Abstract
Objective
We evaluated the activity and safety of the combination of topotecan, cisplatin and bevacizumab in patients with recurrent or persistent carcinoma of the cervix.
Methods
Eligible patients had persistent or recurrent cervical cancer not amenable to curative intent treatment. No prior chemotherapy for recurrence was allowed. Treatment consisted of cisplatin 50 mg/m2 day 1, topotecan 0.75 mg/m2 days 1,2 and 3 and bevacizumab 15 mg/kg day 1 every 21 days until disease progression or limiting toxicity. The primary endpoint was progression free survival at 6 months. We explored PET/CT as a potential early indicator of response to therapy.
Results
Twenty-seven eligible patients received a median of 3 treatment cycles (range, 1–19). Median fallow-up was 10 months (range, 1.7–33.4). The 6-month PFS was 59% (80% CI: 46-70%). in 26 evaluable patients, we observed 1 CR (4%; 80% CI: 0.4–14%) and 8 PR (31%; 80% CI: 19–45%) lasting a median of 4.4 months. Ten patients had SD (39%; 80% CI: 25–53%) with median duration of 2.2 months. Median PFS was 7.1 months (80%; CI: 4.7–10.1) and median OS was 13.2 months (80% CI: 8.0–15.4). All patients were evaluated for toxicity. Grade 3–4 hematologic toxicity was common (thrombocytopenia 82% leukopenia 74%, anemia 63%, neutropenia 56%). Most patients (78%) required unanticipated hospital admissions for supportive care and/or management of toxicities.
Conclusion
The addition of bevacizumab to topotecan and cisplatin results in an active but highly toxic regimen. Future efforts should focus on identification of predictive biomarkers of prolonged response and regimen modifications to minimize toxicity.
Keywords: Ceivical cancer, Chemotherapy, Topotecan, Cisplatin, Bevacizumab, PET/CT
Introduction
Cervical cancer remains the third most common and the fourth most lethal cancer in females worldwide [1]. In the United States, it is estimated that 12,170 women were diagnosed and 4,220 women died from cervical cancer in 2012 [2].
Despite survival improvement since the introduction of chemo-radiatiort. the prognosis for patients with cervical cancer remains poor. Failure rates approach 15–30% for patients with stage I–II disease and increases to 40–60% for those with stage III tumors [3–6]. Only 10% of patients with recurrent disease respond to therapy and are alive at 5 years [3,7].
Cisplatin is considered the most active single agent in the setting of recurrent cervical cancer [8]. The Gynecologic Oncology Group (GOG) protocol 179 was the first study to demonstrate a survival benefit with combination therapy. The combination of cisplatin and topotecan was superior to single-agent cispiatin, with the former showing progression free survival (PFS) of 4.6 months, overall survival (OS) of 9.4 months, and a 27% objective response rate [9]. Recent evaluation of other cisplatin-containing combinations has demonstrated comparable survival figures [10].
Angiogenesis plays a key role in cervical carcinogenesis and progression [11–14]. VEGF expression has been associated with deep tumor invasion, pelvic node metastases, pelvic and distant failures as well as impaired survival [14,15]. VEGF inhibition represents an attractive therapeutic strategy for this disease. The GOG conducted a phase II study of bevacizumab for the treatment of persistent or recurrent cervical cancer. The drug was well tolerated and 24% of patients survived progression free for at least 6 months with an objective response rate of 11% [16]. We previously reported on six patients with heavily pre-treated cervical cancer managed with bevacizumab in combination with cytotoxic chemotherapy. One complete and one partial response were observed and two patients had stable (estimated clinical benefit rate of 67%) [17].
While the anti-tumor activity of these agents is promising, there is a critical need to identify biomarkers of therapeutic response. Positron emission tomography-computed tomography (PET/CT) has emerged as an important tool for the management of patients with cervical cancer [18,19]. Interestingly, there is emerging data on the potential role of PET/CT as a clinically useful biomarker for early prediction of therapeutic oncologic responses [20,21].
We sought to evaluate the activity and safety of the combination of topotecan, cispiatin and bevacizumab in patients with incurable recurrent or persistent carcinoma of the cervix. In addition, we explored the potential role of PET-CT with [18F]-fluorodeoxyglucose (FDG) as an early indicator of response to therapy.
Patients and methods
Patient eligibility
Patients with histologically proven recurrent or persistent squamous, adenosquamous or adenocarcinoma of the uterine cervix not amenable to curative treatment were enrolled. Eligibility criteria included age ≥18 years, disease measurable by Response Evaluation Criteria in Solid Tumors (RECIST Version 1.0). [22]; no prior therapy for recurrence and no prior chemotherapy or biologic therapy other than adjuvant cisplatin; GOG performance status of 0 or 1; adequate bone marrow function (defined as absolute neutrophil count (ANC) ≥ 1,500/μL, and platelets ≥ 100,000/μL); adequate renal function (defined as creatinine ≤1.5× the institutional upper limit normal [ULN] or creatinine clearance >60 mL/minute); normal hepatic function (defined as bilirubin ≤ 1.5 × ULN and AST and alkaline phosphatase ≤2.5 × ULN); normal coagulation parameters (defined as prothrombin time [PT] such that the international normalized ratio [INR] <1,5 [INR between 2 and 3 allowed if a patient was on stable dose of therapeutic warfarin] and a PTT <1.2 X control). Minimal peripheral neuropathy was allowed (sensory and/or motor ≤grade 1).
Patients with severe infection; non-healing wound, ulcer or bone fracture, active bleeding, coagulopathy, significant cardiovascular disease, proteinuria (urine protein–creatinine ratio [UPCR] > 1.0), active central nervous system disease, abdominal fistula or abscess, recent surgery and/or history of other malignancy within 5 years of enrollment were ineligible.
The study was reviewed and approved by the institutional review board of each participating institution and all patients gave informed consent prior to enrollment according to local institutional and federal guidelines (registered under ClinicalTrials.gov under identifier: NCT00548418).
Protocol treatment and evaluation
Patients received topotecan 0.75 mg/m2 intravenously (IV) on days 1, 2 and 3; cisplatin 50 mg/m2 IV on day 1 and bevacizumab 15 mg/kg IV on day 1, every 21 days until disease progression or cumulative adverse effects dictating cessation of therapy. Dose modifications were allowed in cases of >10% change in body weight. Cytokine support was permitted at the treating physician’s discretion. G-CSF was indicated by protocol for subsequent cycles in patients who developed grade 3 or 4 neutropenia despite protocol mandated dose reductions.
Toxicity was monitored clinically and by laboratory assessment before each cycle. The National Cancer Institute’s Common Toxicity Criteria, version 3.0 was used to characterize toxicity [23]. Patients were required to have ANC ≥l,50G/μL, platelet count ≥100,000/μL, and creatinine <2.0 mg/dL (or <1.5 mg/dL if prior grade 2 or higher renal toxicity) on the day of re-treatment. There was no dose modification of bevacizumab. Cispiatin dose reductions of 25% in 2 level increments and topotecan dose reduction (to 0.5 mg/m2) were indicated for specific toxicities defined by protocol.
PET/CT scans were performed at study entry and within 7 days prior to third cycle of therapy. Acquisition and analysis of FDC-PET/CT scans were performed according to accepted NCI guidelines [21]. FDG was closed at 0.14–0.21 mCi/kg (actual dose 10–20 mCi) and image acquisition was started 60 ± 10 minutes after FDG injection. A low-dose CT scan was acquired for anatomic localization and attenuation correction using standard parameters. PET data were corrected for dead time, scatter, random and attenuation using standard algorithms. All images were qualitatively and quantitatively interpreted by a single experienced nuclear medicine physician (F.D.). Results from second PET/CT were not provided to the patient or the treating oncologist. For PET/CT interpretation, up to a maximum of 3 lesions (≥ 1.5 cm in smallest dimension) having the highest intensity uptake were identified as target lesions on the baseline FDG PET/CT study. Standardized uptake values (SUV) based on the patient’s total body weight were measured on each target lesion using 3-dimensional ellipsoidal volumes of interest (VOIs) surrounding the tumor. Maximum SUV (SUVmax) was recorded for each VOI.
Results of the PET/CT scan performed prior to cycle 3 were compared to those of the baseline FDG-PET/CT study. Complete metabolic response (CMR) was defined as complete resolution of all metabolically active target and non-target lesions, and no interval development of new lesions. Partial metabolic response (PMR) was recorded when one or both of the following occurred: 1. 20% or greater decrease in maximum SUV from baseline at target lesion with no unequivocal metabolic progression of non-target disease, and no unequivocal new lesions or 2. decrease in total number of non-target lesions, without complete resolution of metabolically active disease, or unequivocal decrease in degree of FDG activity within >50% of the lesions and unequivocal new lesions. Metabolic progression (MP) was defined as: unequivocal development of one or more new metabolically active lesion(s) or 20% or greater increase in maximum SUV from baseline at target lesion(s) or unequivocal increase in FDG activity within non-target lesions or unequivocal increase in size of target or non-target lesions. Other cases were defined as metabolically stable (MS).
Imaging studies for disease evaluation were obtained at study entry and prior to every other treatment cycle. Best response as defined according to RECIST was recorded for each patient based on previously mentioned dedicated imaging studies [22]. OS was defined as time from study entry until death from any cause or date of last contact. PFS was defined as the period from study entry until documentation of disease progression, death or date of last contact, whichever occurred first.
Statistical considerations
The primary endpoint was anti-tumor activity of the protocol therapy measured by the probability of surviving progression free ≥6 months. Results from GOG protocol 179 revealed a median PFS of 4.6 months with approximately 40% of patients remaining progression free at 6 months [9]. Based on a 50% improvement in 6 month PFS (from 40% to 60% free of progression at 6 months) with a one-sided 0.10 significance and 80% power, accrual of 27 subjects would be required. This sample size was based on a normal approximation to binomial proportions for comparing the null versus the alternative hypothesis.
The Kaplan-Meier product limit was used to estimate OS, PFS and 6-month PFS. Response rates with their associated exact binomial confidence intervals were computed.
For the exploratory analysis of PET data response status was dichotomized (CMR + PMR versus MS + MP). We evaluated the association between baseline and change (Δ) in SUVmax and their overall predictive ability on response status. In patients with multiple lesions, the mean of SUVmax of all lesions was also included in the analyses. A Cox proportional hazards model was fitted for PFS and OS respectively, treating SUVmax as an independent variable. SUVmax changes from pre-treatment to follow-up PET scans were also calculated. Due to small sample size, we anticipated that all model-based assessments related to PET performance would likely be subject to relatively large measurement errors and as such would be considered exploratory.
All statistical analyses were performed using SAS version 9.2 (SAS Institutes, Cary, NC).
Results
Patient characteristics
A total of 27 patients were enrolled at three National Cancer Institute designated Comprehensive Cancer Centers from December 2007 through September 2011. All participants received pelvic radiotherapy primarily or adjuvantly as part of their initial treatment plan. Patient and disease characteristics of the study cohort are presented in Table 1.
Table 1.
Patient and clinical characteristics.
|
n = 27 |
||
|---|---|---|
| n | % | |
| Age (years) | ||
| 20-29 | 2 | 7.4 |
| 30-39 | 5 | 18.5 |
| 40-49 | 5 | 18.5 |
| 50-59 | 7 | 25.9 |
| 60-69 | 6 | 22.2 |
| ≥70 | 2 | 7.4 |
| Race | ||
| White | 23 | 85.2 |
| African American | 4 | 14.8 |
| Performance status | ||
| 0 | 14 | 61.9 |
| 1 | 13 | 48.1 |
| Stage | ||
| I | 9 | 33.3 |
| II | 8 | 29.6 |
| III | 8 | 29.6 |
| IV | 2 | 7.4 |
| Histologic type | ||
| Squamous cell | 18 | 66.6 |
| Adenocarcinoma | 9 | 33.3 |
| Grade | ||
| 1 (well differentiated) | 2 | 7.4 |
| 2 (moderately differentiated) | 11 | 40.7 |
| 3 (poorly differentiated) | 14 | 51.9 |
| Prior hysterectomy | ||
| No | 22 | 81.5 |
| Yes | 5 | 18.5 |
All patients received at least one cycle of study treatment. In total 143 cycles were administered with a median of 3 cycles per patient (range 1–19). As of December of 2012, all patients discontinued protocol treatment; 11 for disease progression, 2 for refusal of additional therapy (both with stable disease), 1 for severe cisplatin hypersensitivity and 13 due to toxicity (4 renal, 4 hematologic, 3 global deterioration, 1 gastrointestinal fistula and 1 hypertension). At last contact, seventeen patients had died of disease progression (63%), 8 were alive with disease (30%) and 2 were alive with no evidence of disease (7%).
Adverse events
All patients were evaluated for toxicity. Sixteen patients (59%) had at least one cycle delayed due to toxicity. A total of 21 patients (78%) required at least one unanticipated hospital admission for supportive therapy and/or management of toxicities derived from protocol treatment. One patient died within 30 days from protocol therapy due to “sudden cardiovascular collapse” possibly related to protocol treatment. Table 2 illustrates all adverse effects observed during the study.
Table 2.
Adverse events.
| Grade (no. of patients) |
||||||
|---|---|---|---|---|---|---|
| Adverse effect | 0 | 1 | 2 | 3 | 4 | 5 |
| Leukopenia | 2 | 2 | 3 | 12 | 8 | 0 |
| Neutropenia | 6 | 2 | 4 | 4 | 11 | 0 |
| Thrombocytopenia | 1 | 1 | 3 | 7 | 15 | 0 |
| Anemia | 0 | 1 | 9 | 15 | 2 | 0 |
| Allergy | 24 | 2 | 1 | 0 | 0 | 0 |
| Auditory | 26 | 0 | 1 | 0 | 0 | 0 |
| Cardiovascular | 12 | 4 | 7 | 2 | 1 | 1 |
| Coagulation | 18 | 8 | 1 | 0 | 0 | 0 |
| Constitutional | 1 | 9 | 11 | 6 | 0 | 0 |
| Dermatologic | 13 | 7 | 7 | 0 | 0 | 0 |
| Endocrine | 26 | 1 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 1 | 8 | 13 | 5 | 0 | 0 |
| Genitourinary/renal | 14 | 2 | 3 | 8 | 0 | 0 |
| Hemorrhage | 21 | 3 | 0 | 3 | 0 | 0 |
| Infection | 15 | 0 | 7 | 5 | 0 | 0 |
| Lymphatic | 20 | 5 | 2 | 0 | 0 | 0 |
| Metabolic | 1 | 5 | 8 | 12 | 1 | 0 |
| Musculoskeletal | 24 | 3 | 0 | 0 | 0 | 0 |
| Neurologic | 12 | 7 | 6 | 2 | 0 | 0 |
| Visual | 26 | 0 | 1 | 0 | 0 | 0 |
| Pain | 3 | 6 | 9 | 6 | 3 | 0 |
| Pulmonary | 16 | 9 | 1 | 1 | 0 | 0 |
All patients (n = 27) were evaluable for toxicities.
Severe hematologic toxicity was frequent. Packed red blood cell transfusions were required by 21 patients (78%) and platelets by 8 patients (30%). Severe (grade 3–4) thrombocytopenia was observed in 22 patients (82%), leukopenia in 20 (74%). anemia in 17 (63%) and neutropenia in 15 patients (56%). Cytokine support (G-CSF) was prescribed for 20 patients (70%).
Serious non-hematologic events included grade 3–4 metabolic/laboratory abnormalities in 12 patients (44%), pain in 9 patients (33%) and genitourinary/renal toxicity in 8 patients (30%). One vesicovaginal and one rectovaginal fistula were documented.
Activity of topotecan, cispiatin and bevacizumab
Twenty six patients were evaluable for response. The overall response rate was 35% (80% CI: 22–49%). One confirmed complete response (4%, 80% CI: 0.4–14%) and 8 partial responses (31%, 80% CI: 19–45%) lasting a median 4.4 months (range 1.6–9.5 months) were documented. Another 10 patients (39%, 80% CI: 25%-53%) had stable disease with a median duration of 2.2 months (range 0.7–9.6 months) and 7 had progressive disease (30%).
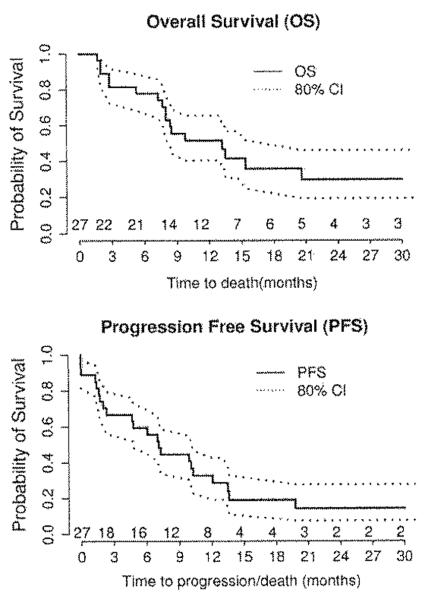
Median follow up for the study cohort was 10.0 months (range 1.7–33.4 months). The probability of surviving progression free at 6 months was 59% (80% CI: 46–70%). Median PFS and OS for all patients were 7.1 months (80% CI: 4.7–10.1 months) and 13.2 months 80% CI: 8.0–15.4 months) respectively (Fig. 1).
Fig. 1.
Kaplan-Meier curves for: A. overall survival (OS) and E. Progression free survival (PFS). Numbers under the curves indicate the number of survivors at each censor point. Dotted lines indicate boundaries of 80% confidence intervals (80% CI).
PET/CT as a predictive biomarker
Relevant PET/CT data are presented in Supplementary Table 1. Pre-treatment average maximal SUV (SUVmax-T0) was 11.05 ± 5.10 among responders (PR/CR) and 12.55 ± 8.12 among non-responders (PD/SD) (P = 0.82). SUVmax-T0 was not associated with OS or PFS (P = 0.74 and P = 0.79, respectively). The baseline average SUVmax (SUVaverage-T0) at all target lesions was also evaluated and was not associated with response (9.74 ± 4.42 and 10.50 ± 6.25 for responders and non-responders respectively, P = 0.86), OS or PFS (P = 0.67 and P = 0.63, respectively).
As part of our analyses, we also estimated the predictive value of the change in SUVmax from baseline to post-treatment scans (Δ SUVmax). We found such variation to be non-predictive of response (P = 0.77) or survival (P = 0.12 and P = 0.26, respectively for OS and PFS).
Discussion
Since the inclusion of cispiatin in the treatment regimens for cervical cancer, various studies have attempted to improve survival rates by incorporating additional cytotoxic drugs. Moore et al. demonstrated that the addition of paclitaxel resulted in significant improvement in response (19% to 36%, P = 0.002) with an associated modest 2 month improvement in median PFS (P < 0.001) [24]. Unfortunately, there was no OS benefit. Long et al., in GOG-179, studied the combination cispiatin and topotecan for patients with advanced disease, The combination resulted in improved response rates (27% vs. 13%, P = 0.004). Notably, an improvement in median PFS (1.7 months. P = 0.007) and median OS (2.9 months, P = 0.02) was verified for the first time in this trial. More recent evaluation of other cisplatin-containing combinations has demonstrated comparable survival figures [10].
Current approaches to novel anticancer therapies have focused on targeted agents. Biologic anti-angiogenic agents have gained remarkable interest for the treatment of a large number of malignancies. There is a clear rationale for incorporating anti-angiogenic agents in the treatment of cervical cancer. It appears that cervical neovascularization begins early in the carcionogenic process. Comparisons of normal cervical tissue, dysplastic epithelium and invasive cervical cancer have shown that MVD progressively increases with advancing disease [12,25]. The importance of VEGF expression during this process has been demonstrated in a number of studies [13–15]. Similarly, hypoxia inducible factor 1 (HIF-1) has been shown to represent a key effector of vasculoneogenesis in cervical cancer. HIF-1 targets VEGF and as such represent an attractive therapeutic target. Interestingly, among the most active HIF-1 targeting compounds are the camptothecin analogs including the topoisomerase I inhibitor topotecan [26]. This biologic rationale along with aforementioned data demonstrating important clinical activity, prompted us to study the combination of topotecan, cispiatin and bevacizumab for patients with incurable cervical cancer.
In this study, we observed significant activity for the protocol combination. We observed an overall response rate of 35% including one (4%) complete response. Furthermore, the observed 6-month PFS of 59% with median PFS of 7.1 and OS of 13.2 months observed in the current study, compare favorably with recent historic data in this patient population [10]. When studied as a single agent by the GOG, bevacizumab was to be considered interesting if an overall response rate of 16% was observed (interestingly, that study observed an overall response rate of 11%) [16]. For reference, combination topotecan and cispiatin yielded response rates of 27% with median PFS and OS of 4.6 and 9.4 months, respectively [9]. The response rate in our study was similar to that seen with combination paclitaxel and cispiatin (36%) [24]. However, our regimen resulted in modestly longer PFS (7.1 vs. 4.8 months) and OS (13.2 vs. 9.7 months). Not surprisingly, the combination of cispiatin. topotecan and bevacizumab had a higher response rate compared to bevacizumab alone (11%) [16].
Unfortunately, the experimental regimen resulted in excessive toxicity, with nearly 80% of patients requiring unanticipated hospital admissions to manage adverse events and/or to provide supportive care. We also observed one death possibly related to therapy and a high rate of severe constitutional toxicities which lead approximately 19% of patients discontinuing protocol treatment (due to severe constitutional deterioration and/or refusal to continue treatment). Despite remarkable activity, the still disappointing OS results and high toxicity suggest that alternate regimens for this poor prognosis population must be developed.
It is important to minimize toxicities in patients with incurable disease unlikely to benefit from therapy. Given the prognostic value of PET in cervical cancer and its ability to predict response in therapeutic cancer trials we explored the potential prognostic value of this imaging modality for patients undergoing treatment in this protocol [18–20,27]. A recently published trial demonstrated the predictive ability of PET among patients being treated for follicular lymphoma 127). The current study was limited by the small number and frequency of missing data for some of the participants. Therefore, definitive trends in relation to PET/CT and prediction of response were not observed. Interestingly, 50% of patients with disease progression had increases in SUVmax. Such increase was only observed in 11% and 25% of patients with stable disease and partial responses respectively. Similarly, we did not observe progression in any case with a ΔSUVmax greater than −42%, suggesting that drops in SUVmax of such magnitude are associated with increased likelihood of deriving clinical benefit (Supplementary Table 1). Similar conclusions have been observed when PET/CT has been utilized to monitor early response to neoadjuvant chemotherapy for the management of head and neck cancer [28].
Although our study demonstrates important activity for the combination of topotecan, cisplatin and bevacizumab in patients with recurrent or persistent cervical cancer, the toxicities associated with this regimen are disappointing. The GOG activated protocol 240 in April of 2009. The design of the trial was based on non-inferiority of cisplatin/paclitaxel (published in 2009 after initiation of this study) [10]. GOG-240 randomized patients with stage IVB. recurrent or persistent carcinoma of the cervix to cisplatin/paclitaxel with or without bevacizumab versus topotecan/paclitaxel with or without bevacizumab. The study completed accrual in January of 2012. We hope that results from that trial will help clarify the added value of bevacizumab and the role of cisplatin re-treatment in the era of chemoradiotherapy for the management of patients with recurrent or persistent cervical cancer.
Supplementary Material
HIGHLIGHTS.
The experimental regimen is very active for advanced cervical cancer.
This regimen results in high toxicity.
Biomarkers of response and regimen modifications to minimize toxicity are needed.
Footnotes
Support: This investigator initiated trial has been supported by research grants from GSK and Genentech. This work was also supported by the Alvin J. Siteman Cancer Center’s Biostatistics Core and Imaging and Response Assessment Core. The Siteman Cancer Center is supported by NCI Cancer Center Support Grant P30 CA91S42.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ygyno.2013.04.009.
Conflict of interest statement This investigator initiated trial has been supported by research grants from GSK and Genentech. Dr. Alvarez Secord reports having received additional research funding from GSK and Genentech. Additionally, she has received honoraria for Advisory Board from Genentech. All other authors have declared that they have no conflicts of interest.
References
- [1].Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [3].Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al. Recurrent squamous cell carcinoma of cervix after definitive radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:249–57. doi: 10.1016/j.ijrobp.2004.02.044. [DOI] [PubMed] [Google Scholar]
- [4].Okawa T, Kita M, Goto M, Tazaki E. Radiation therapy alone in the treatment of carcinoma of the uterine cervix: review of experience at Tokyo Women’s Medical College (1969–1983) Int J Radiat Oncol Biol Phys. 1987;13:1845–9. doi: 10.1016/0360-3016(87)90350-6. [DOI] [PubMed] [Google Scholar]
- [5].Perez CA, Camel HM, Kuske RR, Kao MS, Galakatos A, Hederman MA, et al. Radiation therapy alone in the treatment of carcinoma of the uterine cervix: a 20-year experience. Gynecol Oncol. 1986;23:127–40. doi: 10.1016/0090-8258(86)90216-7. [DOI] [PubMed] [Google Scholar]
- [6].Sommers GM, Grigsby PW, Perez CA, Kamel HM, Kao MS, Galakatos AE, et al. Outcome of recurrent cervical carcinoma following definitive irradiation. Gynecol Oncol. 1989;35:150–5. doi: 10.1016/0090-8258(89)90033-4. [DOI] [PubMed] [Google Scholar]
- [7].Wang CJ, Lai CH, Huang HJ, Hong JH, Chou HH, Huang KG, et al. Recurrent cervical carcinoma after primary radical surgery. Am J Obstet Gynecol. 1999;181:518–24. doi: 10.1016/s0002-9378(99)70486-2. [DOI] [PubMed] [Google Scholar]
- [8].Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1079–85. doi: 10.1200/JCO.1985.3.8.1079. [DOI] [PubMed] [Google Scholar]
- [9].Long HJ, III, Bundy BN, Grendys EC, Jr, Benda JA, McMeekin DS, Sorosky J, Fiorica JV. Gynecologic Oncology Group Study. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2005;23:4626–33. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- [10].Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent or persistent cervical carcinoma: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:4649–55. doi: 10.1200/JCO.2009.21.8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zaghloul MS, El Naggar M, El Deeb A, Khaled H, Mokhtar N. Prognostic implication of apoptosis and angiogenests in cervical uteri cancer. Int J Radiat Oncol Biol Phys. 2000;48:1409–15. doi: 10.1016/s0360-3016(00)00800-2. [DOI] [PubMed] [Google Scholar]
- [12].Ozalp S, Yalcin OT, Oner U, Tanir HM, Acikalin M, Sarac I. Microvessel density as a prognostic factor in preinvasive and invasive cervical lesions. Eur J Gynaecol Oncol. 2003;24:425–8. [PubMed] [Google Scholar]
- [13].Lee JS, Kim HS, Park JT, Lee MC, Park CS. Expression of vascular endothelial growth factor in the progression of cervical neoplasia and its relation to angiogenesis and p53 status. Anal Quant Cytol Histol. 2003;25:303–11. [PubMed] [Google Scholar]
- [14].Lee IJ, Park KR, Lee KK, Song JS, Lee KG, Lee JY, et al. Prognostic value of vascular endothelial growth factor in Stage IB carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 2002;54:768–79. doi: 10.1016/s0360-3016(02)02970-x. [DOI] [PubMed] [Google Scholar]
- [15].Gaffney DK, Haslam D, Tsodikov A, Hammond E, Seaman J, Holden J, et al. Epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) negatively effect overall survival in carcinoma of the cervix treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:922–8. doi: 10.1016/s0360-3016(03)00209-8. [DOI] [PubMed] [Google Scholar]
- [16].Monk BJ, Sill MW, Burger RA, Gray HJ, Buekers TE, Roman LD. Phase II trial of bevacizumab in the treatment of persistent or recurrent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2009;27:1069–74. doi: 10.1200/JCO.2008.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wright JD, Viviano D, Powell MA, Gibb RK, Mutch DG, Grigsby PW, et al. Bevacizumab combination therapy in heavily pretreated. recurrent cervical cancer. Gynecol Oncol. 2006;103:439–93. doi: 10.1016/j.ygyno.2006.03.023. [DOI] [PubMed] [Google Scholar]
- [18].Kidd EA, Siegel BA, Dehdashti F, Rader JS, Mutch DG, Powell MA, et al. Lymph node staging by positron emission tomography in cervical cancer: relationship to prognosis. J Clin Oncol. 2010;28:2103–13. doi: 10.1200/JCO.2009.25.4151. [DOI] [PubMed] [Google Scholar]
- [19].Schwarz JK, Siegel BA, Dehdashti F, Grigsby PW. Metabolic response on post-therapy FDG-PET predicts patterns of failure after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2012;83:185–90. doi: 10.1016/j.ijrobp.2011.05.053. [DOI] [PubMed] [Google Scholar]
- [20].Trotman J, Fournier M, Lamy T, Seymour JF, Sonet A, Janikova A, et al. Positron emission tomography-computed tomography (PET-CT) after induction therapy is highly predictive of patient outcome in follicular lymphoma: analysis of PET-CT in a subset of PRIMA trial patients. J Clin Oncol. 2011;23:3194–200. doi: 10.1200/JCO.2011.35.0736. [DOI] [PubMed] [Google Scholar]
- [21].Shankar LK, Hoffman JM, Bacharach S, Graham MM, Karp J, Lammertsma AA, et al. Sullivan D; National Cancer Institute. Consensus recommendations for the use of 18 F-FOG PET as an indicator of therapeutic response in patients in National Cancer Institute Trials. J Nucl Med. 2006;47:1059–66. [PubMed] [Google Scholar]
- [22].Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- [23].National Cancer Institute: Common Terminology Criteria for Adverse Events v3.0. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/etc.http://etc_30.
- [24].Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB. recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–9. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- [25].Smith-McCune KK, Weidner N. Demonstration and characterization of the angiogenic properties of cervical dysplasia. Cancer Res. 1994;54:800–4. [PubMed] [Google Scholar]
- [26].Chau NM, Rogers P, Aherne W, Carroll V, Collins I, McDonald E, et al. Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible faaor-1alpha induction in response to hypoxic stress and growth factors. Cancer Res. 2005;65:4918–28. doi: 10.1158/0008-5472.CAN-04-4453. [DOI] [PubMed] [Google Scholar]
- [27].Dupuis J, Berriolo-Riedinger A, Julian A, Brice P, Tychyj-Pinel C, Tilly H, et al. Impact of [18F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunotherapy: a prospective study from the Groupe d’Etudes des Lymphomes de I’Adulte and GOELAMS. J Clin Oncol. 2012;30:4317–22. doi: 10.1200/JCO.2012.43.0934. [DOI] [PubMed] [Google Scholar]
- [28].Kikuchi M, Nakamolo Y, Shinohara S, Fujiwara K, Yamazaki H, Kanazawa Y, et al. Early evaluation of chemotherapy response using FDG-PET/CT predicts survival prognosis in patients with head and neck squamous cell carcinoma. Int J Clin Oncol. 2012 doi: 10.1007/s10147-012-0393-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.