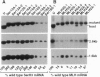
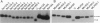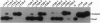Abstract
Although rRNA has a conserved core structure, its size varies by more than 2000 bases between eubacteria and vertebrates, mostly due to the size variation of discrete variable regions. Previous studies have shown that insertion of foreign sequences into some of these variable regions has little effect on rRNA function. These properties make rRNA a potentially very advantageous vehicle to carry other RNA moieties with biological activity, such as "antisense RNAs." We have explored this possibility by inserting antisense RNAs targeted against one essential and two nonessential genes into a site within a variable region in the Tetrahymena thermophila large subunit rRNA gene. Expression of each of the three genes tested can be drastically reduced or eliminated in transformed T. thermophila lines containing these altered rRNAs. In addition, we found that only antisense rRNAs containing RNA sequences complementary to the 5' untranslated region of the targeted mRNA were effective. Lines containing antisense rRNAs targeted against either of the nonessential genes grow well, indicating that the altered rRNAs fulfill their functions within the ribosome. Since functional rRNA is extremely abundant and stable and comes into direct contact with translated mRNAs, it may prove to be an unparalleled vehicle for enhancing the activity of functional RNAs that act on mRNAs.
Full text
PDF

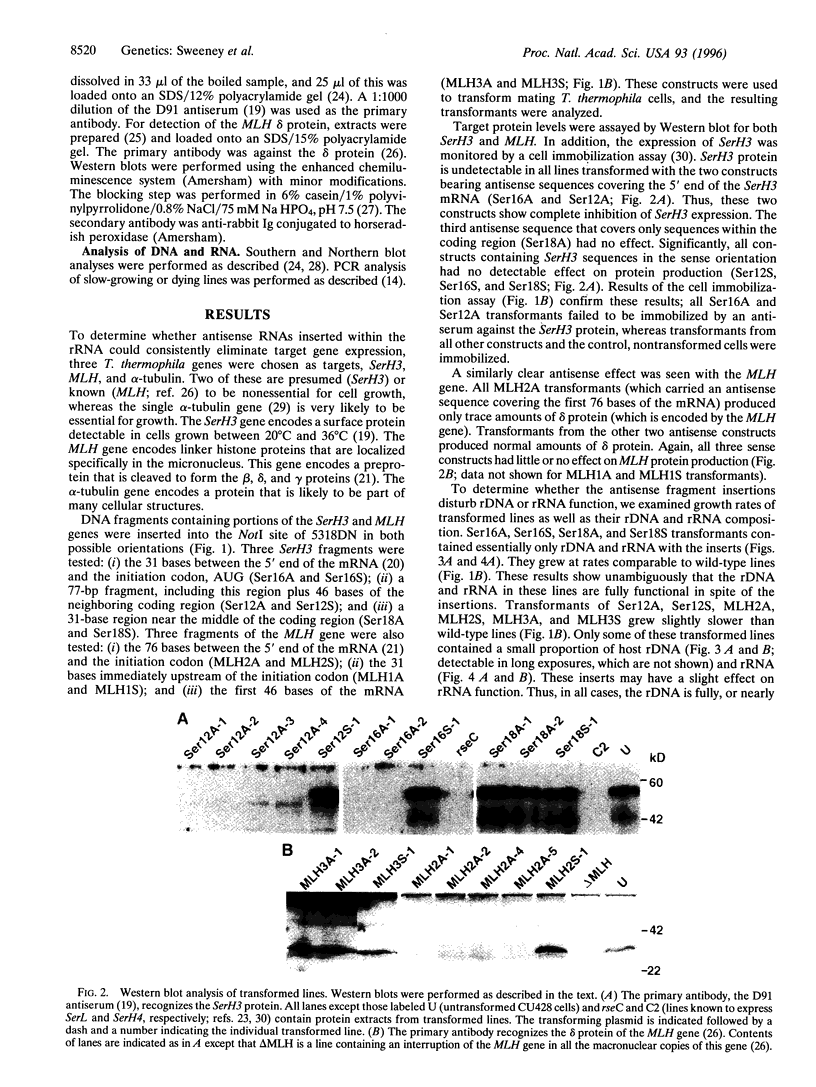



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns P. J., Katzen A. L., Martin L., Blackburn E. H. A drug-resistant mutation in the ribosomal DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1985 May;82(9):2844–2846. doi: 10.1073/pnas.82.9.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan R. C., Shalke G., Gorovsky M. A. Developmental rearrangements associated with a single type of expressed alpha-tubulin gene in Tetrahymena. Cell. 1984 Feb;36(2):441–445. doi: 10.1016/0092-8674(84)90237-x. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S. Purification and partial characterization of the H immobilization antigens of Tetrahymena thermophila. J Protozool. 1986 May;33(2):204–208. doi: 10.1111/j.1550-7408.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Engberg J., Nielsen H. Complete sequence of the extrachromosomal rDNA molecule from the ciliate Tetrahymena thermophila strain B1868VII. Nucleic Acids Res. 1990 Dec 11;18(23):6915–6919. doi: 10.1093/nar/18.23.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaertig J., Gorovsky M. A. Efficient mass transformation of Tetrahymena thermophila by electroporation of conjugants. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9196–9200. doi: 10.1073/pnas.89.19.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie P. G., Hudspeth A. J. Chemiluminescence detection of proteins from single cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2563–2567. doi: 10.1073/pnas.88.6.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorovsky M. A. Studies on nuclear structure and function in Tetrahymena pyriformis. II. Isolation of macro- and micronuclei. J Cell Biol. 1970 Dec;47(3):619–630. doi: 10.1083/jcb.47.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green P. J., Pines O., Inouye M. The role of antisense RNA in gene regulation. Annu Rev Biochem. 1986;55:569–597. doi: 10.1146/annurev.bi.55.070186.003033. [DOI] [PubMed] [Google Scholar]
- Gutell R. R., Fox G. E. A compilation of large subunit RNA sequences presented in a structural format. Nucleic Acids Res. 1988;16 (Suppl):r175–r269. doi: 10.1093/nar/16.suppl.r175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman S. D., Glover C. V., Allis C. D., Gorovsky M. A. Heat shock, deciliation and release from anoxia induce the synthesis of the same set of polypeptides in starved T. pyriformis. Cell. 1980 Nov;22(1 Pt 1):299–307. doi: 10.1016/0092-8674(80)90177-4. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Bruns P. J. Ribosome biosynthesis in Tetrahymena pyriformis. Regulation in response to nutritional changes. J Cell Biol. 1976 Nov;71(2):383–394. doi: 10.1083/jcb.71.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han H., Schepartz A., Pellegrini M., Dervan P. B. Mapping RNA regions in eukaryotic ribosomes that are accessible to methidiumpropyl-EDTA.Fe(II) and EDTA.Fe(II). Biochemistry. 1994 Aug 23;33(33):9831–9844. doi: 10.1021/bi00199a004. [DOI] [PubMed] [Google Scholar]
- Hassouna N., Michot B., Bachellerie J. P. The complete nucleotide sequence of mouse 28S rRNA gene. Implications for the process of size increase of the large subunit rRNA in higher eukaryotes. Nucleic Acids Res. 1984 Apr 25;12(8):3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Hoff A. M., Sardelli A. D., Ford M. Chimeric antisense RNAs. Antisense Res Dev. 1991 Winter;1(4):371–371. doi: 10.1089/ard.1991.1.371. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Wold B. J. Stable reduction of thymidine kinase activity in cells expressing high levels of anti-sense RNA. Cell. 1985 Aug;42(1):129–138. doi: 10.1016/s0092-8674(85)80108-2. [DOI] [PubMed] [Google Scholar]
- McGrath K. E., Yu S. M., Heruth D. P., Kelly A. A., Gorovsky M. A. Regulation and evolution of the single alpha-tubulin gene of the ciliate Tetrahymena thermophila. Cell Motil Cytoskeleton. 1994;27(3):272–283. doi: 10.1002/cm.970270308. [DOI] [PubMed] [Google Scholar]
- Shen X., Yu L., Weir J. W., Gorovsky M. A. Linker histones are not essential and affect chromatin condensation in vivo. Cell. 1995 Jul 14;82(1):47–56. doi: 10.1016/0092-8674(95)90051-9. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Berkowitz M. S., Potoczak D., Krause M., Raab C., Quinn F., Doerder F. P. Characterization of the T, L, I, S, M and P cell surface (immobilization) antigens of Tetrahymena thermophila: molecular weights, isoforms, and cross-reactivity of antisera. J Protozool. 1992 May-Jun;39(3):420–428. doi: 10.1111/j.1550-7408.1992.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Smith D. L., Doerder F. P. Multiple effects of mutation on expression of alternative cell surface protein genes in Tetrahymena thermophila. Genetics. 1992 Jan;130(1):97–104. doi: 10.1093/genetics/130.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spangler E. A., Blackburn E. H. The nucleotide sequence of the 17S ribosomal RNA gene of Tetrahymena thermophila and the identification of point mutations resulting in resistance to the antibiotics paromomycin and hygromycin. J Biol Chem. 1985 May 25;260(10):6334–6340. [PubMed] [Google Scholar]
- Stearns T., Botstein D. Unlinked noncomplementation: isolation of new conditional-lethal mutations in each of the tubulin genes of Saccharomyces cerevisiae. Genetics. 1988 Jun;119(2):249–260. doi: 10.1093/genetics/119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Lee T. C., Smith C. A., Ungers G. E., Gilboa E. Expression of chimeric tRNA-driven antisense transcripts renders NIH 3T3 cells highly resistant to Moloney murine leukemia virus replication. Mol Cell Biol. 1990 Dec;10(12):6512–6523. doi: 10.1128/mcb.10.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney R., Chen L., Yao M. C. An rRNA variable region has an evolutionarily conserved essential role despite sequence divergence. Mol Cell Biol. 1994 Jun;14(6):4203–4215. doi: 10.1128/mcb.14.6.4203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney R., Chen L., Yao M. C. Phenotypic effects of targeted mutations in the small subunit rRNA gene of Tetrahymena thermophila. Mol Cell Biol. 1993 Aug;13(8):4814–4825. doi: 10.1128/mcb.13.8.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney R., Yao M. C. Identifying functional regions of rRNA by insertion mutagenesis and complete gene replacement in Tetrahymena thermophila. EMBO J. 1989 Mar;8(3):933–938. doi: 10.1002/j.1460-2075.1989.tb03454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Small catalytic RNAs. Annu Rev Biochem. 1992;61:641–671. doi: 10.1146/annurev.bi.61.070192.003233. [DOI] [PubMed] [Google Scholar]
- Tondravi M. M., Willis R. L., Love H. D., Jr, Bannon G. A. Molecular characterization of SerH3, a Tetrahymena thermophila gene encoding a temperature-regulated surface antigen. Mol Cell Biol. 1990 Nov;10(11):6091–6096. doi: 10.1128/mcb.10.11.6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. W. Gene inhibition using antisense oligodeoxynucleotides. Nature. 1994 Nov 24;372(6504):333–335. doi: 10.1038/372333a0. [DOI] [PubMed] [Google Scholar]
- Wu M., Allis C. D., Sweet M. T., Cook R. G., Thatcher T. H., Gorovsky M. A. Four distinct and unusual linker proteins in a mitotically dividing nucleus are derived from a 71-kilodalton polyprotein, lack p34cdc2 sites, and contain protein kinase A sites. Mol Cell Biol. 1994 Jan;14(1):10–20. doi: 10.1128/mcb.14.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Kimmel A. R., Gorovsky M. A. A small number of cistrons for ribosomal RNA in the germinal nucleus of a eukaryote, Tetrahymena pyriformis. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3082–3086. doi: 10.1073/pnas.71.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Accurate processing and amplification of cloned germ line copies of ribosomal DNA injected into developing nuclei of Tetrahymena thermophila. Mol Cell Biol. 1989 Mar;9(3):1092–1099. doi: 10.1128/mcb.9.3.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mol J. N., Stuitje A. R. Modulation of eukaryotic gene expression by complementary RNA or DNA sequences. Biotechniques. 1988 Nov-Dec;6(10):958–976. [PubMed] [Google Scholar]