Abstract
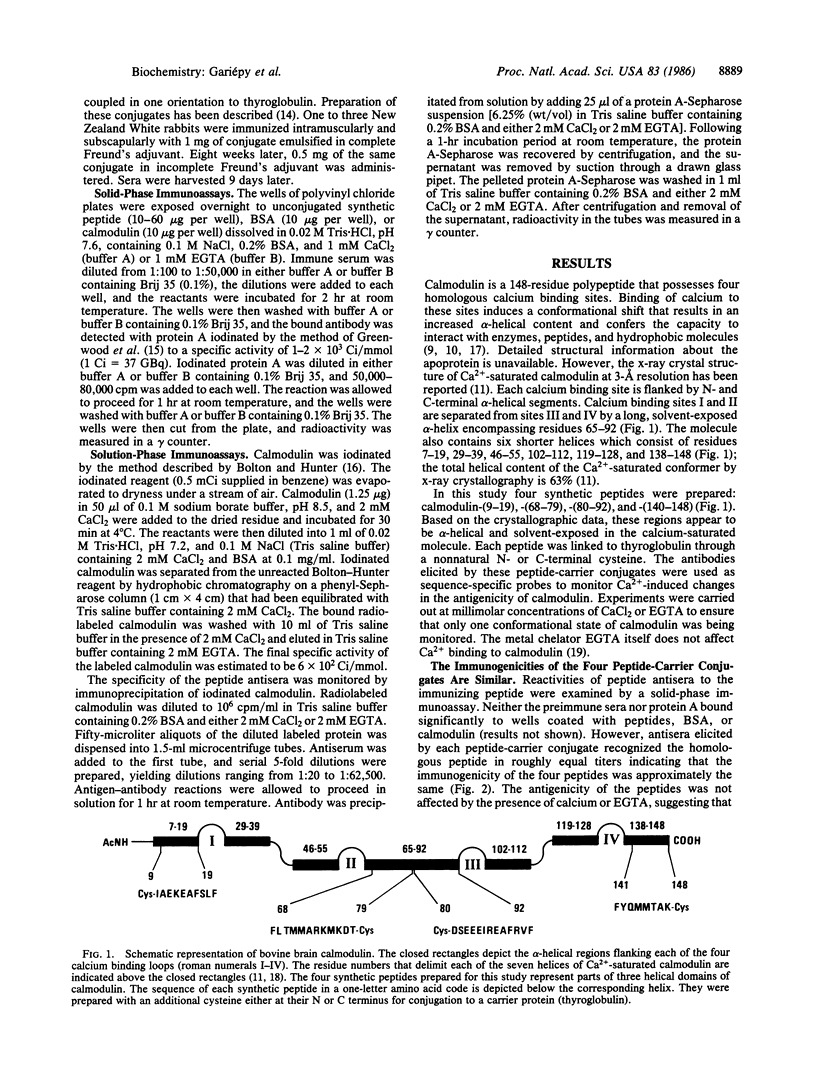
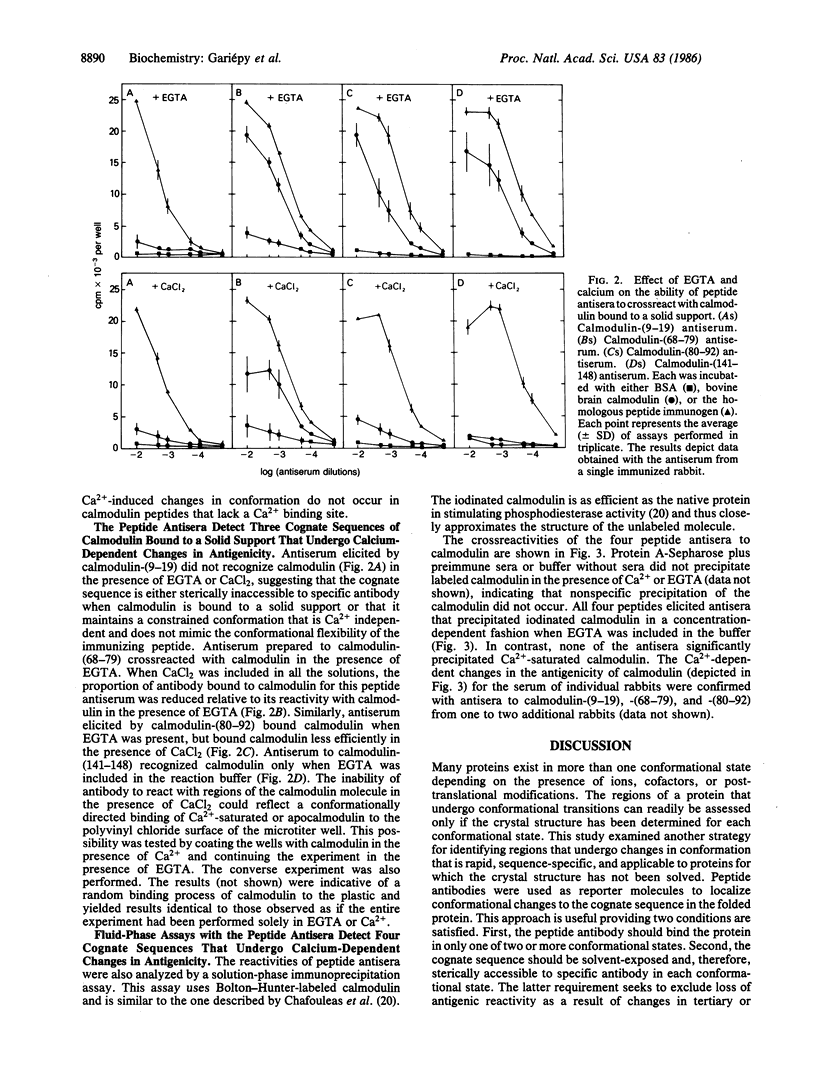
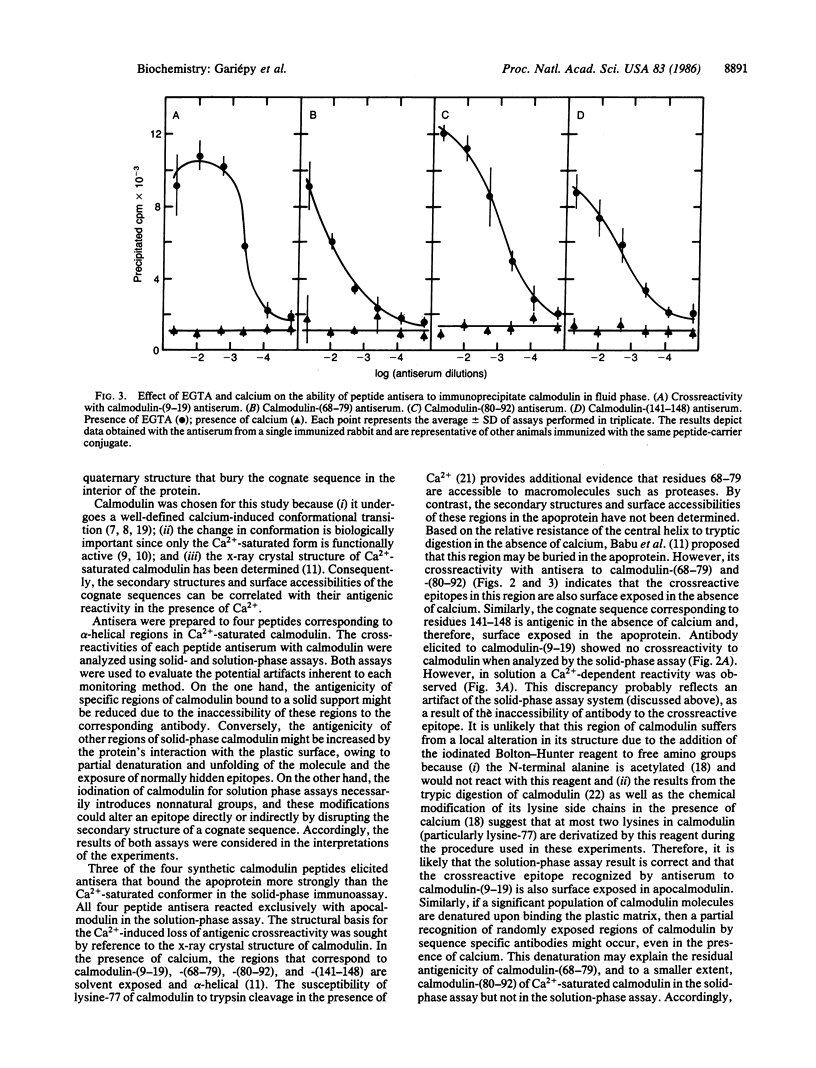
Local changes in conformation between the calcium-saturated and calcium-free forms of calmodulin were monitored using antisera to four peptides corresponding to three helical regions of the calcium-saturated protein. The N-terminal helix was monitored using antiserum to residues 9-19, calmodulin-(9-19); the C-terminal helix using antiserum to residues 141-148, calmodulin-(141-148); and the long central helix with antisera to residues 68-79 and 80-92, calmodulin-(68-79) and -(80-92). Crossreactivities of peptide antisera with calmodulin (either in the presence or absence of calcium) were determined using solution-phase and solid-phase immunoassays. When examined by the fluid-phase assay, all four peptides elicited antibody that precipitated radiolabeled apocalmodulin but not the calcium-saturated form of the protein. Similarly, when calmodulin was immobilized on a solid-support, only the calcium-free form readily bound the antibodies to calmodulin-(80-92) and -(141-148). In addition, the crossreactivity of antiserum to calmodulin-(68-79) with calcium-saturated calmodulin in solid phase was reduced by approximately equal to 40% relative to reactivity with apocalmodulin. According to the x-ray crystal structure of Ca2+-saturated calmodulin and the antigenic reactivity of calmodulin for the peptide antisera in the absence of calcium, the regions of the protein monitored by these antisera are exposed to the surface in both conformational states and probably accessible to specific antibodies. The apparent preference of peptide antibodies for one conformation of the molecule suggests that changes in the conformation of calmodulin occur in cognate sequences that are transformed by calcium from antigenic, flexible structures to less antigenic, relatively helical structures. Peptide antibodies may be employed as sequence-specific reporter molecules to monitor local conformational changes providing the cognate sequence is sterically accessible to antibody in both states but antigenic in only one.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chafouleas J. G., Dedman J. R., Munjaal R. P., Means A. R. Calmodulin. Development and application of a sensitive radioimmunoassay. J Biol Chem. 1979 Oct 25;254(20):10262–10267. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy J., O'Hanley P., Waldman S. A., Murad F., Schoolnik G. K. A common antigenic determinant found in two functionally unrelated toxins. J Exp Med. 1984 Oct 1;160(4):1253–1258. doi: 10.1084/jem.160.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee C. B. Conformational transition accompanying the binding of Ca2+ to the protein activator of 3',5'-cyclic adenosine monophosphate phosphodiesterase. Biochemistry. 1977 Mar 8;16(5):1017–1024. doi: 10.1021/bi00624a033. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Vanaman T. C. Calmodulin. Adv Protein Chem. 1982;35:213–321. doi: 10.1016/s0065-3233(08)60470-2. [DOI] [PubMed] [Google Scholar]
- Liu Y. P., Cheung W. Y. Cyclic 3':5'-nucleotide phosphodiesterase. Ca2+ confers more helical conformation to the protein activator. J Biol Chem. 1976 Jul 25;251(14):4193–4198. [PubMed] [Google Scholar]
- Means A. R., Tash J. S., Chafouleas J. G. Physiological implications of the presence, distribution, and regulation of calmodulin in eukaryotic cells. Physiol Rev. 1982 Jan;62(1):1–39. doi: 10.1152/physrev.1982.62.1.1. [DOI] [PubMed] [Google Scholar]
- Novotný J., Handschumacher M., Haber E., Bruccoleri R. E., Carlson W. B., Fanning D. W., Smith J. A., Rose G. D. Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc Natl Acad Sci U S A. 1986 Jan;83(2):226–230. doi: 10.1073/pnas.83.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter J. D., Strang-Brown P., Walker P. L., Iida S. Ca2+ binding to calmodulin. Methods Enzymol. 1983;102:135–143. doi: 10.1016/s0076-6879(83)02014-5. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Fernandez R., Schoolnik G. K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984 Jul 1;160(1):208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs D. H., Schechter A. N., Eastlake A., Anfinsen C. B. An immunologic approach to the conformational equilibria of polypeptides. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3790–3794. doi: 10.1073/pnas.69.12.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. G., Shinnick T. M., Green N., Lerner R. A. Antibodies that react with predetermined sites on proteins. Science. 1983 Feb 11;219(4585):660–666. doi: 10.1126/science.6186024. [DOI] [PubMed] [Google Scholar]
- Tainer J. A., Getzoff E. D., Alexander H., Houghten R. A., Olson A. J., Lerner R. A., Hendrickson W. A. The reactivity of anti-peptide antibodies is a function of the atomic mobility of sites in a protein. Nature. 1984 Nov 8;312(5990):127–134. doi: 10.1038/312127a0. [DOI] [PubMed] [Google Scholar]
- Teale J. M., Benjamin D. C. Antibody as an immunological probe for studying the refolding of bovine serum albumin. I. The catalysis of reoxidation of reduced bovine serum albumin by glutathione and a disulfide interchange enzyme. J Biol Chem. 1976 Aug 10;251(15):4603–4608. [PubMed] [Google Scholar]
- Teale J. M., Benjamin D. C. Antibody as an immunological probe for studying the refolding of bovine serum albumin. II. Evidence for the independent refolding of the domains of the molecule. J Biol Chem. 1976 Aug 10;251(15):4609–4615. [PubMed] [Google Scholar]
- Teale J. M., Benjamin D. C. Antibody as immunological probe for studying refolding of bovine serum albumin. Refolding within each domain. J Biol Chem. 1977 Jul 10;252(13):4521–4526. [PubMed] [Google Scholar]
- Thornton J. M., Edwards M. S., Taylor W. R., Barlow D. J. Location of 'continuous' antigenic determinants in the protruding regions of proteins. EMBO J. 1986 Feb;5(2):409–413. doi: 10.1002/j.1460-2075.1986.tb04226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Characterization of tryptic fragments obtained from bovine brain protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Nov 10;252(21):7440–7443. [PubMed] [Google Scholar]
- Walsh M., Stevens F. C. Chemical modification studies on the Ca2+-dependent protein modulator of cyclic nucleotide phosphodiesterase. Biochemistry. 1977 Jun 14;16(12):2742–2749. doi: 10.1021/bi00631a024. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Sharief F., Vanaman T. C. The complete amino acid sequence of the Ca2+-dependent modulator protein (calmodulin) of bovine brain. J Biol Chem. 1980 Feb 10;255(3):962–975. [PubMed] [Google Scholar]
- Westhof E., Altschuh D., Moras D., Bloomer A. C., Mondragon A., Klug A., Van Regenmortel M. H. Correlation between segmental mobility and the location of antigenic determinants in proteins. Nature. 1984 Sep 13;311(5982):123–126. doi: 10.1038/311123a0. [DOI] [PubMed] [Google Scholar]


