Abstract
Alcohol abuse suppresses the immune responses of alveolar macrophages (AMs) and increases the risk of a respiratory infection via chronic oxidative stress and depletion of critical antioxidants within alveolar cells and the alveolar lining fluid. Although alcohol-induced mitochondrial oxidative stress has been demonstrated, the oxidation of the mitochondrial thioredoxin redox circuit in response to alcohol has not been examined. In vitro ethanol exposure of a mouse AM cell line and AMs from an ethanol-fed mice demonstrated NADPH depletion concomitant with oxidation of mitochondrial glutathione and oxidation of the thioredoxin redox circuit system including thioredoxin 2 (Trx2) and thioredoxin 2 reductase (Trx2R). Mitochondrial peroxiredoxins (Prdxs), which are critical for the reduction of the thioredoxin circuit, were irreversibly hyperoxidized to an inactive form. Ethanol also decreased the mRNAs for Trx2, Trx2R, Prdx3, and Prdx5 plus the mitochondrial thiol-disulfide proteins glutaredoxin 2, glutathione reductase, and glutathione peroxidase 2. Thus, the mitochondrial thioredoxin circuit was highly oxidized by ethanol, thereby compromising the mitochondrial antioxidant capacity and ability to detoxify mitochondrial reactive oxygen species. Oxidation of the mitochondrial thioredoxin redox circuit would further compromise the transient oxidation of thiol groups within specific proteins, the basis of redox signaling, and the processes by which cells respond to oxidants. Impaired mitochondria can then jeopardize cellular function of AMs such as phagocytosis which may explain the increased risk of respiratory infection in subjects with an alcohol use disorder.
Keywords: chronic alcohol ingestion, alveolar macrophage, mitochondria, thioredoxin, peroxiredoxin hyperoxidation
Introduction
In the alveolar space, alveolar macrophages (AMs) orchestrate the innate immune response through phagocytosis and a respiratory burst to kill infectious microbes. With binge and chronic alcohol abuse, AM immune functions are impaired increasing the risk of pneumonia and tuberculosis, even in those without a medical diagnosis of an alcohol use disorder [1, 2]. In previous studies, we demonstrated that one salient feature for the alcohol-induced impaired immune response by AMs is depletion of the antioxidant glutathione (GSH) and subsequent chronic oxidant stress [3–9]. This persistent alcohol-induced oxidant stress results in multiple cascades that impair AMs phagocytosis and the respiratory burst culminating in a lung highly susceptible to infections. In previous studies, we demonstrated that one mechanism for chronic generation of reactive oxygen species (ROS) is through alcohol-induced upregulation of NADPH oxidase 1, 2, and 4 in AMs [9]. Studies in other cell types demonstrated that alcohol-induced mitochondrial ROS also contributes to the characteristic chronic oxidative stress [10–13]. However, the degree of alcohol-induced mitochondrial oxidant stress in AMs has not been addressed.
Although ROS are implicated in cell signaling and regulation, adequate levels of antioxidant defenses are required to protect against the harmful effects of excessive ROS production. In the mitochondrial electron transport chain, the ROS superoxide (O2•−) is generated as a byproduct during ATP generation but is also related to the concentration of the electron donor NADH, the local O2 concentration, and the second-order rate constants for the electron donor and O2 [14–16]. This is coupled with complex oxidation-reduction (redox) systems that prevent excessive mitochondrial ROS generation and maintain mitochondrial signaling and function [17–20]. For example, mitochondria contain 10% of the cellular GSH pool which can be oxidized to its disulfide form GSSG. In addition to GSH, other critical thiol-disulfide redox molecules that also serve as antioxidants are located within the mitochondria, including glutaredoxin 2 (Grx2), thioredoxin 2 (Trx2), and peroxiredoxin 3 and 5 (Prdx3 and 5), glutathione reductase (GR) and thioredoxin reductase 2 (TrxR2). These sulfur switches coordinately regulate the activities of redox-sensitive proteins containing cysteines and are key components of the low-flux redox circuits critical for cell signaling and metabolism [21]. When the redox environment within the mitochondria is altered, it stimulates further intracellular oxidative stress, which can trigger cell death pathways whereby GSH oxidation and depletion are the earliest events in mitochondrial-dependent apoptosis [22, 23].
Mitochondria are also involved in alcohol metabolism. The oxidative pathway for alcohol metabolism involves alcohol dehydrogenase and mitochondrial aldehyde dehydrogenase which uses nicotinamide adenine dinucleotide (NAD+) as a cofactor, reducing NAD+ to NADH [24]. Therefore, alcohol metabolism can promote NADH accumulation leading to an increased NADH/NAD+ ratio which can subsequently upregulate O2•− production [16]. Increased O2•− production and its dismutation then increases the formation of H2O2 within the mitochondria [16]. Given the critical role of H2O2 flux in redox signaling and the thioredoxin circuit in regulation of that signaling, it is important to determine whether the ethanol-induced increases in mitochondrial ROS [25] is also associated with oxidation of the mitochondrial thioredoxin circuit.
AMs are critical to the impaired innate immune response associated with alcohol abuse. Therefore, we examined whether the mitochondrial oxidative stress associated with chronic ethanol ingestion included oxidation of the major mitochondrial thiol-disulfide antioxidant system, the thioredoxin circuit. With in vitro ethanol exposure or chronic ethanol ingestion, multiple members of the mitochondrial Trx2 redox circuit were oxidized including Nicotinamide adenine dinucleotide phosphate (NADPH), GSH, Trx2, Prdx, and TrxR2. Extensive oxidation of the Trx2 redox system may explain the ethanol-induced mitochondrial ROS generation and subsequent loss of critical AM functions such as phagocytosis.
Materials and Methods
Mouse model of chronic ethanol ingestion
All animal studies were performed in accordance with National Institutes of Health guideline outlined in the Guide for the Care and Use of Laboratory Animals. All protocols were reviewed and approved by the Emory University Institutional Animal Care and Use Committee. Mice (C57BL/6J; age 6–8 weeks) were fed standard laboratory chow ad libitum with incremental increases of ethanol in the drinking water over 3 weeks (5%/week) to a final concentration of 20% (v/v). Mice were then maintained on 20% ethanol in the drinking water for 10–12 weeks (n=5/group) [26, 27]. The controls were pair-fed in order to control for the calories due to ethanol as well as any differences in food intake. This method replicates blood alcohol levels in human subjects with an alcohol use disorder, as assessed by studies using pair-fed ethanol-treated female or male C57BL/6J mice [28, 29]. After sacrifice, tracheas from all mice were cannulated and the lungs lavaged (3X with 1 ml of PBS). Mouse AMs (mAMs) were then isolated from the lavage fluid by centrifugation at 8000 rpm for 5 min. The cell pellet was resuspended in RPMI 1640 medium containing 2% FBS plus 1% penicillin/streptomycin and then incubated at 37°C in 5% CO2 atmosphere before analysis. The cell population was 95% macrophage as determined by MAC1 staining and Diff Quik analysis with cell viability >95% as determined by calcein-ethidium iodide staining. This method routinely yielded ∼1 × 106 mAMs per mouse and did not vary between the experimental groups.
MHS cell culture and ethanol exposure
The AM cell line, MHS (American Type Culture Collection, Manassas, VA), was used as a model system for studying the effects of in vitro ethanol exposure. Cells were cultured in RPMI 1640 medium containing 10% FBS plus 1% penicillin/streptomycin at 37°C in a 5% CO2 atmosphere. MHS cells were treated with 0.2% (v/v) ethanol for 5 consecutive days with the media changed daily.
RNA isolation and quantitative RT-PCR
Total RNA was extracted using RNAeasy Mini Kit (Qiagen, Valencia, CA) and cDNA synthesized using 1 µg of RNA and the RT² First Strand Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction. Primers were designed and purchased from Invitrogen (Carlsbad, CA) for the detection of Trx2, TrxR2, Prdx3, Grx2, GR, and Gpx1. Quantitative real-time (qRT)-PCR was performed using RT² SYBR® Green qPCR Mastermix (Qiagen, Valencia, CA) on the Applied Biosystems ABI Prism 7500 version 1.4 sequence detection system. The following conditions were used: 95°C for 10 min and 40 cycles entailing 95°C for 10 s followed by annealing at 60°C for 1 min. After analysis, values are expressed as the relative expression of mRNA normalized to 18s mRNA.
Mitochondrial GSH, GSSG, and oxidant stress analysis
For analysis of GSH and GSSG pools by HPLC analysis, mitochondria were rapidly isolated using a Percoll density gradient as previously described by this laboratory [30]. Briefly, the mitochondrial samples were immediately acidified with perchloric acid (5%, v/v, total) containing the internal standard γ-glutamyl-glutamate (5 µM; final concentration). After derivatization with iodoacetic acid and dansyl chloride, the GSH and GSSG fractions were separated on an amino µBondaPak column by HPLC with fluorescence detectors (Waters, Milford, MA). GSH and GSSG concentrations were determined relative to the γ-glutamyl-glutamate internal standard. Some freshly isolated mAMs were allowed to attach to a glass slide (1 h) and then loaded with MitoTracker Red CM-H2XRos (2 µM; 15 min; Molecular Probes, Eugene, OR) as described in the product literature. Cells were also loaded with MitoTracker Green FM (2 µM) for colocalization of the MitoTracker Red CM-H2XRos dye to the mitochondria. After staining, fluorescence was measured by fluorescence microscopy and the results expressed as relative fluorescence units (RFU) per 106 cells.
Mitochondrial NAD+/NADH and NADP+/NADPH ratio
Some mitochondria were isolated from MHS cells or AMs using the Mitochondria Isolation Kit (Thermo Fisher Scientific, Rockford, IL). Mitochondrial NAD(P)+ and NAD(P)H were then extracted with the appropriate extraction buffer, mixed with assay buffer, and absorbance read at 565 nm. The EnzyChrom™ NAD(P)+/NAD(P)H assay kit (Bioassay Systems, Hayward, CA) was used to determine the mitochondrial ratios of NAD+/NADH and NADP+/NADPH.
Trx2 redox western blot analysis
The reduced and oxidized forms of Trx2 were assessed by redox western blot analysis [31]. This assay is based on derivatization of the reduced cysteine sites on Trx2 which would be available for modification. In contrast, the oxidized sites are unavailable for derivatization. In brief, cellular proteins were acid precipitated with ice-cold 10% (v/v) trichloric acetic acid (30 min; 4°C). After an acetone wash (30 min; 4°C), samples were resuspended in 20 mM Tris (pH 8.0) containing 15 mM 4-acetoamido−4’-maleimidylstilbene−2,2’-disulfonic acid (AMS, Invitrogen, Carlsbad, CA) which derivatizes the free thiols on Trx2 and increases the protein molecular weight by 1 kDa per two Cys [32, 33]. After a 3 hr incubation at room temperature, the oxidized and reduced forms of Trx2 were separated by nonreducing native polyacrylamide gel electrophoresis, detected by western blot analysis, and quantitated by an Odyssey Scanning system (Li-Cor; Lincoln, NE). Some samples were treated with 5 mM dithiothreitol (30 min) or 1 mM H2O2 (30 min) to obtain the fully reduced and oxidized forms of Trx2, respectively.
TrxR2 redox western blot
To distinguish between the reduced monomers and oxidized dimers of TrxR2, the reduced monomers were alkylated with N-ethylmaleimide (NEM) using previously described methods [34]. In brief, cells were harvested and treated with 100 µl of lysis buffer (40 mM HEPES, 50 mM NaCl, 1 mM EGTA, pH 7.4) containing NEM (50 µM) plus 4 µl of 25% CHAPS (1% final). The cell lysis mixture was incubated (room temperature; 10 min), periodically vortexed, and then centrifuged at 16,000×g for 5 min to remove insoluble materials. After nonreducing electrophoresis, the reduced and oxidized forms of TrxR2 were detected by western blot analysis and analyzed by an Odyssey Scanning system (Li-Cor; Lincoln, NE).
Redox western blot of Prdx hyperoxidation
Analysis of peroxiredoxin hyperoxidation was performed using the method as previously described [35]. In brief, cells were harvested and resuspended in 100 µl of lysis buffer (40 mM HEPES, 50 mM NaCl, 1 mM EGTA, pH 7.4) plus 4 µl of 25% CHAPS (1% final). The lysis buffer did not contain NEM since Prdx monomers undergo dimerization after cell lysis. In contrast, the hyperoxidized forms of Prdxs are unable to dimerize and remain in the monomer form. The cell lysates were incubated at room temperature for 10 min, periodically vortexed, and centrifuged at 16,000×g for 5 min to remove insoluble materials. After nonreducing electrophoresis, Prdx-SO3H was detected by western blotting, and quantitated using the Odyssey Scanning system (Li-Cor; Lincoln, NE).
Results
Chronic ethanol ingestion promoted mitochondrial ROS and oxidation of GSH/GSSG
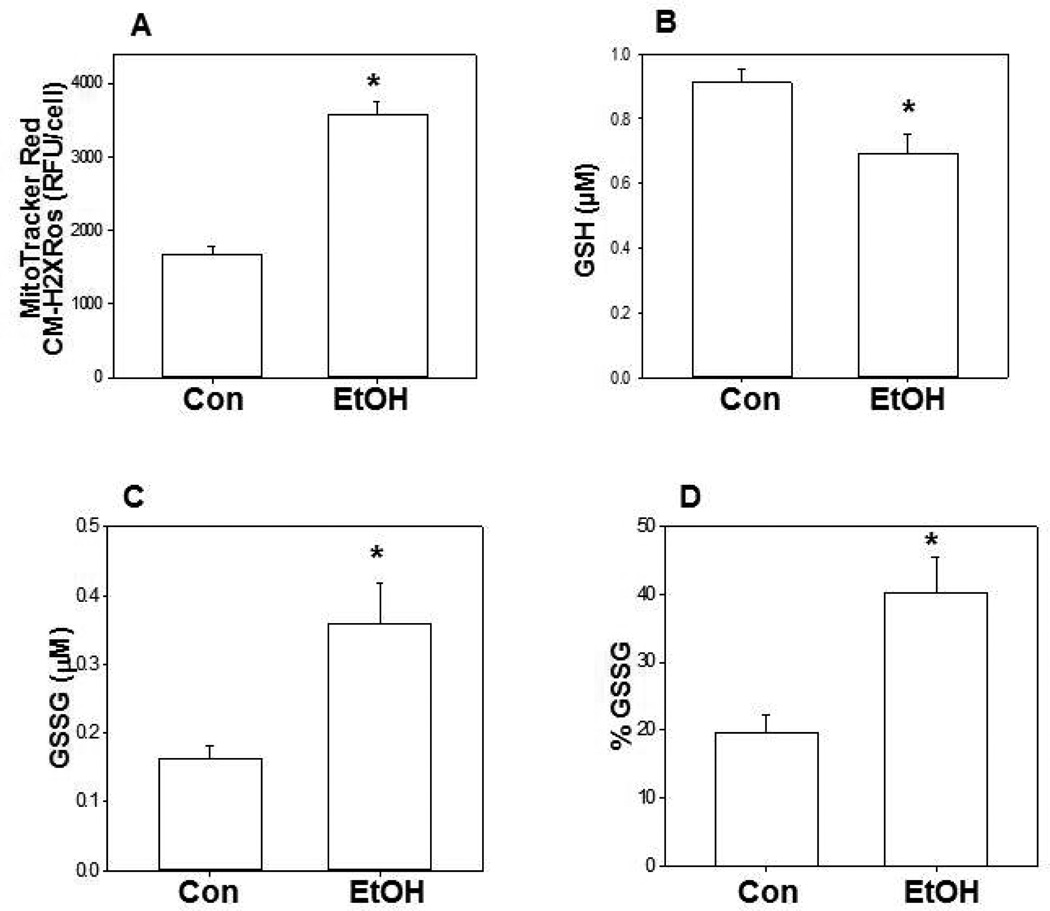
Using MitoTracker Red CM-H2XRos as as the indicator, chronic ethanol ingestion increased mitochondrial ROS in mAMs by ∼2-fold when compared to cells from control mice (Figure 1A). With chronic ethanol ingestion, the mitochondrial GSH pool was decreased by 25% (Figure 1B) while the GSSG pool increased 2.5-fold (Figure 1C) when compared to the control group. This resulted in a 2-fold increase in the percentage of the total pool (GSH + GSSG) present in the oxidized form GSSG (Figure 1D).
Figure 1. Chronic ethanol ingestion oxidized mitochondrial GSH/GSSG.
Mice were given drinking water (with and without 20% ethanol) for 12 wk. The mAMs were isolated and after loading with MitoTracker Red CM-H2XRos, the RFU/cells were determined as an indicator of mitochondrial ROS in mAMs (A). Some isolated AMs were permeabilized with digitonin, and the mitochondria isolated by a Percoll gradient as described in the Methods section for analysis of mitochondrial GSH (B) and GSSG (C) by HPLC analysis. The percentage of GSSG over total reduced and oxidized glutathione was also determined (D). Each value represents the mean ± SE of 5 or more different cell preparations. “Con” denotes the control group and “EtOH” denotes the ethanol-fed group. *P ≤ 0.05 compared with the control group.
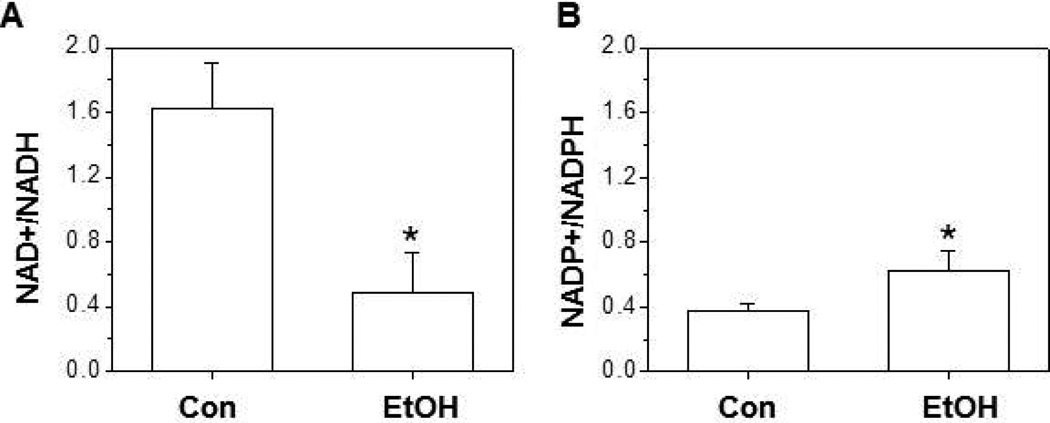
Chronic ethanol exposure decreased the mitochondrial NAD+/NADH ratio but increased the NADP+/NADPH ratio
Inside the cell, the predominant form is oxidized NAD+ where it facilitates the oxidative reactions in the citric acid cycle and glycolysis [36]. In the mAMs from control mice, the mitochondrial ratio of NAD+/NADH was ∼4 times greater than the NADP+/NADPH ratio. However, chronic ethanol ingestion increased the mitochondrial NADH levels by ∼2-fold when compared to the control group (Figure 2A) resulting in a 75% decrease in the NAD+/NADH ratio when compared to the mitochondrial pool of control AMs. In the control mAMs, the mitochondrial pool of NADPH was ∼5-fold lower that then the NADH pool. In contrast, chronic ethanol ingestion promoted a 2-fold increase in the NADP+/NADPH ratio (Figure 2B), primarily driven by the depletion of NADPH.
Figure 2. In the mitochondria of mAMs, chronic ethanol ingestion decreased NAD+/NADH but increased NADP+/NADPH.
mAMs were collected from control (Con) and ethanol (EtOH) fed mice, pooled, and underwent mitochondrial isolation using the Mitochondria Isolation Kit as described in the Methods section. The ratios of NAD+/NADH (A) and NADP+/NADPH (B) were determined by a colorimetric assay (n=5). All values are expressed as mean ± SE and normalized to control conditions. *p<0.05; Ethanol (EtOH) vs Control.
Chronic ethanol exposure induced oxidation of mitochondrial Trx2, Prdxs, and TrxR2
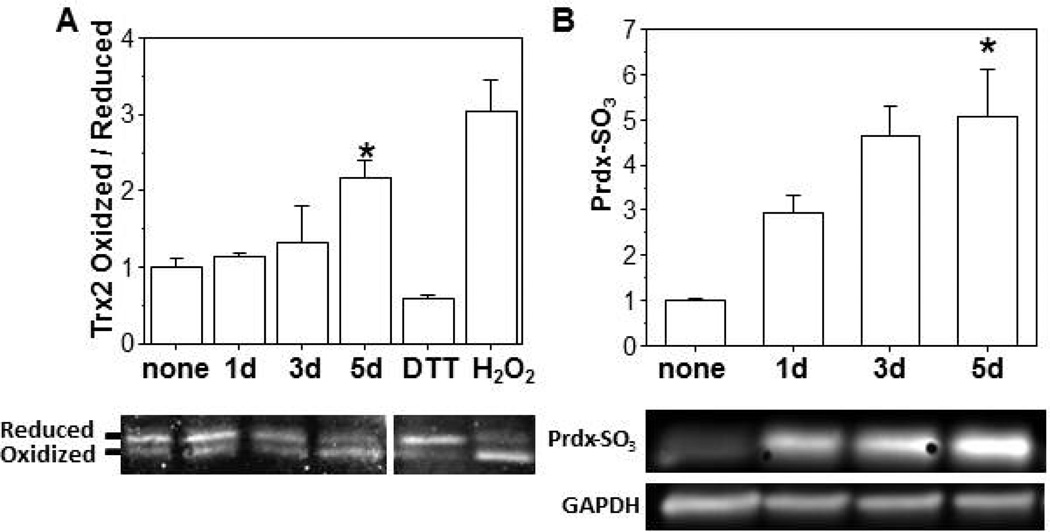
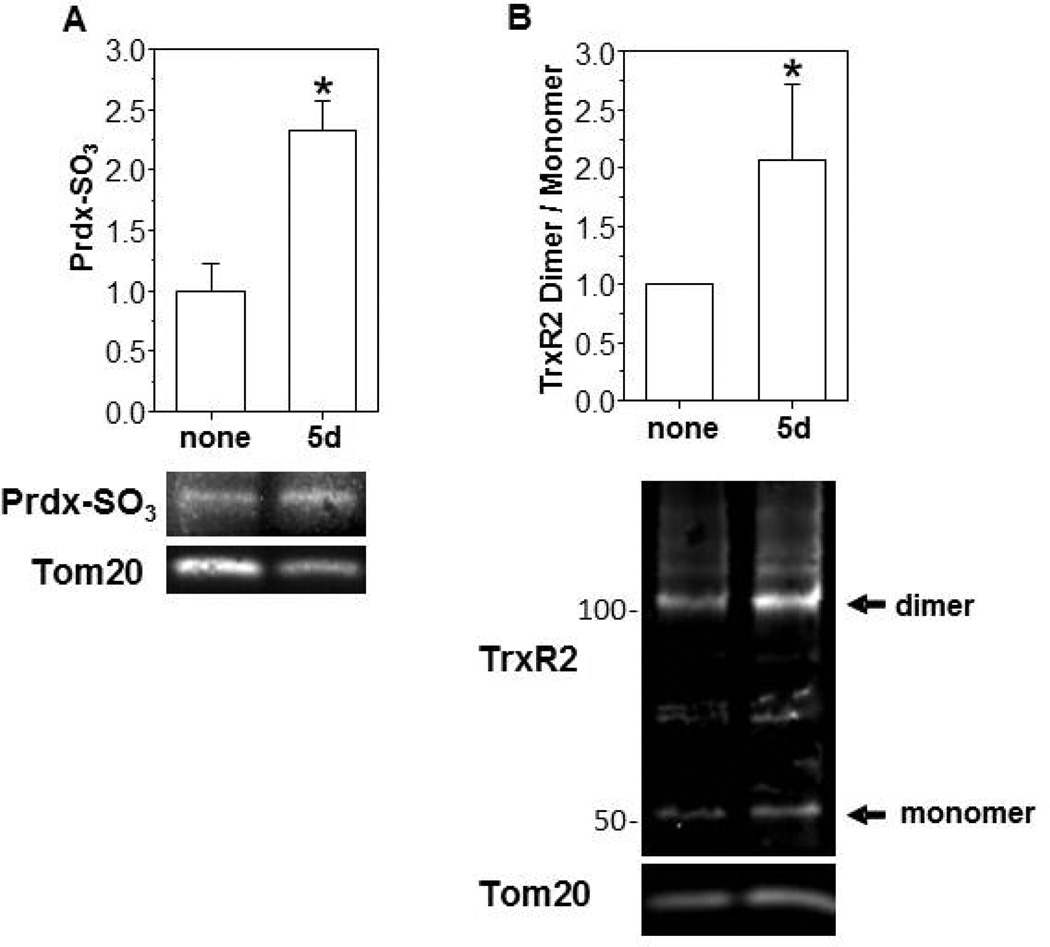
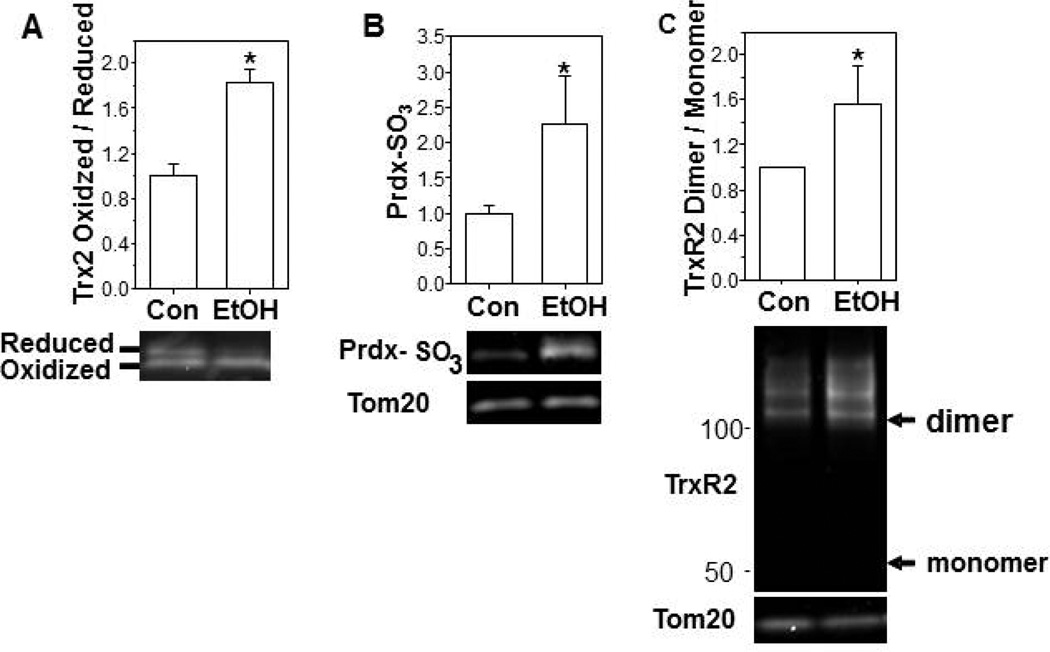
In MHS cells, ethanol (0.2%) exposure promoted the oxidation of Trx2 in a time dependent manner and increased the ratio of oxidized to reduced Trx2 ∼ 2-fold when compared to the control group on day 5 (Figure 3A). The total protein pool of Trx2 was not significantly altered. Over the same 5 d time course, ethanol exposure also promoted a 5-fold increase in Prdx hyperoxidation when compared to controls (Figure 3B) and a ∼2.5-fold increase in the hyperoxidized form of mitochondrial Prdxs (Figure 4A). In addition to oxidation of Trx2, in vitro ethanol exposure also increased TrxR2 oxidation resulting in a 2-fold increase in the ratio of the oxidized (dimer) to reduced (monomer) forms (Figure 4B) but did not alter the total TrxR2 protein pool. We next examined whether the oxidation of the Trx2 redox circuit obtained with in vitro ethanol exposure of an AM cell line was physiologically relevant to chronic ethanol ingestion. Indeed, the ratio of oxidized/reduced Trx2 (Figure 5A) and the hyperoxidized form of Prdx (Figure 5B) were increased ∼2-fold in the mitochondria of mAMs after chronic ethanol ingestion. Likewise, chronic ethanol ingestion increased the ratio of the oxidized (dimer) to reduced (monomer) form of TrxR2 by ∼2-fold (Figure 5C). The total mitochondrial protein pools for Trx2, Prdx, and TrxR2 were not statistically different between the two groups.
Figure 3. In vitro ethanol treatments induced Trx2 oxidation and Prdx hyperoxidation.
MHS cells were treated with 0.2% ethanol (1d, 3d, or 5d; EtOH) and then underwent redox western blot analysis to obtain the reduced and oxidized forms of Trx2 (A). The hyperoxidized form of Prdx was determined by western blot analysis (B). Exposure to dithiolthreitol (DTT; 5 mM) or H2O2 (1 mM) were used as redox controls for the reduced and oxidized forms, respectively. N=3 with each point obtained on a different day. All values are expressed as mean ± SE, and normalized to untreated condition. *p<0.05; 5d of ethanol (EtOH) treatment vs no treatment.
Figure 4. In vitro ethanol treatments induced mitochondrial Prdx hyperoxidation and TrxR2 oxidation.
Cultured MHS cells were either untreated (None) or treated with ethanol (5d, 0.2% EtOH). After the exposure period, mitochondria were isolated with the Mitochondria Isolation Kit from the ethanol treated and untreated MHS cells and the hyperoxidized forms of mitochondrial Prdxs determined (A). Formation of TrxR2 dimers were determined by redox western blot analysis (B). Mitochondrial protein, Tom20, was used as a loading control. N=3 with each point obtained on a different day. All values are expressed as mean ± SE and normalized to the untreated condition. *p<0.05; 5d of ethanol (EtOH) treatment vs no treatment.
Figure 5. Chronic ethanol ingestion induced mitochondrial thiol-disuldide protein oxidation in vivo.
mAMs were collected from control or ethanol-fed mice and cells. For Trx2, the whole cell lysate was used for thiol-disulfide protein redox analysis (A). For analysis of Prdx, the mAM from 3 mice were pooled before isolation of mitochondria by the Mitochondria Isolation Kit and determination of hyperoxidation (B). For TrxR2, the whole AM cell lysate was used for redox western blot analysis (C). The analysis represents at least three independent analyses. All values are expressed as mean ± SE and normalized to control condition. *p<0.05; Ethanol-fed (EtOH) vs Control.
Chronic ethanol ingestion decreased mRNA expression of mitochondrial redox proteins
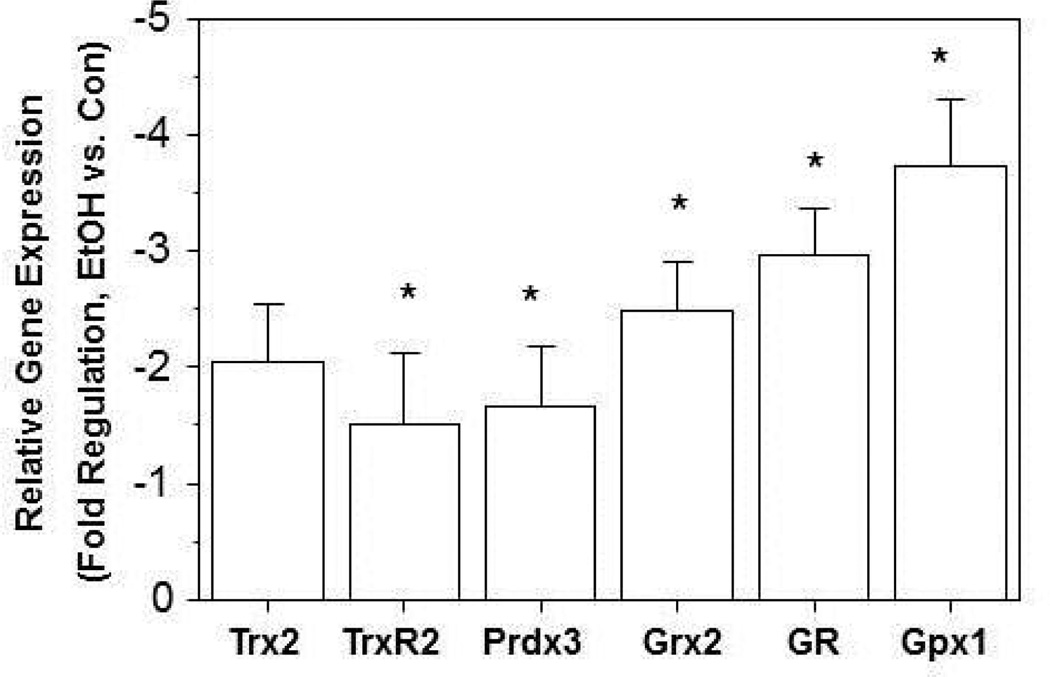
In addition to oxidation, we also examined whether ethanol altered mRNA expression of the proteins involved in the mitochondrial thiol-disulfide antioxidant system. When compared to the mAMs from the control group, chronic ethanol ingestion significantly decreased mRNA expression of Prdx3, and TrxR2 by ∼2-fold (Figure 6). Furthermore, chronic ethanol ingestion decreased the mRNA expression for the GSH related enzymes GSH reductase (GR), GSH peroxidase (Gpx), and glutaredoxin by ∼3-fold when compared to the control mAMs (Figure 6).
Figure 6. Chronic ethanol ingestion decreased mRNA expression of thiol-disulfide proteins.
After mAMs were collected from control and ethanol-fed mice, mRNA levels of Trx2, TrxR2, Prdx3, Grx2, GR and Gpx1 were determined (n ≥ 5). All mRNA values were measured by qRT-PCR, normalized to 18s mRNA and expressed as mean ± SE. *p<0.05 for Ethanol-fed (EtOH) vs Control.
Discussion
In the current study, multiple diverse strategies demonstrated that chronic ethanol ingestion promoted mitochondrial oxidative stress in mAMs. The mitochondrial targeted redox sensitive fluorophore MitoTracker Red CM-H2XRos demonstrated a 2-fold increase in fluorescence suggesting increased mitochondrial ROS. Other markers such as concomitant decreases in GSH, increases in GSSG, and an oxidation of this thiol-disulfide ratio within the mitochondria fraction further validated ethanol-induced mitochondrial oxidative stress. These ethanol-induced decreases in mitochondrial GSH/GSSG in the mAMs were consistent with our previous studies in alveolar type II cells from ethanol-fed rats [30]. Whether the ethanol-induced decreases in the GSH mitochondrial pool are due decreased uptake by the mitochondria, decreased GSH availability to the AM because of decreased GSH in the epithelial lining fluid [37] or increased utilization remains to be determined.
Although oxidized NAD+ is the predominant form in control cells [36], chronic ethanol ingestion decreased the NAD+/NADH ratio, primarily due to increases in the mitochondrial pool of NADH. It is unclear if this increase in NADH is strictly due to ethanol metabolism. However, decreases in the NAD+/NADH ratio can subsequently interrupt the mitochondrial electron chain reactions, the citric acid cycle, and glycolysis resulting in increased ROS production and an imbalance in the redox state of the mitochondria or the cell [38]. In contrast to NAD+/NADH, the reduced form of NADPH predominates where it promotes the reductive reactions involved in biosynthesis [36]. Unlike the ethanol-induced increases in NADH, chronic ethanol ingestion decreased the mitochondrial pool of NADPH, potentially through increased NADPH consumption during the reduction of the oxidized moieties within the redox sensitive thiol-disulfide enzymes. Other possibilities include ethanol-induced impairment of the different mechanisms which maintain NADPH pools in the mitochondria such as the NADP-linked malic enzyme and NADP-linked isocitrate dehydrogenase (outlined in the Graphical Abstract) [39]. There is also inter-conversion of NADH and NADPH within the matrix space of mitochondria through nicotinamide nucleotide transhydrogenase (NNT). Deletion of the NNT gene has been reported for C57BL/6J mice [40] but the lung tissue from the C57BL/6J mice used in the current study expressed the NNT gene (data not shown). Additional studies are needed to determine the impact of chronic ethanol ingestion on these different mechanisms involved in maintaining the mitochondrial NADPH pool in mAMs.
Whatever the mechanisms by which chronic ethanol ingestion results in decreased NADPH, it plays a key role as a donor of electrons that provide reducing equivalents and maintain thiol-disulfide protein activities. Therefore, the ethanol-induced decreases in NADPH could have extensive ramifications in the ability of the mitochondria to sustain these critical redox sensitive signaling pathways such as the thioredoxin redox circuit. In addition to decreased NADPH, in vitro ethanol exposure of MHS cells promoted oxidation of several members of the thioredoxin circuit including Trx2, TrxR2, and Prdx. More physiologically relevant, chronic ethanol ingestion resulted in the oxidation of these same members of the mitochondrial thioredoxin circuit in mAMs. As outlined in the Graphical Abstract, these redox sensitive mitochondrial switches are dynamically interactive. Trx2, the mitochondrial specific form, is a protein-disulfide oxidoreductase which forms an intra-molecular disulfide bonds within its active site when oxidized. As shown in Figure 5, chronic ethanol ingestion promoted Trx2 oxidation and decreased availability of NADPH which would translate into decreased reducing equivalents available to return the oxidized Trx2 to its active reduced state. Reduction of Trx2 to its functional form is also dependent on TrxR2, a FAD-containing seleno-protein belonging to the pyridine nucleotide-disulfide oxidoreductase family [41]. It is the carboxy terminal seleno-cysteine residue of TrxR2 that catalyzes the reduction of oxidized Trx2 using NADPH as the electron donor. With ethanol-induced oxidation of TrxR2 and decreased reducing equivalents from NADPH, the ethanol-induced increases in Trx2 oxidation are not unexpected.
During energy metabolism, the mitochondria are continuously exposed to a high flux of O2•−, H2O2, and related ROS. O2•− is not membrane permeable and is mostly released into the mitochondrial matrix where it undergoes spontaneous or enzyme-catalyzed dismutation to H2O2. Depending on its concentration, H2O2 can either act as a second messenger or as a destructive oxidant, with the cellular switch between these two functions supposedly controlled by Prdx [42]. Therefore, rapid removal and destruction of H2O2 are pivotal in maintaining normal cellular signaling and functions. To protect against oxidative damage, there is an integrated set of thiol-based antioxidant systems within the matrix comprised of the GSH- and Trx2-based systems that support the activities of GSH peroxidase and Prdx systems, respectively.
Within the mitochondria, Prdx3 and Prdx5 are the first line of defense against H2O2 by reducing it to water using reducing equivalents from NADPH supplied by Trx2 and, ultimately, TrxR2 (NAPDH→TrxR2 →Trx2 →Prdx; Graphical Abstract) [42, 43]. The active site for Prdx contains a redox-active cysteine which is oxidized by H2O2 or other peroxides to a sulfenic (-SOH) acid which then reacts with a conserved cysteine on another Prdx subunit to form an intermolecular disulfide. Reduction of this Prdx disulfide is dependent on Trx2 which, in turn, is oxidized to form an intermolecular disulfide bond. Extended high concentrations of H2O2 can lead to an over-oxidized sulfinic (-SO2H) form of Prdx which can be reduced by sulphiredoxin. However, an inability to reduce the oxidized form back to its active state can lead to hyperoxidized Prdx (Prdx-SO3), a form that is irreversible and catalytically inactive [44, 45]. Since the Prdx catalytic cycles require functional Trx2 and TrxR2 as well as NADPH availability [46], ethanol-induced increases in the hyperoxidized form of mitochondrial Prdxs were not unexpected. Equally important, the hyperoxidized form is irreversible and protein synthesis is required to obtain functional mitochondrial Prdxs [44, 45]. However, the ethanol-induced decreases in its mRNA expression suggest that there will also be limited Prdx protein synthesis in mAMs after chronic ethanol ingestion, further exacerbating impaired Prdx clearance of H2O2. The thioredoxin circuit is further compromised by decreased mRNA expression for Trx2 and TrxR2. The inability of the mitochondria to efficiently remove H2O2 may account for the ethanol-induced increases in mitochondrial ROS detected by the mitochondrial-targeted redox sensitive fluorophore.
The other mechanism to remove H2O2 from the mitochondrial matrix is through Grx2 [42]. In contrast to the Trx2 circuit, Grx2 is not reduced by a specific reductase but is non-enzymatically dependent on GSH [42]. Since chronic ethanol ingestion decreased GSH and promoted its oxidation to GSSG, the capacity of oxidized Grx2 to be reduced back to its active form is compromised with chronic ethanol ingestion. The other redox-regulatory function of Grx2, modulation of protein S-glutathionylation, would also be compromised. Since NADPH is the ultimate reductant for Grx2 activity, ethanol-induced decreases in NADPH would also contribute to compromised capacity of Grx2 to respond to S-glutathionylation and altered redox status. Oxidation of the redox-regulatory functions of Grx2 is further complicated by ethanol-induced decreases in mRNA for Grx2 as well as Gpx1 and GR.
In summary, a role for alcohol in mitochondrial oxidative stress has been well documented [47]. However, there is a growing interest in the roles of reversible oxidation of cysteine residues as a means of redox regulation of normal biological signaling within the mitochondria and other subcellular compartments. This is the first study to examine the effects of chronic ethanol ingestion on the oxidation of the mitochondrial thioredoxin circuit. Both in vitro and in vivo ethanol exposures resulted in significant increases in mitochondrial ROS and oxidation of the GSH/GSSG thiol pair within AMs. In addition to NADPH depletion, oxidation of multiple points along the mitochondrial thioredoxin circuit were observed including mitochondrial Prdx hyperoxidation, Trx2 and TrxR2 oxidation, and oxidation of NADP+/NADPH. This integrated network of mitochondrial thiols regulates steady-state H2O2 levels, mitochondrial-dependent metabolism, and modulation of redox-sensitive signaling. For AMs, mitochondria play a critical role in supporting cellular redox signaling and providing the ATP necessary for the energy-dependent processes of phagocytosis and its accompanying respiratory burst. Thus, oxidation of these critical mitochondrial thiol-disulfide redox switches by ethanol may impair AM redox signaling and explain the compromised immune functions in subjects that abuse alcohol.
Highlights.
NADPH and mitochondrial GSH were oxidized by chronic ethanol ingestion.
Mitochondrial Trx2 and TrxR2 were oxidized by chronic ethanol ingestion.
Mitochondrial Prdx3 was irreversibly oxidized by chronic ethanol ingestion.
Oxidation of the Trx2 redox circuit by alcohol would further compromise mitochondrial redox signaling
Acknowledgements
Research reported in this publication was supported by NIAAA of the National Institutes of Health under a T32 training grant T32AA013528 (LY), the Emory Alcohol and Lung Biology Center 1P50AA135757 (LAB), and R01 AA12197 (LAB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AMs
Alveolar macrophages
- Grx
Glutaredoxin
- GSH
Glutathione (reduced)
- GSSG
Glutathione (oxidized)
- Gprx
Glutathione peroxidase
- GR
Glutathione reductase
- NAD
Nicotinamide adenine dinucleotide
- NADP
Nicotinamide adenine dinucleotide phosphate
- Prdx
Peroxiredoxin
- ROS
Reactive oxygen species
- RFU
Relative fluorescent units
- Trx2
Thioredoxin 2
- TrxR2
Thioredoxin Reductase 2
- AMS
4-acetoamido-4’-maleimidylstilbene-2,2’-disulfonic acid
- NNT
Nicotinamide nucleotide transhydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Brown LA, Cook RT, Jerrells TR, Kolls JK, Nagy LE, Szabo G, Wands JR, Kovacs EJ. Acute and chronic alcohol abuse modulate immunity. Alcohol Clin Exp Res. 2006;30:1624–1631. doi: 10.1111/j.1530-0277.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 2.Mehta AJ, Guidot DM. Alcohol abuse, the alveolar macrophage and pneumonia. The American journal of the medical sciences. 2012;343:244–247. doi: 10.1097/MAJ.0b013e31823ede77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown LAS, Ping X-D, Harris FL, Gauthier TW. Glutathione availability modulates alveolar macrophage function in the chronic ethanol-fed rat. Am J Physiol Lung Cell Mol Physiol. 2007;292:L824–L832. doi: 10.1152/ajplung.00346.2006. [DOI] [PubMed] [Google Scholar]
- 4.Brown SD, Gauthier TW, Brown LA. Impaired terminal differentiation of pulmonary macrophages in a Guinea pig model of chronic ethanol ingestion. Alcohol Clin Exp Res. 2009;33:1782–1793. doi: 10.1111/j.1530-0277.2009.01017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown SD, Brown LA. Ethanol (EtOH)-Induced TGF-beta(1) and Reactive Oxygen Species Production Are Necessary for EtOH-Induced Alveolar Macrophage Dysfunction and Induction of Alternative Activation. Alcohol Clin Exp Res. 2012;36:1952–1962. doi: 10.1111/j.1530-0277.2012.01825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi PC, Applewhite L, Ritzenthaler JD, Roman J, Fernandez AL, Eaton DC, Brown LA, Guidot DM. Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol. 2005;175:6837–6845. doi: 10.4049/jimmunol.175.10.6837. [DOI] [PubMed] [Google Scholar]
- 7.Tang SM, Gabelaia L, Gauthier TW, Brown LA. N-acetylcysteine improves group B streptococcus clearance in a rat model of chronic ethanol ingestion. Alcohol Clin Exp Res. 2009;33:1197–1201. doi: 10.1111/j.1530-0277.2009.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner MC, Yeligar SM, Brown LA, Michael Hart C. PPARgamma ligands regulate NADPH oxidase, eNOS, and barrier function in the lung following chronic alcohol ingestion. Alcohol Clin Exp Res. 2012;36:197–206. doi: 10.1111/j.1530-0277.2011.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. J Immunol. 2012;188:3648–3657. doi: 10.4049/jimmunol.1101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CC, Liou SW, Chen WC, Hu FR, Wang IJ, Lin SJ. Coenzyme Q 10 rescues ethanol-induced corneal fibroblast apoptosis through the inhibition of caspase-2 activation. J Biol Chem. 2013 doi: 10.1074/jbc.M112.401844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell T, Chacko B, Ballinger S, Bailey S, Zhang J, Darley-Usmar V. Convergent mechanisms for dysregulation of mitochondrial quality control in metabolic disease: implications for mitochondrial therapeutics. Biochem Soc Trans. 2013:127–133. doi: 10.1042/BST20120231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knockaert L, Fromenty B, Robin MA. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity. FEBS J. 2011;278:4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. [DOI] [PubMed] [Google Scholar]
- 13.Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer F, Hamann A, Osiewacz HD. Mitochondrial quality control: an integrated network of pathways. Trends Biochem Sci. 2012;37:284–292. doi: 10.1016/j.tibs.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Shabalina I, Nedergaard J. Mitochondrial ('mild') uncoupling and ROS production: physiologically relevant or not? Biochem Soc Trans. 2011;39:1305–1309. doi: 10.1042/BST0391305. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial Metabolism of Reactive Oxygen Species. Biochemistry (Moscow) 2005;70:246–264. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 18.Wallace DC, Fan W, Procaccio V. Mitochondrial Energetics and Therapeutics. Annu Rev Pathol Mech Dis. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu S-S. Calcium, ATP and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;2004:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 20.Patenaude A, Murthy MRV, Mirault M-E. Mitochondrial Thioredoxin System. J Biol Chem. 2004;279:27302–27314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP. Disruption of mitochondrial redox circuitry in oxidative stress. Chem Biol Interact. 2006;163:38–53. doi: 10.1016/j.cbi.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Petit PX, Gendron M-C, Schrabtz N, Metivier D, KROEMER G, Maciorowska Z, Sureau F, Koester S. Oxidation of pyridine nucleotides during Fasand ceramide-induced apoptosis in Jurkat cells : correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation. Biochem J. 2001;353:357–367. [PMC free article] [PubMed] [Google Scholar]
- 23.Franco R, Cidlowski JA. Glutathione Efflux and Cell Death. Antioxid Redox Signal. 2012;17:1694–1713. doi: 10.1089/ars.2012.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 25.Manzo-Avalos S, Saavedra-Molina A. Cellular and Mitochondrial Effects of Alcohol Consumption. Int J Environ Res Public Health. 2010;7:4281–4304. doi: 10.3390/ijerph7124281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cook RT, Schlueter AJ, Coleman RA, Tygrett L, Ballas ZK, Jerrells TR, Nashelsky MB, Ray NB, Haugen TH, Waldschmidt TJ. Thymocytes, Pre-B Cells, and Organ Changes in a Mouse Model of Chronic Ethanol Ingestion-Absence of Subset-Specific Glucocorticoid-Induced Immune Cell Loss. Alcohol Clin Exp Res. 2007;31:1746–1758. doi: 10.1111/j.1530-0277.2007.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook RT, Zhu X, Coleman RA, Ballas ZK, Waldschmidt TJ, Ray NB, LaBrecque DR, Cook BL. T-cell activation after chronic ethanol ingestion in mice. Alcohol. 2004;33:178–181. doi: 10.1016/j.alcohol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 28.Jerrells TR, Pavlik JA, DeVasure J, Vidlak D, Costello A, Strachota JM, Wyatt TA. Association of chronic alcohol consumption and increased susceptibility to and pathogenic effects of pulmonary infection with respiratory syncytial virus in mice. Alcohol. 2007;41:357–369. doi: 10.1016/j.alcohol.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song K, Coleman RA, Zhu X, Alber C, Ballas ZK, Waldschmidt TJ, Cook RT. Chronic ethanol consumption by mice results in activated splenic T cells. J Leukoc Biol. 2002;72:1109–1116. [PubMed] [Google Scholar]
- 30.Brown LA, Harris FL, Guidot DM. Chronic ethanol ingestion potentiates TNF-alpha-mediated oxidative stress and apoptosis in rat type II cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L377–L386. doi: 10.1152/ajplung.2001.281.2.L377. [DOI] [PubMed] [Google Scholar]
- 31.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–145. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 32.Damdimopoulos AE, Miranda-Vizuete A, Pelto-Huikko M, Gustafsson J-Å, Spyrou G. Human mitochondrial thioredoxin. Involvement in mitochondrial membrane potential and cell death. J Biol Chem. 2002;277:33249–33257. doi: 10.1074/jbc.M203036200. [DOI] [PubMed] [Google Scholar]
- 33.Halvey PJ, Watson WH, Hansen JM, Go Y-M, Samali A, Jones DP. Compartmental oxidation of thiol–disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–219. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go YM, Pohl J, Jones DP. Quantification of redox conditions in the nucleus. Methods Mol Biol. 2009;464:303–317. doi: 10.1007/978-1-60327-461-6_17. [DOI] [PubMed] [Google Scholar]
- 35.Andrew G, Cox; Christine C, Winterbourn; Hampton MB. Measuring the Redox State of Cellular Peroxiredoxins by Immunoblotting. Methods Enzymol. 2010;474:51–66. doi: 10.1016/S0076-6879(10)74004-0. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls DG, Ferguson SJ, Ferguson S. Bioenergetics. Third Edition. Academic Press; 2002. [Google Scholar]
- 37.Yeh MY, Burnham EL, Moss M, Brown LA. Chronic alcoholism alters systemic and pulmonary glutathione redox status. Am J Respir Crit Care Med. 2007;176:270–276. doi: 10.1164/rccm.200611-1722OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- 39.Hanukoglu I, Rapoport R. Routes and regulation of NADPH production in steroidogenic mitochondria. Endocr Res. 1995;21:231–241. doi: 10.3109/07435809509030439. [DOI] [PubMed] [Google Scholar]
- 40.Ripoll VM, Meadows NA, Bangert M, Lee AW, Kadioglu A, Cox RD. Nicotinamide nucleotide transhydrogenase (NNT) acts as a novel modulator of macrophage inflammatory responses. Faseb J. 2012;26:3550–3562. doi: 10.1096/fj.11-199935. [DOI] [PubMed] [Google Scholar]
- 41.Zhong L, Arner ESJ, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: The active site is a redox-active selenolthiolyselenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proc Natl Acad Sci USA. 2000;97:5854–5859. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bindoli A, Fukuto JM, Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peskin AV, Low FM, Paton LN, Maghzal GJ, Hampton MB, Winterbourn CC. The High Reactivity of Peroxiredoxin 2 with H2O2 Is Not Reflected in Its Reaction with Other Oxidants and Thiol Reagents. J Biol Chem. 2007;282:11885–11892. doi: 10.1074/jbc.M700339200. [DOI] [PubMed] [Google Scholar]
- 44.Rhee SG, Kang SW, Jeong W, Chang T-S, Yang K-S, Woo HA. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr Opin Cell Biol. 2005;17:183–189. doi: 10.1016/j.ceb.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Kim SY, Jo H-Y, Kim MH, Cha Y-y, Choi SW, Shim J-H, Kim TJ, Lee K-Y. H2O2-dependent Hyperoxidation of Peroxiredoxin 6 (Prdx6) Plays a Role in Cellular Toxicity via Up-regulation of iPLA2 Activity. J Biol Chem. 2008;283:33563–33568. doi: 10.1074/jbc.M806578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Go Y-M, Jones DP. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch Biochem Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Feldstein AE, Bailey SM. Emerging role of redox dysregulation in alcoholic and nonalcoholic fatty liver disease. Antioxid Redox Signal. 2011;15:421–424. doi: 10.1089/ars.2011.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]