have shown that CA-125 decline in response to surgery before initiation of primary chemotherapy is associated with subsequent disease progression after controlling for factors such as baseline CA-125 values, surgical outcome, postoperative chemotherapy regimen administered, age, and ascites. The effect of CA-125 decline on PFS was powerful in patients who underwent optimal cytoreduction, while no prognostic effect was observed in patients who were left with suboptimal residual disease. Preliminary data indicate an even more pronounced prognostic effect of perioperative serum CA-125 changes in patients with microscopic residual disease (increase versus ≥ 80%- decline; HR=5.9; 95% CI, 2.676-13.199; P<0.0001). Our findings suggest that serum CA-125 variations after optimal cytoreduction may, in addition to reflecting changes in tumor volume, be regarded as inherent biomarkers, identifying patients who respond individually (by means of CA-125 changes) to an optimal reduction of tumor burden.
It is important to recognize several limitations of this study. The primary limitations of the current study are the retrospective nature of the analysis, the non-standardized timing of CA-125 collection, as well as a lack of a central laboratory review. Furthermore, perioperative CA-125 variations are subject to complex kinetics of serum CA-125 levels in the immediate postoperative period. The composite action of surgical cytoreduction, surgical trauma as well as the presence and burden of remaining CA-125 producing cancer cells likely contribute to changes of serum CA-125 levels; the exact weight of each confounding factor is unknown. After controlling for known prognostic factors, however, our results indicate that there is a strong relationship between perioperative serum CA-125 changes and the risk of progression in patients who were left with minimal residual disease after the surgical procedure. By investigating the percent decline of perioperative CA-125 levels rather than absolute values, we control for a wide variety of absolute serum CA-125 levels among individual advanced ovarian cancer patients and individual cancers. In order to obtain a meaningful interpretation of the % decline relative to the preoperative (baseline) value of CA-125, our model accounts for both the baseline CA-125 value and the % decline. The results suggest that the % decline remains predictive after adjusting for the individual patient's preoperative CA-125.
Several perioperative prognostic variables in advanced-stage ovarian cancer have been studied over the years [21]. Of all identified early prognostic factors before initiation of chemotherapy, cytoreductive outcome is so powerful that in today's practice, patients are stratified to postoperative chemotherapy treatments or randomized clinical trials based on the amount of residual disease remaining after cytoreductive surgery [22]. The findings of this study are important because they suggest that, for patients with small-volume disease, there may be a novel, powerful early biomarker that can identify patients who are at a low or high risk of recurrence.
Perhaps more importantly, the results implicate that patients who respond strongly to an optimal cytoreduction by means of a steep perioperative serum CA-125 decline may harbor ovarian cancers with less aggressive phenotypes (e.g., tumors with the presence of intratumoral T cells [23], BRCA germ-line mutation carriers [24-26], or other slowly developing ovarian cancers [27]). Similarly, patients who have a less pronounced perioperative CA-125 serum decline or increase in response to optimal cytoreduction may have ovarian cancers with rapidly progressing phenotypes driven by the augmentation of CA-125 producing tumor cells in the time interval between surgery and initiation of primary chemotherapy. Thus, the results of this study provide meaningful support for additional translational research correlating perioperative serum CA-125 responses of patients with molecular tumor characteristics.
If confirmed prospectively, CA-125 dynamics in response to surgery may allow a subset of patients with higher risk of recurrence to be identified prior to starting chemotherapy. This may be useful for patient counseling with regards to participation in protocols investigating novel therapies. Furthermore, perioperative CA-125 dynamics could be incorporated into novel, more accurate prognostic models, such as nomograms.
Footnotes
Presented in part at the 39th Annual Meeting on Women's Cancer, March 9-12, 2008, Tampa, Florida, USA.
- Oliver Zivanovic, MD: no conflicts of interest to declare
- Camelia S. Sima, MS: no conflicts of interest to declare
- Alexia Iasonos, PhD: no conflicts of interest to declare
- Katherine M. Bell-McGuinn, MD: no conflicts of interest to declare
- Paul J Sabbatini, MD: no conflicts of interest to declare
- Mario M. Leitao, MD: consultant and speaker for Genzyme; surgical proctor for Intuitive Surgical
- Douglas A. Levine, MD: no conflicts of interest to declare
- Ginger J. Gardner, MD: no conflicts of interest to declare
- Richard R. Barakat, MD: no conflicts of interest to declare
- Dennis S. Chi, MD: no conflicts of interest to declare
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bast RC, Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–7. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bast RC, Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR, Jr, Knapp RC. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983;309:883–7. doi: 10.1056/NEJM198310133091503. [DOI] [PubMed] [Google Scholar]
- 4.Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, Mitchell H, Lambert HE. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996;14:1545–51. doi: 10.1200/JCO.1996.14.5.1545. [DOI] [PubMed] [Google Scholar]
- 5.Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, Eisenhauer E, Sagae S, Greven K, Vergote I, Cervantes A, Vermorken J. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst. 2004;96:487–8. doi: 10.1093/jnci/djh081. [DOI] [PubMed] [Google Scholar]
- 6.van der Burg ME, Lammes FB, van Putten WL, Stoter G. Ovarian cancer: the prognostic value of the serum half-life of CA125 during induction chemotherapy. Gynecol Oncol. 1988;30:307–12. doi: 10.1016/0090-8258(88)90244-2. [DOI] [PubMed] [Google Scholar]
- 7.Gadducci A, Zola P, Landoni F, Maggino T, Sartori E, Bergamino T, Cristofani R. Serum half-life of CA 125 during early chemotherapy as an independent prognostic variable for patients with advanced epithelial ovarian cancer: results of a multicentric Italian study. Gynecol Oncol. 1995;58:42–7. doi: 10.1006/gyno.1995.1181. [DOI] [PubMed] [Google Scholar]
- 8.Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, Dalifard I, Hacene K, Pichon MF. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006;17:1234–8. doi: 10.1093/annonc/mdl120. [DOI] [PubMed] [Google Scholar]
- 9.Crawford SM, Peace J. Does the nadir CA125 concentration predict a long-term outcome after chemotherapy for carcinoma of the ovary? Ann Oncol. 2005;16:47–50. doi: 10.1093/annonc/mdi012. [DOI] [PubMed] [Google Scholar]
- 10.Markman M, Federico M, Liu PY, Hannigan E, Alberts D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol. 2006;103:195–8. doi: 10.1016/j.ygyno.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Juretzka MM, Barakat RR, Chi DS, Iasonos A, Dupont J, Abu-Rustum NR, Poynor EA, Aghajanian C, Spriggs D, Hensley ML, Sabbatini P. CA125 level as a predictor of progression-free survival and overall survival in ovarian cancer patients with surgically defined disease status prior to the initiation of intraperitoneal consolidation therapy. Gynecol Oncol. 2007;104:176–80. doi: 10.1016/j.ygyno.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 12.Markman M, Liu PY, Rothenberg ML, Monk BJ, Brady M, Alberts DS. Pretreatment CA-125 and risk of relapse in advanced ovarian cancer. J Clin Oncol. 2006;24:1454–8. doi: 10.1200/JCO.2005.04.7373. [DOI] [PubMed] [Google Scholar]
- 13.Zorn KK, Tian C, McGuire WP, Hoskins WJ, Markman M, Muggia FM, Rose PG, Ozols RF, Spriggs D, Armstrong DK. The prognostic value of pretreatment CA 125 in patients with advanced ovarian carcinoma : a Gynecologic Oncology Group study. Cancer. 2009;115:1028–35. doi: 10.1002/cncr.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sevelda P, Rosen A, Barrada M, Gober S, Vavra N, Salzer H. [Does follow-up of false-negative CA-125 serum values in tumor after-care of ovarian cancer make sense?]. Gynakol Rundsch. 1990;30(Suppl 1):210–1. [PubMed] [Google Scholar]
- 15.Yedema CA, Kenemans P, Thomas CM, Massuger LF, Wobbes T, Verstraeten R, van Kamp GJ, Hilgers J. CA 125 serum levels in the early post-operative period do not reflect tumour reduction obtained by cytoreductive surgery. Eur J Cancer. 1993;29A:966–71. doi: 10.1016/s0959-8049(05)80203-5. [DOI] [PubMed] [Google Scholar]
- 16.Gadducci A, Landoni F, Maggino T, Sartori E, Zola P, Fanucchi A. The relationship between postoperative decline of serum CA 125 levels and size of residual disease after initial surgery in patients with advanced ovarian cancer: a CTF study. Gynecol Oncol. 1996;63:234–7. doi: 10.1006/gyno.1996.0312. [DOI] [PubMed] [Google Scholar]
- 17.Vergote I, Rustin GJ, Eisenhauer EA, Kristensen GB, Pujade-Lauraine E, Parmar MK, Friedlander M, Jakobsen A, Vermorken JB. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000;92:1534–5. doi: 10.1093/jnci/92.18.1534. [DOI] [PubMed] [Google Scholar]
- 18.Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol. 1996;7:361–4. doi: 10.1093/oxfordjournals.annonc.a010602. [DOI] [PubMed] [Google Scholar]
- 19.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T. Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol. 2001;19:4054–7. doi: 10.1200/JCO.2001.19.20.4054. [DOI] [PubMed] [Google Scholar]
- 20.Lee E, Wei L, Amato D. Cox-Type Regression Analysis for Large Numbers of Small Groups of Correlated Failure Time Observations. In: Klein JP, Goel PK, editors. In Survival Analysis: State of the Art. Kluwer Academic Publishers; Dordrecht: 1992. pp. 237–47. [Google Scholar]
- 21.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, Armstrong DK, Muggia F, McGuire WP. Gynecologic Oncology Group Study. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–7. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 22.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA. Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 24.Cass I, Baldwin RL, Varkey T, Moslehi R, Narod SA, Karlan BY. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer. 2003;97:2187–95. doi: 10.1002/cncr.11310. [DOI] [PubMed] [Google Scholar]
- 25.Rubin SC, Benjamin I, Behbakht K, Takahashi H, Morgan MA, LiVolsi VA, Berchuck A, Muto MG, Garber JE, Weber BL, Lynch HT, Boyd J. Clinical and pathological features of ovarian cancer in women with germ-line mutations of BRCA1. N Engl J Med. 1996;335:1413–6. doi: 10.1056/NEJM199611073351901. [DOI] [PubMed] [Google Scholar]
- 26.Boyd J, Sonoda Y, Federici MG, Bogomolniy F, Rhei E, Maresco DL, Saigo PE, Almadrones LA, Barakat RR, Brown CL, Chi DS, Curtin JP, Poynor EA, Hoskins WJ. Clinicopathologic features of BRCA-linked and sporadic ovarian cancer. JAMA. 2000;283:2260–5. doi: 10.1001/jama.283.17.2260. [DOI] [PubMed] [Google Scholar]
- 27.Levanon K, Crum C. DrapkinR. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]