SUMMARY
Nowadays, drug-induced sleep endoscopy (DISE) is performed widely and its validity and reliability has been demonstrated by several studies; in fact, it provides clinical information not available by routine clinical inspection alone. Its safety and utility are promising, but still needs to be improved to reach the level of excellence expected of gold standard tests used in clinical practice. Our study compares the results of clinical and diagnostic evaluation with those of sleep endoscopy, evaluating the correlation between clinical indexes of routine clinical diagnosis and sites of obstruction in terms of number of sites involved, entity of obstruction and pattern of closure. This study consists in a longitudinal prospective evaluation of 138 patients who successfully underwent sleep endoscopy at our institution. Patients were induced to sleep with a low dose of midazolam followed by titration with propofol. Sedation level was monitored using bispectral index monitoring. Our results suggest that the multilevel complete collapse was statistically significantly associated with higher apnoea hypopnea index values. By including partial sites of obstruction greater than 50%, our results also suggest that multilevel collapse remains statistically and significantly associated with higher apnoea hypopnoea index values. Analyzing BMI distribution based on number of sites with complete and partial obstruction there was no significant difference. Finally, analyzing Epworth Sleepiness Score distribution based on number of sites with complete obstruction, there was a statistically significant difference between patients with 3-4 sites of obstruction compared to those with two sites or uni-level obstruction. In conclusion, our data suggest that DISE is safe, easy to perform, valid and reliable, as previously reported. Furthermore, we found a good correlation between DISE findings and clinical characteristics such as AHI and EPS. Consequently, adequate assessment by DISE of all sites of obstruction is very important, not only in patients with low-moderate AHI and EPS, but also in patients with a high AHI or/and high EPS, in particular to plan multilevel surgery that in these latter situations is more demanding since success may be harder to achieve.
KEY WORDS: Obstructive sleep apnoea syndrome, Drug-induced sleep endoscopy, AHI, BMI, EPS
RIASSUNTO
Attualmente, la sleep endoscopy indotta per via farmacologica (DISE) è ampiamente eseguita e la validità ed affidabilità di tale metodica sono state dimostrate in numerosi studi. La sua sicurezza e utilità sono promettenti, tuttavia, tale esame necessita di essere migliorato per raggiungere il livello di eccellenza tipico di un test gold standard utilizzato nella pratica clinica. Obiettivo del nostro studio è stato paragonare i risultati della valutazione clinica e diagnostica tradizionale con quelli della sleep endoscopy, mettendo a confronto i parametri clinici con i siti di ostruzione, ed in particolare prendendo in considerazione il numero di siti implicati, il livello di ostruzione e il pattern di chiusura. In questo studio abbiamo effettuato una valutazione prospettica longitudinale di 138 pazienti che sono stati sottoposti a sleep endoscopy con induzione farmacologica del sonno presso la nostra clinica. Ai pazienti è stato indotto il sonno mediante un basso dosaggio di propofol seguito da una somministrazione di boli progressivi. La profondità della sedazione è stata monitorata mediante il monitoraggio BIS. Sulla base dei risultati ottenuti è stato possibile stabilire che l'ostruzione completa multilivello si associa in modo statisticamente significativo ad un indice più elevato di apnea/ipopnea (AHI). Includendo nell'analisi anche i siti di ostruzione parziale con chiusura > del 50% abbiamo potuto dimostrare che l'associazione fra il collasso multilivello ed un valore di indice apnea/ipopnea più elevato rimane statisticamente significativa. Per quanto riguarda il BMI invece non sono state riscontrate correlazioni significative con il numero di siti di ostruzione completi o parziali. Infine, analizzando la scala della sonnolenza di Epworth e correlandola con il numero di siti con ostruzione completa abbiamo trovato una differenza statisticamente significativa tra i paziente con 3-4 siti di ostruzione rispetto ai pazienti con 2 o 1 livello di ostruzione. In conclusione, i nostri dati suggeriscono che la sleep endoscopy indotta per via farmacologica (DISE) costituisce una procedura sicura, di facile esecuzione, valida ed affidabile, come già descritto in altri studi. Inoltre abbiamo dimostrato una buona correlazione e coerenza tra i risultati della sleep endoscopy e gli indici di AHI e di Epworth. Concludendo riteniamo che un'adeguata valutazione dei siti di ostruzione mediante sleep endoscopy sia molto importante non solo nei pazienti con AHI ed indice di Epworth lieve/moderato, ma anche in quelli con AHI e/o indice di Epworth elevati, soprattutto nella pianificazione di una chirurgia multilivello che in questi casi è più impegnativa con un indice di successo più difficile da raggiungere.
Introduction
Nowadays, drug-induced sleep endoscopy (DISE) is performed widely and its validity has been demonstrated by several studies; in fact, it provides clinical information not available by routine clinical inspection alone 1. Even though sleep endoscopy during natural sleep is an ideal test, it is an impractical diagnostic tool because the study needs to be carries out during the night and the endoscope may disturb the sleeping patient. For these reasons, in 1991 Croft and Pringle 2 introduced drug-induced sleep nasendoscopy, which provides direct visualization of structural collapses of the upper airway under anaesthesia. The study during the awake state may offer erroneous information regarding upper airway obstruction. In fact, Campanini A et al. 1 recently demonstrated that awake and sedation ENT evaluation was identical in only the 25% of cases. In particular, discrepancies involved the oropharyngeal and laryngo-hypopharyngeal sites. Laryngeal obstruction was misunderstood in almost 33% of cases. These results are very important because diagnostically inaccurate information can lead to relevant negative implications in terms of treatment choices, particularly for surgical cases.
Identification of the site of obstruction and pattern of upper airway changes during sleep is a key point essential in guiding therapeutic approaches of obstructive sleep apnoea syndrome (OSAS). Several studies have demonstrated that DISE may help to specify therapy individually, leading to an increased surgical success rate 3 4. For these reasons, DISE has been an essential breakthrough in evaluation of OSA patients. Nevertheless, it has been widely criticized due to the use of sedation that may cause false-positive results, as it assesses sleep in only a short interval that is not representative of a night's snoring, and that the patient's snoring quality and quantity of apnoea occurrences varies with sleeping position and sleep stages 5.
The reliability of DISE is good, and studies on its safety and utility are promising. Nevertheless, it needs to be improved to reach the level of excellence expected of gold standard tests used in clinical practice. In particular, additional research is required to determine the correlations between data obtained during DISE and results of standard clinical evaluation. Finally, some limitations remain unresolved: the lack of standardized protocols and validated grading scales; potential differences with natural sleep; effects of intravenous anaesthetic agents; test-retest reliability among different examiners 6 7.
Our study compares results of clinical and diagnostic evaluation with those of sleep endoscopy, evaluating the correlation between clinical indexes and sites of obstruction in terms of number of sites involved, entity of obstruction and pattern of closure. Furthermore, we analyze the validity of the sleep endoscopy to assess the best treatment for patients with OSAS, particularly those who are undergoing surgical treatment or receiving specific treatment such as oral devices.
Materials and methods
Subjects
From January 2011 to February 2013, we included 138 patients aged between 18 and 72 years (mean age 47.36 years) who successfully underwent sleep endoscopy at our institution (Department of Head and Neck Surgery, Institute of Otorhinolaryngology "A. Gemelli Hospital", Catholic University School of Medicine and Surgery, Rome). There were 108 males and 30 females. BMI ranged from 22.6 to 36.4 kg/m2 (mean BMI 27.2 ± 3.04); mean neck circumference was 40.5 ± 2.6 cm with values ranging from 31 to 47 cm; mean waist circumference was 101±8.4 cm with range between 12 and 87 cm. Mean Epworth index was 9.8 ± 2.75. Mean apnoea-hypopnoea index (AHI) was 35 ± 9 events/hour. Mean ODI 9.8 ± 4.75 events/hour.
Study design
Single institution, longitudinal prospective evaluation of a consecutive group of patients that underwent DISE for management of OSAS. All patients were willing to participate and consented to their inclusion.
Baseline assessment
Each subject was evaluated with a baseline Epworth Sleepiness Scale (ESS) and Berlin Questionnaire (BQ). All patients received a full otolaryngologic examination to evaluate awake status of upper airway and in particular, size of uvula, soft palate and tonsils, tongue base, vallecula, size and shape of epiglottis and Mallampati Score. Following this preliminary evaluation, a full-night comprehensive PSG was scheduled.
Polysomnography
Each patient underwent a full-night ambulatory cardiorespiratory monitoring (A-PSG), performed to assess the sleep-related respiratory pattern. A-PSG was monitored continuously during the sleep-endoscopy procedure using a portable device. A-PSG recordings included airflow (nasal cannula trasducer), respiratory effort (chest and abdominal belts), oxygen saturation, snoring sound, heart rate and body position. An experienced sleep technician performed the application sensors for instructing the patients in their correct application. The frequency of obstructive events is reported as AHI. OSA severity was defined as mild for AHI ≥ 5 and < 15, moderate for AHI ≥ 15 and ≤ 30 and severe for AHI > 30.
PSG monitoring was also performed in continuous mode during the sleep endoscopy procedure. The PSG setup included 3 EEG (F3, C3, O1 referred to the contra-lateral mastoid), 2 EOG, surface EMG of submental and intercostal muscles, airflow (nasal cannula trasducer), respiratory effort (chest and abdominal belts), oxygen saturation, snoring sound, heart rate and body position. A PSG technician attended the recording. Central, mixed and obstructive events were classified according to standardized criteria. Wake, drowsiness and sleep stages were scored on the basis of EEG, EOG and EMG traces, according to standardized criteria. This monitoring allowed performing sleep endoscopy during night, NREM sleep, and to visually identify obstructive apnoeas during the procedure 8-10.
Drug-induced sleep endoscopy
In a controlled, monitored setting (continuous PSG monitoring, as specified in the previous paragraph) with the help of an anaesthesiologist, all patients underwent DISE performed by a specialist ENT. Patients were induced to sleep with a low dose of propofol (0.01 mg/kg) followed by a titration of propofol (3 mg/kg/hr).
Sedation level was monitored using bispectral index (BIS) monitoring (Aspect Medical Systems, Newton, MA). When the patient was asleep and actively snoring with BIS between 50 and 70, a video-recorded fibreoptic nasopharyngoscope was used to assess the upper airway. In our procedures, the average BIS was 55.78 with a mean minimum BIS of 43.15 and a mean maximum BIS of 72.14 5.
When the patient begins to snore and demonstrate airway collapse, the flexible endoscope is inserted through one of the nostrils and is placed on different levels of the upper airways to visualize the site of collapse in real time. Areas of obstruction (velum including soft palate and tonsils, tongue base, larynx and hypopharynx) were classified using a DISE scoring sheet reporting type of obstruction [complete (100%) or partial (grade I: 0-25%; grade II: 25-50%; grade III: 50-75%: grade IV: > 75%)] and dynamic pattern of closure (anterior–posterior, concentric, laterolateral). The DISE scoring sheet was completed in a second time by the principal investigator based on DISE assessment. To characterize the dynamic pattern of closure, DISE findings were reported using the VOTE classification system 11.
During the examination, we also performed a mandibular pull-up (MPU) manoeuvre. The manoeuvre consists in moving the jaw forward by 6-8 mm, and pulling-up the lower dental arch to evaluate the increase in the air space of the areas under examination while breathing improved or snoring disappeared 12.
In our institution, drug induced sleep endoscopy is generally contraindicated in patients with a high American Society of Anesthesiologists (ASA) score and propofol or midazolam allergies (albeit rare), owing to high risk. Relative contraindications are also severe OSA (an AHI > 70 events/hour) and severe obesity, as these patients are regarded as poor candidates for sleep surgery or an MRA.
Statistical analysis
Statistical analysis was performed using SPSS for Windows. Continuous variables were expressed as mean ± SD. Comparisons between groups were performed by Mann-Whitney U-test and t-test. The results were considered statistically significant for p values < 0.05.
Results
Taking into consideration grade of the sites of obstruction observed during DISE, it was possible to establish their prevalence. Considering a complete obstruction of 100%, we found that palatal obstruction was the most frequently observed site of obstruction (73%), followed by tongue base obstruction (43%), laryngeal obstruction (31%) and hypopharyngeal obstruction (23%). Data are shown in Table I in all patients at different AHI and BMI. Partial obstruction greater than 50% was present at a palatal site in 23%, at a base tongue site in 34%, at a laryngeal site in 37% and at a hypo-pharyngeal site in 26% of patients. Data are shown in Table II in all patients at AHI and BMI.
Table I.
Patient distribution considering site of complete (100%) obstruction and AHI and BMI.
| Complete retro palatal collapse | Complete retro lingual collapse | Complete oro-hypopharyngeal lateral wall collapse | Complete laryngeal collapse | |
|---|---|---|---|---|
| All patients (n = 138) | 102 (73.9%) | 60 (43.47%) | 32 (23.18%) | 44 (31.88%) |
| AHI < 30 (n = 72 ) | 42 (58.33%) | 14 (19.44%) | 4 (5.55%) | 18 (25%) |
| AHI ≥ 30 (n = 66) | 60 (90.90%) | 46 (69.69%) | 28 (42.42%) | 26 (39.39%) |
| BMI < 30 (n = 108) | 82 (75.92%) | 42 (38.88%) | 22 (20.37%) | 36 (33.33%) |
| BMI ≥ 30 (n = 30) | 20 (66.66%) | 18 (60%) | 10 (33.33%) | 8 (26.66%) |
Table II.
Patient distribution considering sites of partial (greater than 50%) obstruction and AHI and BMI.
| Partial retro palatal collapse | Partial retro lingual collapse | Partial oro-hypopharyngeal lateral wall collapse | Partial laryngeal collapse | |
|---|---|---|---|---|
| All patients (n = 138) | 34 (24.63%) | 48 (34.78%) | 52 (37.68%) | 36 (26.08%) |
| AHI < 30 (n = 72 ) | 22 (30.55%) | 32 (44.44%) | 32 (44.44%) | 18 (25%) |
| AHI > 30 (n = 66) | 10 (15.15%) | 14 (21.21%) | 22 (32.33%) | 18 (27.27%) |
| BMI < 30 (n = 108) | 26 (24.07%) | 44 (40.74%) | 48 (44.44%) | 28 (25.92%) |
| BMI > 30 (n = 30) | 6 (20%) | 4 (13.33%) | 6 (20%) | 8 (26.66%) |
The prevalence of cases based on number of sites of complete obstruction and different AHI and BMI is shown in Table III. The partial sites with obstructions greater than 50% are shown in Table IV.
Table III.
Patient distribution based on number of complete sites of obstruction and AHI and BMI.
| 1 site | 2 sites | 3 sites | 4 sites | |
|---|---|---|---|---|
| All patients (n = 138) | 54 (39.13%) | 52 (37.68%) | 16 (11.59%) | 16 (11.59%) |
| AHI < 30 (n = 72 ) | 38 (52.7%) | 22 (30.5%) | 4 (2.8%) | 0 |
| AHI ≥ 30 (n = 66) | 14 (9.24%) | 30 (45.45%) | 12 (18.18%) | 16 (24.24%) |
| BMI < 30 (n = 108) | 50 (46.29%) | 40 (37.03%) | 8 (7.40%) | 12 (11.11%) |
| BMI ≥ 30 (n = 30) | 6 (20%) | 10 (33.33%) | 6 (20%) | 4 (13.33%) |
Table IV.
Patient distribution based on number of partial (greater than 50%) sites of obstruction and AHI and BMI.
| 1 site | 2 sites | 3 sites | 4 sites | |
|---|---|---|---|---|
| All patients (n = 138) | 6 (4.34%) | 42 (30.43%) | 30 (21.73%) | 58 (42.02%) |
| AHI < 30 (n = 72 ) | 4 (5.5%) | 26 (36.11%) | 12 (16.66%) | 24 (33.33%) |
| AHI > 30 (n = 66) | 2 (3.03%) | 16 (24.24%) | 18 (27.27%) | 34 (51.51%) |
| BMI < 30 (n = 108) | 6 (5.55%) | 34 (31.48%) | 18 (16.66%) | 48 (44.44%) |
| BMI > 30 (n = 30) | 0 (%) | 6 (20%) | 14 (46.66%) | 10 (33.33%) |
Table V details prevalence based on patterns of obstruction for each site following the VOTE classification system.
Table V.
Distribution of patients based on patterns of obstruction for each site.
| Antero – posterior | Lateral | Concentric | ||||
|---|---|---|---|---|---|---|
| Partial | Complete | Partial | Complete | Partial | Complete | |
| Velum | 18/138 (13.04%) | 40/138 (28.98%) | 6/138 (4.34%) | 40/138 (28.98%) | 28/138 (20.28%) | 42/138 (30.43%) |
| Oropharynx | / | / | 24/138 (17.39%) | 14/138 (10.14%) | / | / |
| Tongue base | 48/138 (34.78%) | 60/138 (43.47%) | / | / | / | / |
| Epiglottis | 20/138 (14.49%) | 28/138 (20.28%) | 16/138 (11.59%) | 16/138 (11.59%) | / | / |
Correlating results of DISE with polysomnographic parameters, we found that the AHI was significantly higher in patients with multilevel obstruction compared to those with a unilevel one. In fact, our results suggest that the multilevel complete collapse was significantly associated with higher AHI values as shown in Figure 1a. Including partial sites of obstructions greater than 50%, our results suggest that multilevel collapse remains statistically and significantly associated with higher AHI values (Fig. 1b).
Fig. 1.
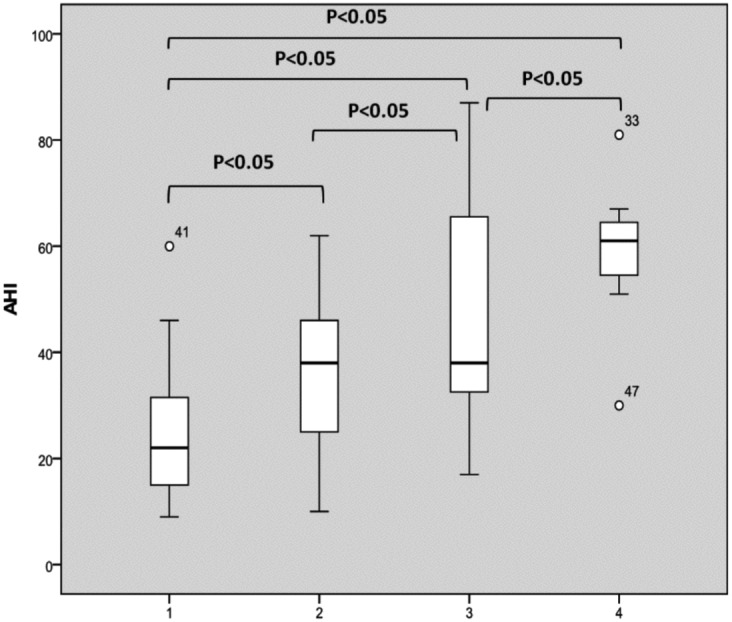
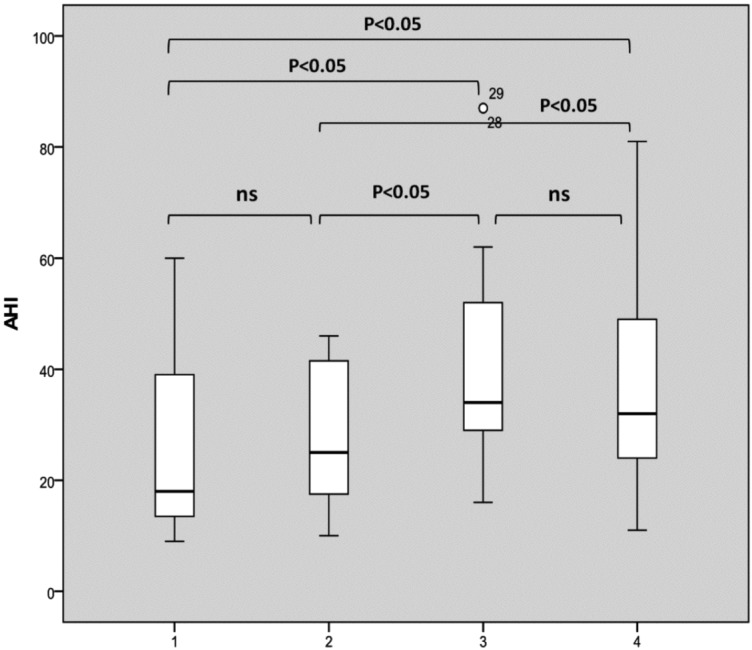
AHI distribution based on the number of sites of complete collapse (a) and complete plus partial collapses greater than 50% (b). The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
a. 1: one complete site of obstruction of 100%; 2: two complete sites of obstruction of 100%; 3: three complete sites of obstruction of 100%; 4: four complete sites of obstruction of 100%).
b. 1: one site of obstruction of 100% or > 50%; 2: two sites of obstruction of 100% and/or > 50%; 3: three sites of obstruction of 100% and/or > 50%; 4: four sites of obstruction of 100% and/or > 50%).
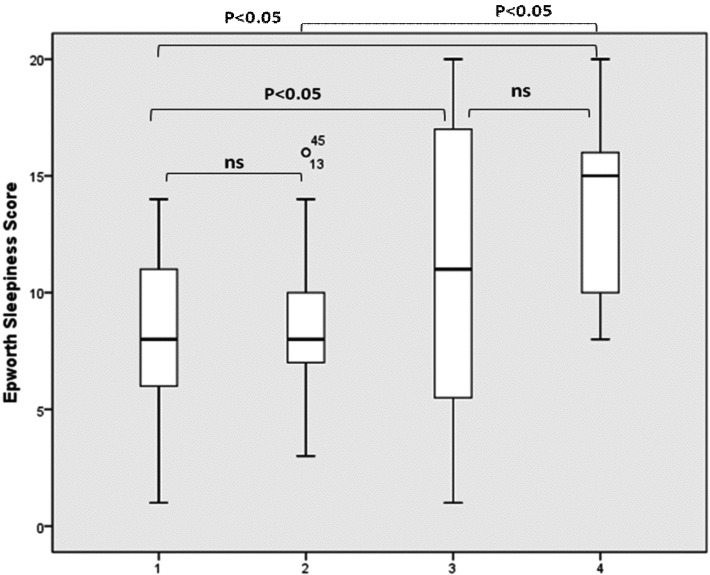
Considering BMI distribution basing on number of sites with complete obstruction, there was no significant difference (Fig. 2a). Considering partial (greater than 50%) sites of collapse, multilevel collapse was not significantly associated with higher BMI as shown in Figure 2b. Finally, analyzing Epworth Sleepiness Score distribution based on number of sites with complete obstruction, we found a significant difference between patients with 3-4 sites of obstruction compared to those with two sites or uni-level obstruction as shown in Figure 3. We did not observe any significant difference in terms of ESS considering sites of partial obstruction greater than 50%.
Fig. 2.
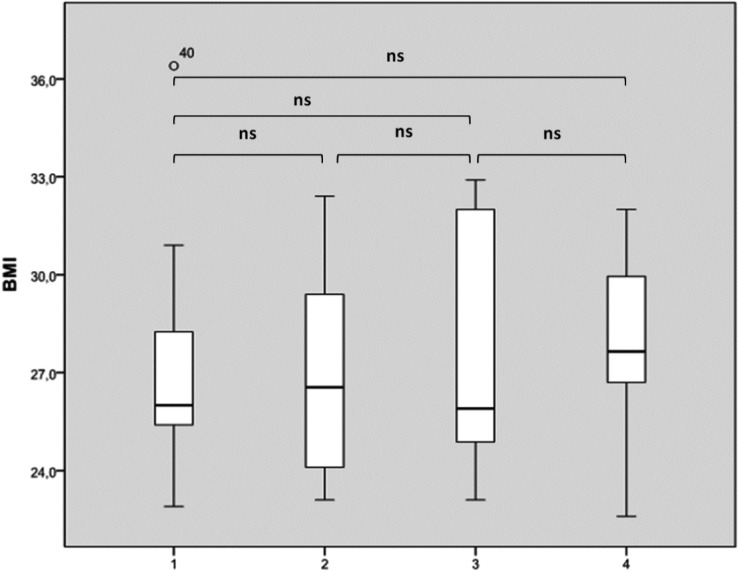
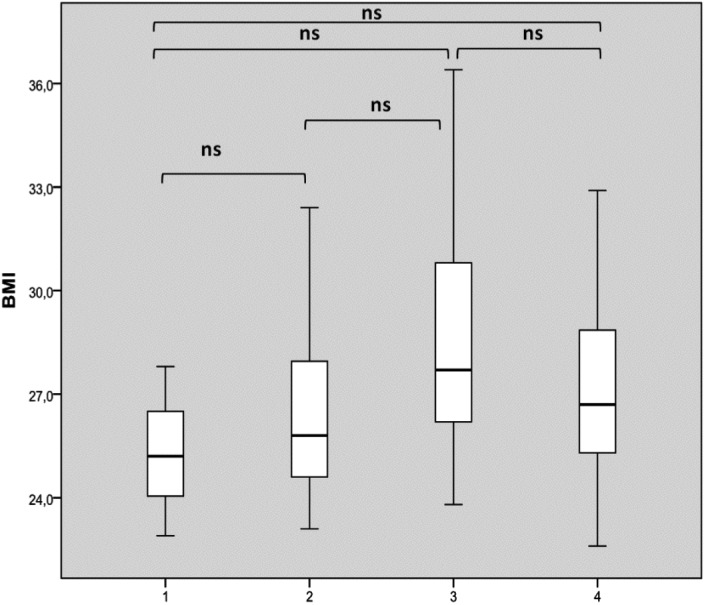
BMI distribution based on the number of sites of complete collapse (a) and complete plus partial collapses greater than 50% (b). The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
a. 1: one complete site of obstruction of 100%; 2: two complete sites of obstruction of 100%; 3: three complete sites of obstruction of 100%; 4: four complete sites of obstruction of 100%).
b. 1: one site of obstruction at 100% or > 50%; 2: two sites of obstruction of 100% and/or > 50%; 3: three sites of obstruction of 100% and/or >5 0%; 4: four sites of obstruction of 100% and/or > 50%).
Fig. 3.
Epworth Sleepiness Score distribution based on the number of sites of complete collapse. The box plots show the median and inter-quartile range and the error bars show the 5th and 95th percentiles.
1: one complete site of obstruction of 100%; 2: two complete sites of obstruction of 100%; 3: three complete sites of obstruction of 100%; 4: four complete sites of obstruction of 100%).
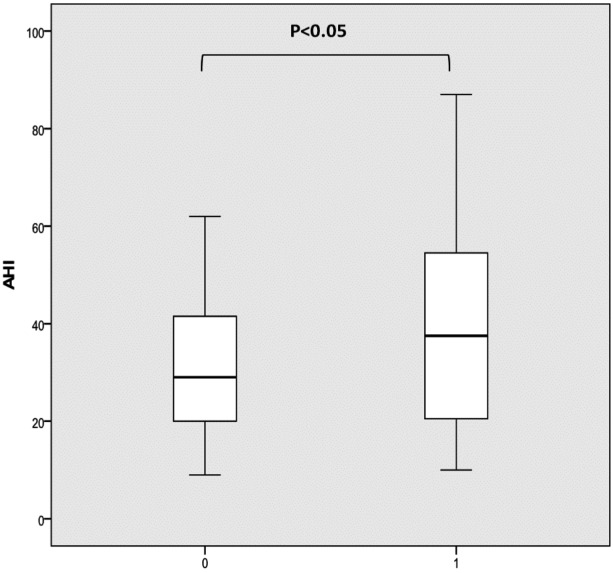
Comparing patients with or without a concentric complete oropharyngeal collapse, there was significantly higher AHI (40.42 versus 31.76 per hour) and higher BMI (26.3 versus 28.3) in the former group compared to the latter (Fig. 4).
Fig. 4.
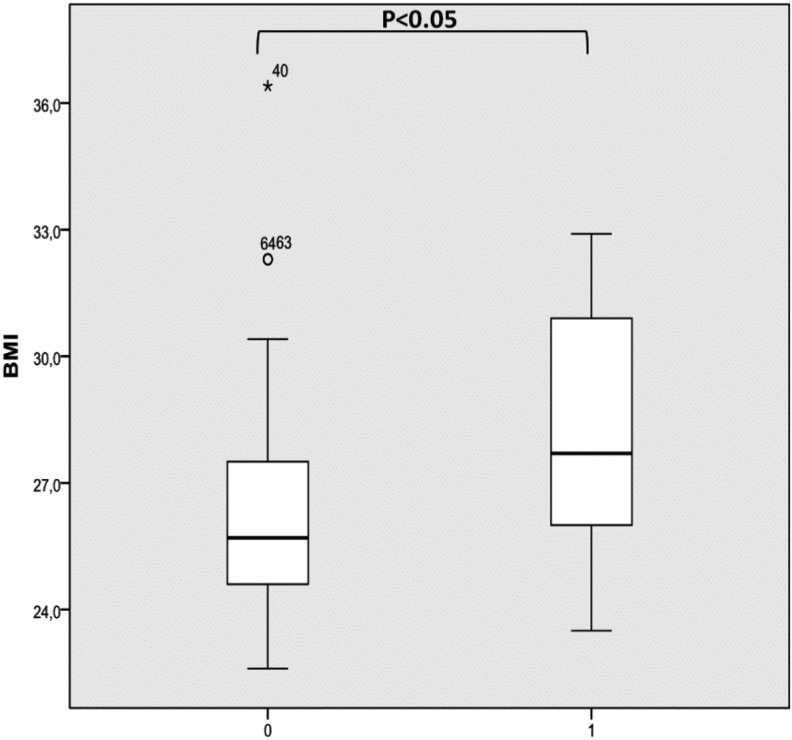
AHI (a) and BMI (b) in patients with (1) or without (0) concentric complete oropharyngeal collapse.
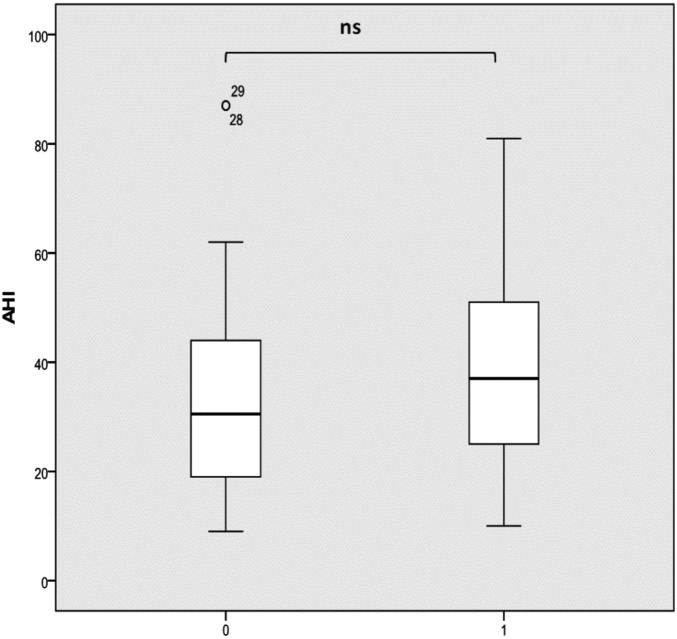
A difference was found in AHI in patients with or without an epiglottis-level obstruction, although this was not statistically significant (33.48 versus 38.22 per hour, P > 0.05; Fig. 5).
Fig. 5.
AHI in patients with (1) or without (0) laryngeal site collapse.
Table VI shows the data of the most appropriate treatment (oral device, surgery or CPAP) chosen for each patient based on the clinical information obtained during drug induced sleep endoscopy.
Table VI.
Distribution of patients based on treatment options chosen considering clinical information obtained during drug induced sleep endoscopy and subdivided based on AHI and BMI. In the last column, the number of cases referred to repeat polysomnography due to inconsistencies between DISE and AHI is reported.
| Oral device | Surgery | CPAP | Repeat PSNG | |
|---|---|---|---|---|
| All patients (n = 138) | 42 (30.43%) | 28 (20.28%) | 56 (40.57%) | 4 (2.89%) |
| AHI < 30 (n = 72) | 30 (41.66%) | 18 (25%) | 18 (25%) | 3 (4.16%) |
| AHI ≥ 30 (n = 66) | 14 (21.21%) | 12 (11.32%) | 38 (57.57%) | 1 (1.51%) |
| BMI < 30 (n = 108) | 34 (31.48%) | 26 (24.07%) | 44 (40.74%) | 2 (1.85%) |
| BMI ≥ 30 (n = 30) | 6 (20%) | 4 (13.33%) | 16 (53.33%) | 2 (6.66%) |
Discussion
Patients with OSAS experience excessive daytime sleepiness, impaired concentration, snoring, recurring nocturnal awakenings and non-restorative sleep with an increased risk of obesity and cardiovascular disease leading to substantial socio-economic implications 13. Diagnostic evaluation is crucial to choose the best treatment options 14-16. Baseline evaluation is very important, but it needs to be integrated with data obtained by polysomnography and sleep endoscopy in order to obtain, for each patient, all relevant information about the physiopathology of the sleep airway obstruction.
Currently, DISE is the most reliable standard method to determine the level, number and severity of sites of obstruction of the airway during the night in sleep apnoea patients and to specify the exact dynamic pattern of closure. In fact, even though overnight polysomnograph is the gold standard diagnostic study for sleep-disordered breathing, it fails to establish the precise site of collapse. Furthermore, the Müller manoeuvre has been questioned because it does not predict the success of surgery, location of upper airway obstruction or severity of the disease 1. In fact, measurement of the collapsibility of the upper airway during the awake state is not a good indicator of collapsibility during sleep, due to differences in term of muscle tone, sensitivity of reflexes and respiratory physiology, between the conscious and the unconscious states. Finally, plain film cephalometry and CT scans only provide static information about the bony structure without evidence of soft tissue collapse. For this reason, new insight is expected from tests such as DISE that add a dynamic evaluation to classical static ones during induced sleep. Dynamic magnetic resonance imaging (MRI) is promising, and is increasingly used in the evaluation of the site of collapse in OSA. In this field, newer studies have also led to a computational model of the human upper airway by signal averaging of MRI. Based on this model, various surgical interventions have been simulated, offering new possibilities in the improvement of surgical and nonsurgical approaches to OSA patients. Future studies will suggest the clinical value of its application in the standard diagnostic work-up 17 18.
Our study compares the results of routine clinical and diagnostic evaluation with DISE results, evaluating the correlation between clinical indexes with number, dynamic pattern and severity of sites of obstruction. We scored four separate obstruction sites differentiating between partial and complete obstruction and defining the dynamic pattern of closure. We statistically correlated AHI, BMI and EPS not only with the number of sites of complete obstruction but also with partial ones, even though literature data are not consistent about this point. Salamanca et al. 19 recently published a large series of patients that underwent DISE without including partial sites of obstruction. Bachar et al. 20 recently proposed a novel grading system for quantifying upper-airway obstruction including partial sites.
In our series, the distribution of sites of obstruction coincides with the results published in the literature 21. In fact, we observed that a retropalatal site is the most frequently involved in the obstruction followed by a retrolingual site. Multilevel obstruction is more common than a single-level one. In addition, the results of our study show that multilevel obstruction was significantly associated with higher AHI. We demonstrated that an higher number of sites of obstruction significantly correlates with higher AHI, not only considering complete obstruction but also including sites with partial obstruction greater than 50%. Taking into account the dynamic pattern of closure, there is a significantly higher AHI only when a concentric pattern of closure is present, and in particular at an oropharyngeal site. Finally, our data suggest that multilevel obstruction was significantly associated with higher EPS as a higher number of sites of obstruction significantly correlated with higher EPS scores. Thus, our study shows the appropriateness and reliability of the results obtained by DISE and their correlation with those obtained by polysomnography.
We obtained different results when analyzing the results of DISE and BMI. Moreover, the available data about correlations between BMI and number of sites of obstruction is not homogenous. Abdullah et al. observed that a trend of a higher BMI was associated with four or more sites of obstruction compared to single-site obstruction 22. In contrast, other authors 23 did not observe the same correlation. In our series, we found that a trend of higher BMI was associated with a higher number of sites of complete obstruction, and that this trend was consistent when including partial sites of obstruction, even though these differences did not reach statistical significance. Nonetheless, expert opinion and various reports 24-26 concur that concentric obstruction is related to higher BMI. In agreement with this, we found a significantly higher BMI in patients with a concentric pattern of closure, and in particular at an oropharyngeal-velum site compared to patients with other patterns of closure.
In our series, the depth of induced sleep was assessed using a BIS Monitor. In fact, the degree of upper airway narrowing can be intensified according to the depth of sedation. The monitoring of sedation during DISE is critical, especially in patients with mouth breathing 5. BIS, originally developed to validate the depth of anaesthesia, has begun to be used in sleep research. Babar-Craig et al. reported on the efficacy of the bispectral index (BIS) monitoring system to validate the depth of sedation during DISE 27. Sleigh et al. 11 found that light sleep was associated with BIS values of 75 to 90, and that slow-wave sleep was associated with BIS values of 20 to 70 28. Therefore, in agreement with literature data, we agree that while performing DISE the depth of sedation must be monitored, and deeper sleep must be included in the test period. In our procedures, the average BIS was 55.78 with a range between a mean minimum BIS of 43.15 and a mean maximum BIS of 72.14.
The gold-standard treatment of OSAS is currently continuous positive airway pressure (CPAP), relegating other treatments – oral device and surgery – in case of failure of CPAP and only in moderate OSAS as the first option treatment. The introduction of DISE in helping to define the pathogenesis of the site of obstruction individually offers more targeted and tailored information that is changing the indications of initial treatment. Unfortunately, a significant number of patients do not tolerate CPAP (30- 50%) 4. For this reason, many patients try to find surgical therapy as an alternative solution. Based on the available data in cases in which surgery is indicated after standard pre-operative evaluation, surgeons still consider sleep endoscopy as knowledge of the precise sites of obstruction is critical for successful sleep surgery; DISE, in fact, allows real-time identification of the levels of collapse and the severity and pattern of collapse at each level, offering an important predictive value of surgical success. Finally, sleep endoscopy shows potential for other applications and has been proposed for selecting candidates for other site specific interventions such as oral appliances and endoscopy-assisted CPAP titration 29. In our series, as a final outcome of the diagnostic work up, surgery, oral device and CPAP were chosen based on DISE results. In particular, when surgery was indicated DISE appears to be fundamental in defining the type of intervention. In four cases, we preferred DISE over repeat polysomnography due to discrepancies between DISE results and AHI before suggesting the best treatment option. Several surgical approaches have been suggested for OSAS patients 30-33, although the surgeon should consider DISE results before planning the optimal surgical treatment.
In conclusion, our data suggest that DISE is safe, easy to perform, valid and reliable, as previously reported. Furthermore, we found a good correlation between DISE findings and clinical characteristics such as AHI and EPS, in agreement with literature data. In particular, we observed a significant association between higher AHI and higher number of sites of obstruction in terms not only of complete obstruction, but also of partial obstruction greater than 50%. In particular, for this latter point, larger study should better characterize the association with clinical indexes to understand its clinical significance. Based on our data, we suggest that adequate assessment by DISE of all sites of obstruction is very important, not only in patients with low-moderate AHI and EPS, but also in patients with high AHI or/and high EPS, which is important to plan multilevel surgery as it is more demanding and success may be harder to achieve. Moreover, it has recently been demonstrated 34 that the test-retest reliability of DISE is good and that DISE has a relevant influence on treatment recommendations, positively influencing success rates of OSA therapy by addressing airway obstruction in a targeted fashion, leading to surgical treatment that is tailored to a specific pattern of obstruction 3. Future challenges for DISE remain standardizing the method of sedation, identification of a uniform DISE classification system and definition of a reproducible and titratable standard mandibular advancement manoeuvre. Future research should focus on these topics to increase the already well-recognized role of DISE.
References
- 1.Campanini A, Canzi P, Vito A, et al. Awake versus sleep endoscopy: personal experience in 250 OSAHS patients. Acta Otorhinolaryngol Ital. 2010;30:70–73. [PMC free article] [PubMed] [Google Scholar]
- 2.Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16:504–509. doi: 10.1111/j.1365-2273.1991.tb01050.x. [DOI] [PubMed] [Google Scholar]
- 3.Eichler C, Sommer JU, Stuck BA, et al. Does drug-induced sleep endoscopy change the treatment concept of patients with snoring and obstructive sleep apnea? Sleep Breath. 2013;17:63–68. doi: 10.1007/s11325-012-0647-9. [DOI] [PubMed] [Google Scholar]
- 4.Vanderveken OM. Drug-induced sleep endoscopy (DISE) for non-CPAP treatment selection in patients with sleep-disordered breathing. Sleep Breath. 2013;17:13–14. doi: 10.1007/s11325-012-0671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong SD, Dhong HJ, Kim HY, et al. Change of obstruction level during drug-induced sleep endoscopy (DISE) according to sedation depth in obstructive sleep apnea. Laryngoscope. 2013 Aug 05; doi: 10.1002/lary.24045. doi: 10.1002/lary.24045. [DOI] [PubMed] [Google Scholar]
- 6.Vroegop AV, Vanderveken OM, Wouters K, et al. Observer variation in drug-induced sleep endoscopy: experienced versus nonexperienced ear, nose, and throat surgeons. Sleep. 2013;36:947–953. doi: 10.5665/sleep.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gillespie MB, Reddy RP, White DR, et al. A trial of drug-induced sleep endoscopy in the surgical management of sleepdisordered breathing. Laryngoscope. 2013;123:277–282. doi: 10.1002/lary.23506. [DOI] [PubMed] [Google Scholar]
- 8.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–276. [PMC free article] [PubMed] [Google Scholar]
- 9.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8:597–619. doi: 10.5664/jcsm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 11.Kezirian EJ, Hohenhorst W, Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268:1233–1236. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 12.Milano F, Mondini S, Billi MC, et al. The impact of a multidisciplinary approach on response rate of mandibular advancing device therapy in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:337–342. [PMC free article] [PubMed] [Google Scholar]
- 13.Fusetti M, Fioretti AB, Valenti M, et al. Cardiovascular and metabolic comorbidities in patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:320–325. [PMC free article] [PubMed] [Google Scholar]
- 14.Passali D, Caruso G, Arigliano LC, et al. Database application for patients with obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2012;32:252–255. [PMC free article] [PubMed] [Google Scholar]
- 15.Passali FM, Bellussi L, Mazzone S, et al. Predictive role of nasal functionality tests in the evaluation of patients before nocturnal polysomnographic recording. Acta Otorhinolaryngol Ital. 2011;31:103–108. [PMC free article] [PubMed] [Google Scholar]
- 16.Piumetto E, Sammartano AM, Meinardi G, et al. Diagnostic and therapeutic iter in paediatric OSAS: personal experience. Acta Otorhinolaryngol Ital. 2011;31:149–153. [PMC free article] [PubMed] [Google Scholar]
- 17.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper air way anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, White DP, Malhotra A. The impact of anatomic manipulations on pharyngeal collapse: results from a computational model of the normal human upper airway. Chest. 2005;128:1324–1330. doi: 10.1378/chest.128.3.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salamanca F, Costantini F, Bianchi A, et al. Identification of obstructive sites and patterns in obstructive sleep apnoea syndrome by sleep endoscopy in 614 patients. Acta Otorhinolaryngol Ital. 2013;33:261–266. [PMC free article] [PubMed] [Google Scholar]
- 20.Bachar G, Nageris B, Feinmesser R, et al. Novel grading system for quantifying upper-airway obstruction on sleep endoscopy. Lung. 2012;190:313–318. doi: 10.1007/s00408-011-9367-3. [DOI] [PubMed] [Google Scholar]
- 21.Borek RC, Thaler ER, Kim C, et al. Quantitative airway analysis during drug-induced sleep endoscopy for evaluation of sleep apnea. Laryngoscope. 2012;122:2592–2599. doi: 10.1002/lary.23553. [DOI] [PubMed] [Google Scholar]
- 22.Abdullah VJ, Wing YK, Hasselt CA. Video sleep nasendoscopy: the Hong Kong experience. Otolaryngol Clin North Am. 2003;36:461–471. doi: 10.1016/s0030-6665(02)00176-7. [DOI] [PubMed] [Google Scholar]
- 23.Ravesloot MJ, Vries N. One hundred consecutive patients undergoing drug-induced sleep endoscopy: results and evaluation. Laryngoscope. 2011;121:2710–2716. doi: 10.1002/lary.22369. [DOI] [PubMed] [Google Scholar]
- 24.Kezirian EJ, Malhotra A, Goldberg AN, et al. Changes in obstructive sleep apnea severity, biomarkers, and quality of life after multilevel surgery. Laryngoscope. Laryngoscope;120:1481–1488. doi: 10.1002/lary.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kezirian EJ, Goldberg AN. Hypopharyngeal surgery in obstructive sleep apnea: an evidence-based review. Arch Otolaryngol Head Neck Surg. 2006;132:206–213. doi: 10.1001/archotol.132.2.206. [DOI] [PubMed] [Google Scholar]
- 26.Iwanaga K, Hasegawa K, Shibata N, et al. Endoscopic examination of obstructivesleep apnea syndrome patients during drug-induced sleep. Acta Otolaryngol Suppl. 2003;550:36–40. doi: 10.1080/0365523031000055. [DOI] [PubMed] [Google Scholar]
- 27.Babar-Craig H, Rajani NK, Bailey P, et al. Validation of sleep nasendoscopy for assessment of snoring with bispectral index monitoring. Eur Arch Otorhinolaryngol. 2012;269:1277–1279. doi: 10.1007/s00405-011-1798-1. [DOI] [PubMed] [Google Scholar]
- 28.Sleigh JW, Andrzejowski J, Steyn-Ross A, et al. The bispectral index: a measure of depth of sleep? Anesth Analg. 1999;88:659–661. doi: 10.1097/00000539-199903000-00035. [DOI] [PubMed] [Google Scholar]
- 29.Civelek S, Emre IE, Dizdar D, et al. Comparison of conventional continuous positive airway pressure to continuous positive airway pressure titration performed with sleep endoscopy. Laryngoscope. 2012;122:691–695. doi: 10.1002/lary.22494. [DOI] [PubMed] [Google Scholar]
- 30.Giarda M, Brucoli M, Arcuri F, et al. Efficacy and safety of maxillomandibular advancement in treatment of obstructive sleep apnoea syndrome. Acta Otorhinolaryngol Ital. 2013;33:43–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Vicini C, Frassineti S, La Pietra MG, et al. Tongue Base Reduction with Thyro-Hyoido-Pexy (TBRTHP) vs. Tongue Base Reduction with Hyo-Epiglottoplasty (TBRHE) in mildsevere OSAHS adult treatment. Preliminary findings from a prospective randomised trial. Acta Otorhinolaryngol Ital. 2010;30:144–148. [PMC free article] [PubMed] [Google Scholar]
- 32.Mantovani M, Minetti A, Torretta S, et al. The velo-uvulopharyngeal lift or "roman blinds" technique for treatment of snoring: a preliminary report. Acta Otorhinolaryngol Ital. 2012;32:48–53. [PMC free article] [PubMed] [Google Scholar]
- 33.Tarsitano A, Marchetti C. Unusual presentation of obstructive sleep apnoea syndrome due to a giant mandible osteoma: case report and literature review. Acta Otorhinolaryngol Ital. 2013;33:63–66. [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Bruno K, Goldberg AN, McCulloch CE, et al. Test-retest reliability of drug-induced sleep endoscopy. Otolaryngol Head Neck Surg. 2009;140:646–651. doi: 10.1016/j.otohns.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vito A, Agnoletti V, Berrettini S, et al. Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques - a randomized controlled study. Eur Arch Otorhinolaryngol. 2011;268:457–462. doi: 10.1007/s00405-010-1376-y. [DOI] [PubMed] [Google Scholar]