About 10% of total hip prostheses need revision within a decade after surgery due to mechanical aseptic loosening of the implant, and the number of revisions is expected to rise due to the increasing number of arthroplasties.[1, 2] To address the issue of implant failure, there has been an increased interest in moving to a fixation model that does not involve poly (methyl methacrylate) based bone cement and instead promotes direct integration of the implant with the host tissue by bone in-growth into the porous implant.[3]
Hydroxyapatite (HAP, Ca10(PO4)6(OH)2), the principal inorganic component of human hard tissues such as cortical bone and teeth to which it has a high binding affinity[4], has been investigated as a coating of orthopedic and dental implants[5], and as a component of biopolymer composite films.[6] Various physical coating processes, such as plasma spray coating, sputtering, and pulsed laser deposition on implant surfaces, have been used for the deposition of HAP films on artificial materials.[7] Although the deposition rates of these physical and reactive chemical processes are relatively high, it is often difficult to tune the composition and structure of the products and to obtain a uniform and stable HAP coating because of the thermally unstable character of HAP.[8] Plasma deposited hydroxyapatite coating, which can be up to several hundred microns thick, has a decreased resistance to mechanical shear and a lack of bonding with inefficient stress relief between the layers has been suggested as a possible cause.[9] Additionally, high temperature processes are unfavorable for the incorporation of biologics such as bone morphogenetic protein (BMP) which are potent mediators of bone tissue formation.[10–12]
Electrostatic multilayer assemblies fabricated using the layer-by-layer (LbL) technique[13] offer a unique platform for creating a biomimetic environment by incorporating materials of superior biological and mechanical properties to create a system that supports the growth and proliferation of target cells and allows them to assemble into a functional tissue unit.[14, 15] Precursor stem cells residing in the bone marrow, commonly known as mesenchymal stem cells (MSCs) can differentiate into osteoblasts which are responsible for laying down new bone. In the context of bone tissue engineering, it is crucial to have a substrate with appropriate signals that can support the attachment and direct the growth, proliferation and differentiation of these osteoprogenitor cells into osteoblasts.[16, 17] Incorporation of hydroxyapatite into LbL architectures can make them highly osteoconductive; i.e., capable of specifically supporting the attachment and proliferation of new osteoblasts and osteoprogenitor cells, providing an interconnected structure through which new cells can migrate and new vessels can form.[18] In vivo, the dissolution of HAP releases calcium and phosphate ions from the coating to the surrounding fibrous tissue and leads to the formation of a crystalline carbonated calcium phosphate layer, with the conversion and incorporation into the bone collagenous matrix.[3, 19] Additionally, these hybrid electrostatic films allow the localized introduction of bone morphogenetic protein-2 (BMP-2) which has the ability to differentiate MSCs into osteoblasts, a property referred to as osteoinduction, and has been demonstrated to greatly enhance bone tissue regeneration.[10] The benefits of release of growth factors such as rhBMP-2 include rapid healing of tissue around the implant and greater bone remodeling over shorter time periods[20]; hence there is a therapeutic advantage to the controlled introducing growth factors from the surface of implants during the healing process.[21–23]
Towards developing a versatile implant coating which would confer osteophilic properties, we incorporated hydroxyapatite nanoparticles complexed with chitosan into nanoscale non-degradable electrostatic multilayers which were capped with a degradable poly(β-amino ester) based film incorporating physiological amounts of rhBMP-2. By incorporating elements that mimicked native wound healing processes, we demonstrated an upregulation of osteogenic markers which increased the rate of MSC differentiation and resulted in greater calcium deposition over shorter time periods when compared to a control substrate with no multilayer coating and multilayer films containing only hydroxyapatite or rhBMP-2.
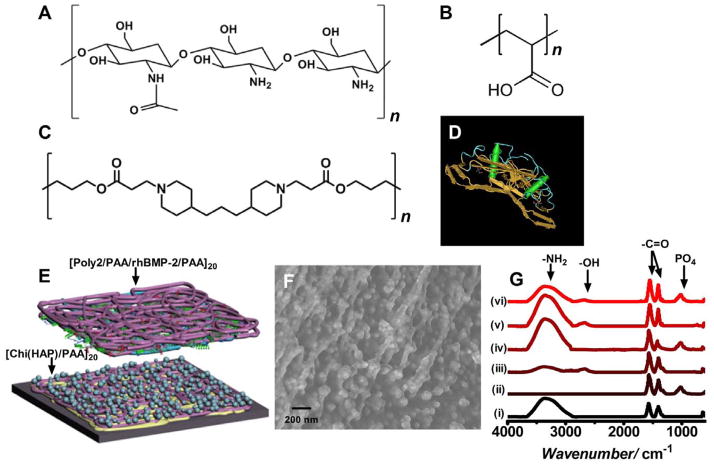
The multicomponent film consisted of a set of osteoconductive base layers under a poly(β-amino ester) based hydrolytically degradable multilayer film for promoting differentiation by introducing controlled amounts of osteoinductive rhBMP-2 (Figure 1). Hydroxyapatite nanoparticles were complexed with chitosan, a linear polysaccharide; in these complexes, it is reported that the calcium ion in HAP interacts with the primary amine group of chitosan.[24] Chitosan is known to be a highly osteoconductive[25, 26], hemostatic material that speeds up wound healing and bone reformation.[27, 28] At acidic and physiological conditions, the amine group in the chitosan structure is protonated (pKa ~ 6.5)[29] conferring a net positive charge to the macromolecule which is necessary for electrostatic film assembly. We anticipated that using poly(acrylic acid) (PAA), a weak polyanion (pKa ~ 4.5), as the complimentary polyanion for this system would yield an ionically crosslinked film which would further assist in stabilizing the HAP complex.[30] ATR IR absorbance spectroscopy indicated the prevalence of a peak at wavenumber 1000cm−1 corresponds to a phosphate group[31] and is present only when hydroxyapatite is a component of the film (Figure 1G). The carbonyl peak present in the spectra can be explained by the use of sodium acetate salt which is present in the polyelectrolyte solutions for partial charge shielding. Substrates coated with HAP particles less than 100 nm in size have been demonstrated to have a greater bone bonding strength compared to the control.[32] Hence, particle aggregation was another consideration, since a uniform surface coating is necessary for creating an optimal osteoconductive surface.[33] A hydrolytically degradable poly(β-amino ester), (Poly2)[34] based multilayer was fabricated atop the osteoconductive [Chi(HAP)/PAA]20 base layers. Under neutral to acidic pH conditions of film fabrication, Poly2 is stable and the amines present along the backbone of Poly2 are protonated, yielding a positive charge necessary for electrostatic LbL assembly. Similarly, rhBMP-2 (Figure 1D; structure extracted using NCBI’s Cn3D MMDB program[35]) is positively charged and stable under acidic conditions (pI ~8.5).[36] The presence of an osteoinductive agent is necessary for bone regeneration.[10] During bone tissue formation and repair processes, rhBMP-2 induces osteoblast differentiation which results in the laying down of new bone that progressively expands to replace damaged tissue.
Figure 1.
Design and fabrication of the osteophilic multilayer architecture. Components of the film (A) Chitosan (75%–85% deacetylated chitin), a polycation (B) Poly(acrylic acid) (PAA), (C) A hydrolytically degradable poly(β-amino ester) (Poly2), a polycation and (D) osteoinductive recombinant human bone morphogenetic protein 2 (rhBMP-2) (E) Schematic of the modular electrostatic assembly. Osteoconductive hydroxyapatite is complexed with chitosan and incorporated into nanoscale thick films along with poly(acrylic acid) in a bilayer architecture. A hydrolytically degradable poly(β-amino ester) based multilayer film incorporating osteoinductive rhBMP-2 lays atop the osteoconductive layer. (F) Scanning electron micrograph of a [Chi(HAP)/PAA]20 bilayer film with the complexed hydroxyapatite nanoparticles. HAP particles complexed to chitosan strands are interwoven in the multilayer architecture (G) IR absorbance spectra of the different components of the osteoconductive layer (i) chitosan (ii) hydroxyapatite (iii) poly(acrylic acid) (iv) [Chitosan/HAP]1 (v) [Chitosan/PAA]20 and (vi) [Chi(HAP)/PAA]20.
These coatings contain osteoconductive and osteoinductive elements, both of which are necessary for bone formation, and are conformal to the complex geometry of the implant for complete biological fixation. We envisioned the controlled degradation of the Poly2 containing layers and the release of rhBMP-2 into the surrounding media and the differentiation of MSCs into osteoblasts which subsequently preferentially adhere to the underlying osteoconductive module consisting of [Chi(HAP)/PAA]20, abutting the substrate, which is not degradable over the time scale of the growth factor release from the [Poly2/PAA/rhBMP-2/PAA]20 multilayers and remains adhered to the substrate, as demonstrated (Figure 2). The growth factor release would not be tunable if chitosan was the only polycation used in the system because it would not be readily degradable; on the other hand, using Poly2 as the only polycation would result in the degradation of the entire multilayer structure, eliminating the ability to enhance bone growth with the permanent osteoconductive base layer. This is also true if a composite [Chi(HAP)/PAA/Poly2/PAA/rhBMP-2/PAA] system had been employed. Rigid hydroxyapatite nanocomposites[37, 38] are used as bone fillers and not suitable as coatings to form new bone tissue independently and hence fix implants to the host tissue.
Figure 2.
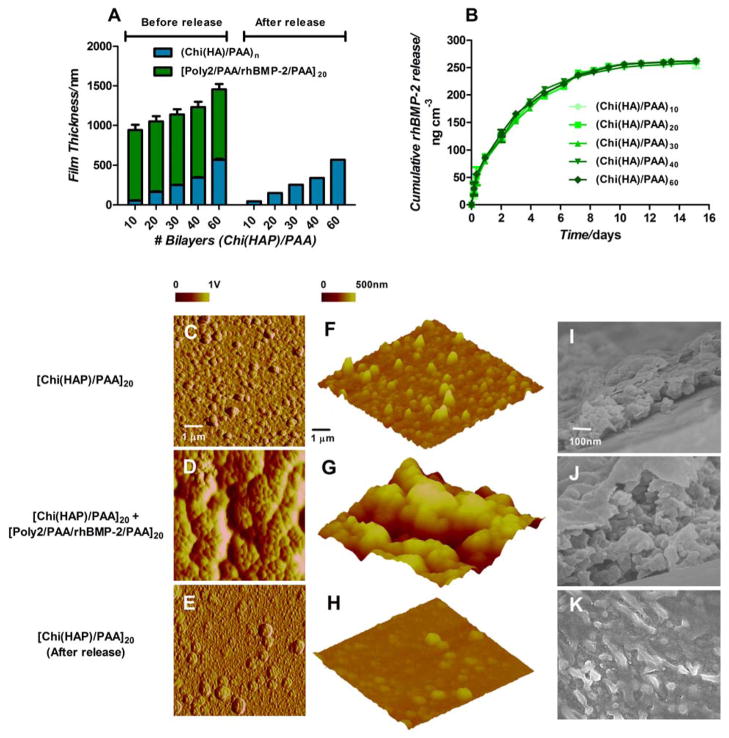
Characteristics of multilayer properties during assembly and degradation. (A) Multilayer film thickness increases linearly with incremental osteoconductive [Chi(HAP)/PAA] layers. Contributions of film thickness from the growth factor eluting layers (green) and hydroxyapatite containing layers (blue) are provided. (B) rhBMP-2 released from the films over 2 weeks. Surface and bulk morphologies of [Chi(HAP)/PAA]20 + [Poly2/PAA/rhBMP-2/PAA]20 multilayer films (C, F) HAP particles are distributed uniformly in the osteoconductive [Chi(HAP)/PAA]20 multilayer surface, and the features are made rougher in (D, G) by the conformal coating of the [Poly2/PAA/rhBMP-2/PAA]20 layers. (E, H, K) Once the growth factor is released by the degradation of the poly(β-amino ester) layers, there is an observable reduction in surface roughness. (F, G, H) Surface height profiles confirm that HAP particles are uniformly distributed with a lack of sequestration in a particular area. (I, J) Corresponding cross sectional scanning electron microscopy images confirm the presence of particles throughout the bulk of the film (I, J, K are at the same scale). C–H are atomic force microscopy images, where C–E are phase contrast images, F–H are height images. I–K are scanning electron microscopy images.
In these osteophilic multilayers, the electrostatic assembly grows linearly with the number of [Chi(HAP)/PAA] baselayers, increasing at 8.98 nm ± 0.13 nm per bilayer (Figure 2A). Consistent surface charge reversal observed after periodic streaming ξ-potential measurements indicated uniform surface charge density consistent with linear film growth (Figure S1, S2). Surface roughness was proportional to film thickness, which increases as particle density within the film increases. We observed that under the conditions of film assembly, HAP particles dispersed uniformly throughout the base film at [Chi(HAP)/PAA]20 without aggregating, as confirmed by EDX and calcium mapping (Figure S4–S8). The nanofilm thickness for [Chi(HAP)/PAA]20 was 163.6 ± 27.3 nm with a roughness of 36.7 ± 4.5 nm (Figure 2A, S3). Simultaneously controlling the quantity of growth factor release from the film is critical, as a large excess can lead to incidences of elevated bone resorption and hematoma.[39] Constant localized extended release of rhBMP-2 represents a more physiological dosing and a top layer of [Poly2/PAA/rhBMP-2/PAA]20 coated on silicon, contained approximately 250 ng/cm3 of growth factor which eluted from the films and thus this architecture was suitable for eluting active growth factor over 2 weeks (Figure 2E). The release of active growth factor from the degradable multilayer films had two phases, as observed previously.[40] The rhBMP-2 released at a relatively constant rate of approximately 30 ng cm−3 day−1 for the first 7 days of release, which was then reduced to 5 ng cm−3day−1 until complete elution. Comparable amount of growth factor was incorporated and released over the same time scale from films independent of the number of osteoconductive base layers. Surface profiles of the films in different states of assembly and degradation indicate a uniform distribution of HAP particles (Figure 2F, 2I) within and on the osteoconductive base layers. The degradable growth factor releasing multilayers coat over the osteoconductive base layers; generally, these films have a consistent roughness of about 200 nm regardless of the size of the features in the underlying base film (Figure 2D, 2G, 2J). After the release of the growth factors, the osteoconductive layer persists and there is a reduction in the surface roughness (Figure 2H, 2K, S3) from the original base layer, suggesting the release of calcium and phosphate ions from the hydroxyapatite into the surrounding media, and thus a reorganization of HAP particles after an exchange of calcium and phosphate ions with the surrounding media which decreases the size and number of particles in the base film. This release is beneficial to the recruitment of MSCs in an in vivo environment.[3] Interestingly, after the release of growth factors, the osteoconductive base layer persists at the same thickness in the dry state.
Pluripotent adult mesenchymal stem cells have the ability to differentiate into a variety of different cell types such as osteocytes, chondrocytes, cardiac muscle and endothelial cells. However, the repair of damaged bone tissue requires migration of MSCs into the damaged area via chemoattractive signals followed by proliferation and differentiation of MSCs to osteoblasts.[41] Such a favorable biological environment is created by the presence of HAP particles which stimulate osteoconduction and rhBMP-2 which provides the primary signal for non-committed MSCs to differentiate into mineral depositing osteoblasts. MSCs were seeded on glass cover slips coated with the osteophilic films. Cells attached to the coated substrate and subsequently responded to substrate signals by appropriate proliferation and differentiation behavior. Alkaline phosphatase (ALP) is a membrane bound glycoprotein which hydrolyzes various monophosphate esters and is expressed at relatively high levels in osteoblasts; it serves as a standard early marker of induction of new bone differentiation from progenitor cells. Alizarin red (AR) stains the calcium deposits and allows quantification of the total deposition in vitro. While the presence of either HAP or rhBMP-2 upregulates ALP and AR production, a maximum in the ALP/AR signal is observed when both HAP and rhBMP-2 are present in the LbL film architecture, indicating a synergistic response by the osteogenic materials after 5 days of culture (Figure 3A, 3B). This is especially pertinent in light of the observation that the AP/AR signals were statistically insignificant for the [Chi(HAP)/PAA]20 and [Poly2/PAA/rhBMP-2/PAA]20.
Figure 3.
Mesenchymal stem cells differentiating into the osteoblast lineage. (A) Alkaline phosphatase (AP) quantification at 5 days after the differentiation cascade is initiated. (B) Alizarin red (AR) staining and quantification for calcium deposits 14 days after mesenchymal stem cells have been in culture under different conditions as indicated. Both AP and AR signals have been represented as fold increase or decrease of the Control +L-AA/β-GP case. A single factor ANOVA test allowed rejection of the null hypothesis for all assays; and a Tukey test between all groups was performed (s.d., n=9, ** p < 0.01; * p < 0.05; ns = not significant all others are p < 0.001). Temporal expression patterns of osteogenic markers in mesenchymal stem cells. Quantification of osteogenic markers (C) Osteocalcin, (D) visualized (green) from differentiating cells by fluorescence over the course of the study. (E) Osteoprotonegrin and (F) Osteopontin expression follows a similar trend to osteocalcin. In all the assays, the synergistic effect of having an osteoconductive and an osteoinductive layer maximizes AP and AR signals. Expression of osteogenic markers may be accelerated and amplified by the introduction of the osteophilic multilayers leading to an environment conducive for bone formation. Peak and cumulative expression levels of the combination osteoconductive and an osteoinductive multilayers are statistically significant compared to other groups (p<0.001, Fig S9–S11).
In order to determine the ability this system to mimic a favorable bone forming environment, we examined the expression of 3 specific protein markers expressed by osteoprogenitor cells during differentiation. Osteocalcin is a small calcium binding protein that tightly adsorbs to hydroxyapatite mineral surfaces, comprising about 10% of the total non-collagen proteins in bone.[42] Similarly, osteopontin is a cell and HAP binding protein synthesized by osteoblasts implicated in bone resorption.[43] Osteoprotonegrin is an osteoblast-secreted receptor, which acts as an antagonist to osteopontin.[44] Together, these form the major group of proteins controlling the bone deposition and remodeling processes. In this study, aliquots of conditioned media, in which the cells were cultured, were collected every two days and assayed for the 3 markers. In all cases there was a peak in the expression levels of the different markers. When [Chi(HAP)/PAA]20 and [Poly2/PAA/rhBMP-2/PAA]20 were presented together, there was a 2 fold upregulation in the peak and cumulative secretion levels of all 3 osteoblast differentiation markers, compared to the control cells in differentiation media and a 20–50% upregulation compared to the individual [Chi(HAP)/PAA]20 and [Poly2/PAA/rhBMP-2/PAA]20 controls indicating that simultaneous presentation of an osteoconductive and an osteoinductive agent may be most beneficial to active bone deposition and remodeling (Figures 3C – 3F and S9 – S11). Additionally, the peak expression of these markers was up to 48 hours earlier when both HAP and rhBMP-2 were present, compared to all the other groups, indicating that the differentiation cascade may be accelerated. This observation is consistent with greater alkaline phosphatase production and calcium deposition for this multilayer combination. Control cells proliferating on plain coverslips do not express any of the markers. In all cases, cell morphology as observed by nuclear (hoechst) and actin (phalloidin) staining indicates that the cells adhere well to the films and are able to grow and proliferate in layers over the course of the study.
In conclusion, we have demonstrated the ability to create a biomimetic modular coating for substrates that simultaneously present hydroxyapatite, an inorganic component of the bone complexed with biocompatible polymers, and rhBMP-2, a bone growth factor to accelerate the regeneration of stable bone tissue. Our findings highlight the characteristics of this alternative uniform coating method for hydroxyapatite, which can be performed at ambient conditions, without the need for high temperature processes. This osteoconductive base layer persists and offers an osteophilic platform on which MSCs can proliferate and differentiate, while the utility of rhBMP-2 has been well documented in mediating interactions between host tissue and implant. This electrostatic multilayer coating delivers physiological nanogram amounts of active rhBMP-2 over two weeks and induces a differentiation response on physiologically responsive MSCs. Introduction of hydroxyapatite and rhBMP-2 upregulated osteogenic markers at earlier times in MSCs which resulted in greater calcium deposition in culture. We have presented an alternative method for the fabrication of hybrid multilayer films consisting of organic and inorganic components for bone tissue healing. The ease of fabrication and the scalability of this platform approach make it easily translatable to a variety of orthopedic implants and devices, with the goal of offering successful and lasting joint replacement, wound healing and repair.
Experimental Section
Materials
Poly (β-aminoester) (Poly2) was synthesized as previously described [34]. All other materials were purchased from Sigma Aldrich (St. Louis, MO) or Invitrogen (Carlsbad, CA) unless otherwise noted.
Preparation of electrostatic films
Hydroxyapatite nanoparticle suspension (10 wt%) was diluted 5 fold in sodium acetate buffer (0.1 M), sterile filtered through a 0.45 μm cellulose acetate membrane (VWR Scientific, Edison, NJ) and added 1:1 (v/v) to chitosan solution in sodium acetate buffer (2 mg/ml). Poly(acrylic acid) and Poly2 dipping solutions were prepared in sodium acetate buffer (1 mg/ml). rhBMP-2 solution, a gift from Pfizer Inc. (Cambridge, MA), was diluted to 250 μg/ml in sodium acetate buffer from a 10mg/ml stock solution. Plasma treated glass or silicon substrates were alternatively dipped into these solutions with an intermediate washing step in water, first to fabricate the osteoconductive [Chi(HAP)/PAA] base layers followed by the degradable [Poly2/PAA/rhBMP-2/PAA] layers. Film thickness was measured using a Dektak 150 Profilometer and surface characterization was performed using a Nanoscope IV atomic force microscope. Scanning electron microscope (SEM) images and energy dispersive X-Ray spectroscopy (EDX) line-scans were performed in a JEOL JSM 6700 scanning electron microscope. All films were fabricated and characterized at least in triplicate.
In vitro cellular assays
Adult mesenchymal stem cells (Lonza, Hopkinton, MA) were cultured in α-MEM supplemented with 20% FBS, 1% penicillin-streptomycin solution and plated on glass cover slips coated with the electrostatic films as described. Alkaline phosphatase and alizarin red assays were routinely performed using a previously described protocol [40, 45]. Media was changed and aliquots from culture were frozen down every 48 hours and assayed for specific bone marker proteins using human bone panel assay 1B (Millipore, Billerica, MA) according to the manufacturer’s protocol. In parallel, cells were fixed and stained for morphology and osteocalcin production for visualization at predetermined time points. All assays were performed at least three times in triplicate.
Supplementary Material
Acknowledgments
This work was supported by the NIH-NIA (5R01AG029601-04), the Institute for Soldier Nanotechnologies (supported by the U.S. Army Research Office under contract W911NF-07-D-0004), the core facilities at the Koch Institute (supported by the NCI under grant 2P30CA014051-39). The authors acknowledge the Langer Lab at MIT for use of equipment and Pfizer Inc. for the supply of rhBMP-2.
Footnotes
Supporting Information is available online from Wiley InterScience or from the author
References
- 1.Ince A, Rupp J, Frommelt L, Katzer A, Gille J, Lohr JF. Clin Infect Dis. 2004;39:1599. doi: 10.1086/425303. [DOI] [PubMed] [Google Scholar]
- 2.Harris WH, Sledge CB. N Engl J Med. 1990;323:725. doi: 10.1056/NEJM199009133231106. [DOI] [PubMed] [Google Scholar]
- 3.Soballe K, Overgaard S. J Bone Joint Surg Br. 1996;78:689. [PubMed] [Google Scholar]
- 4.Lee BH, Oyane A, Tsurushima H, Shimizu Y, Sasaki T, Koshizaki N. ACS Appl Mater Interfaces. 2009;1:1520. doi: 10.1021/am900183e. [DOI] [PubMed] [Google Scholar]
- 5.Knabe C, Klar F, Fitzner R, Radlanski RJ, Gross U. Biomaterials. 2002;23:3235. doi: 10.1016/s0142-9612(02)00078-9. [DOI] [PubMed] [Google Scholar]
- 6.Uchida M, Oyane A, Kim HM, Kokubo T, Ito A. Adv Mater. 2004;16:1071. [Google Scholar]
- 7.Garcia-Sanz FJ, Mayor MB, Arias JL, Pou J, Leon B, Perez-Amor M. J Mater Sci Mater Med. 1997;8:861. doi: 10.1023/a:1018549720873. [DOI] [PubMed] [Google Scholar]
- 8.Raynaud S, Champion E, Bernache-Assollant D, Thomas P. Biomaterials. 2002;23:1065. doi: 10.1016/s0142-9612(01)00218-6. [DOI] [PubMed] [Google Scholar]
- 9.Lynn AK, DuQuesnay DL. Biomaterials. 2002;23:1937. doi: 10.1016/s0142-9612(01)00321-0. [DOI] [PubMed] [Google Scholar]
- 10.Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Science. 1988;242:1528. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- 11.Ai H, Jones SA, Lvov YM. Cell Biochem Biophys. 2003;39:23. doi: 10.1385/CBB:39:1:23. [DOI] [PubMed] [Google Scholar]
- 12.Facca S, Cortez C, Mendoza-Palomares C, Messadeq N, Dierich A, Johnston APR, Mainard D, Voegel JC, Caruso F, Benkirane-Jessel N. Proc Natl Acad Sci U S A. 2010;107:3406. doi: 10.1073/pnas.0908531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decher G. Science. 1997;277:1232. [Google Scholar]
- 14.Kotov NA, Tang ZY, Wang Y, Podsiadlo P. Adv Mater. 2006;18:3203. [Google Scholar]
- 15.Hammond PT. Adv Mater. 2004;16:1271. [Google Scholar]
- 16.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 17.Murphy WL, Hsiong S, Richardson TP, Simmons CA, Mooney DJ. Biomaterials. 2005;26:303. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 18.Greenwald AS, Boden SD, Goldberg VM, Khan Y, Laurencin CT, Rosier RN, Implants CB. J Bone Joint Surg Am. 2001;83A:98. doi: 10.2106/00004623-200100022-00007. [DOI] [PubMed] [Google Scholar]
- 19.Soballe K, Hansen ES, Brockstedt-Rasmussen H, Bunger C. J Bone Joint Surg Br. 1993;75:270. doi: 10.1302/0301-620X.75B2.8444949. [DOI] [PubMed] [Google Scholar]
- 20.Jennissen HP. Ann N Y Acad Sci. 2002;961:139. doi: 10.1111/j.1749-6632.2002.tb03067.x. [DOI] [PubMed] [Google Scholar]
- 21.Linkhart TA, Mohan S, Baylink DJ. Bone. 1996;19:1S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 22.Luginbuehl V, Meinel L, Merkle HP, Gander B. Eur J Pharm Biopharm. 2004;58:197. doi: 10.1016/j.ejpb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Boudou T, Crouzier T, Ren K, Blin G, Picart C. Adv Mater. 2010;22:441. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- 24.Chai C, Leong KW. Mol Ther. 2007;15:467. doi: 10.1038/sj.mt.6300084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muzzarelli RA, Biagini G, Bellardini M, Simonelli L, Castaldini C, Fratto G. Biomaterials. 1993;14:39. doi: 10.1016/0142-9612(93)90073-b. [DOI] [PubMed] [Google Scholar]
- 26.Di Martino A, Sittinger M, Risbud MV. Biomaterials. 2005;26:5983. doi: 10.1016/j.biomaterials.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Bueno EM, Glowacki J. Nat Rev Rheumatol. 2009;5:685. doi: 10.1038/nrrheum.2009.228. [DOI] [PubMed] [Google Scholar]
- 28.Wedmore I, McManus JG, Pusateri AE, Holcomb JB. J Trauma. 2006;60:655. doi: 10.1097/01.ta.0000199392.91772.44. [DOI] [PubMed] [Google Scholar]
- 29.Liu W, Sun S, Cao Z, Zhang X, Yao K, Lu WW, Luk KD. Biomaterials. 2005;26:2705. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 30.Sailaja GS, Ramesh P, Varma HK. J Appl Polym Sci. 2006;100:4716. [Google Scholar]
- 31.Yamaguchi I, Tokuchi K, Fukuzaki H, Koyama Y, Takakuda K, Monma H, Tanaka T. J Biomed Mater Res A. 2001;55:20. doi: 10.1002/1097-4636(200104)55:1<20::aid-jbm30>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 32.Li TT, Lee JH, Kobayashi T, Aoki H. J Mater Sci Mater Med. 1996;7:355. [Google Scholar]
- 33.Habibovic P, Barrere F, van Blitterswijk CA, de Groot K, Layrolle P. J Am Ceram Soc. 2002;85:517. [Google Scholar]
- 34.Lynn DM, Langer R. J Am Chem Soc. 2000;122:10761. [Google Scholar]
- 35.Wang YL, Addess KJ, Chen J, Geer LY, He J, He SQ, Lu SN, Madej T, Marchler-Bauer A, Thiessen PA, Zhang NG, Bryant SH. Nucleic Acids Res. 2007;35:D298. doi: 10.1093/nar/gkl952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheufler C, Sebald W, Hulsmeyer M. J Mol Biol. 1999;287:103. doi: 10.1006/jmbi.1999.2590. [DOI] [PubMed] [Google Scholar]
- 37.Hu QL, Li BQ, Wang M, Shen JC. Biomaterials. 2004;25:779. doi: 10.1016/s0142-9612(03)00582-9. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Zhuang J, Peng Q, Li YD. Adv Mater. 2006;18:2031. [Google Scholar]
- 39.Zara J, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung JU, Kwak J, Wu B, Ting K, Soo C. Tissue Eng Part A. 2011;17:1389. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah NJ, Macdonald ML, Beben YM, Padera RF, Samuel RE, Hammond PT. Biomaterials. 2011;32:6183. doi: 10.1016/j.biomaterials.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruder SP, Kurth AA, Shea M, Hayes WC, Jaiswal N, Kadiyala S. J Orthop Res. 1998;16:155. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 42.Lian JB, Gundberg CM. Clin Orthop Relat Res. 1988;226:267. [PubMed] [Google Scholar]
- 43.Reinholt FP, Hultenby K, Oldberg A, Heinegard D. Proc Natl Acad Sci U S A. 1990;87:4473. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wan M, Shi X, Feng X, Cao X. J Biol Chem. 2001;276:10119. doi: 10.1074/jbc.M006918200. [DOI] [PubMed] [Google Scholar]
- 45.Gregory CA, Gunn WG, Peister A, Prockop DJ. Anal Biochem. 2004;329:77. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.