SUMMARY
There are seven distinct β-tubulin isotypes and eight α-tubulin isotypes in mammals that are hypothesized to have tissue- and cell-specific functions. There is an interest in the use of tubulin isotypes as prognostic markers of malignancy. βV-tubulin, like βIII-tubulin, has been implicated in malignant transformation and drug resistance, however little is known about its localization and function. Thus, we generated for the first time, a rabbit polyclonal antibody specific for human βV-tubulin. The antibody did not cross-react with mouse βV-tubulin or other human β-tubulin isotypes and specifically labeled βV-tubulin by immunoblotting, immunofluorescence and immunohistochemistry. Immunohistochemistry of various human normal tissues revealed that βV-tubulin was expressed in endothelial cells, muscle and cells with muscle differentiation, structures with transport and/or secretary function such as renal tubules, pancreatic ducts and bile ducts, and epithelium with secretary function such as prostate. βV-tubulin was also specifically expressed in pancreatic islets and intratubular germ cell neoplasia, where it may have diagnostic utility. In a small number of malignancies, breast, lung and ovarian cancers, βV-tubulin was aberrantly expressed, suggesting that this isoform may be associated with tumorigenesis. Thus, βV-tubulin expression is a potentially promising prognostic marker of malignancy.
INTRODUCTION
Mammals express seven distinct β-tubulin isotypes, I, II, III, IVa, IVb, V, and VI and eight α-tubulin isotypes 1–3. Heterodimers of α- and β-tubulin assemble head to tail to form protofilaments whose lateral assembly constitutes the microtubule wall. Each of the multiple α- and β-tubulin isotypes are highly conserved, and are identified primarily by their specific C-terminus sequence 2, 4. Several isotype specific antibodies have been made by designing epitopes to these unique regions 5.
Abnormal distribution and expression of α- and β- tubulin isotypes have been reported in numerous malignancies 6, thus altered tubulin isotype expression may promote a more aggressive and drug resistant tumor phenotype 7. For example, βIII-tubulin is overexpressed in ovarian, lung, prostate, and breast cancer cell lines 7, 8, and numerous studies have identified it as a prognosticator of poor survival 9, 10 while others have shown that βIII overexpression may be associated with response to microtubule interacting drugs11, 12. Furthermore, βIII-tubulin overexpression is associated with cell-based models of acquired Taxol resistance 7, 11, 12, and more recently resistance to DNA-damaging drugs 13. Most of the evidence that has led to the association between βIII-tubulin expression and poor survival were derived from immunohistochemistry using βIII-tubulin specific antibodies 9, 12, 14. Therefore, studies addressing the distribution and expression of the various tubulin isotypes in normal and malignant tissue are limited by availability and specificity of antibodies. For this reason, little is known about the expression of α-tubulin isotypes or some of the less well-characterized β-tubulin isotypes, such as βV. A mouse BV-antibody has been developed and well characterized5, however due to the specificity of the antibody it cannot be used to detect human BV-tubulin.
βV-tubulin mRNA has been detected in most human tissue types using qRT-PCR15 and it has been proposed that it is required for progression through mitosis 16. It has also been suggested that βV-tubulin overexpression may mediate Taxol-dependence 17, a characteristic of some Taxol-resistant cells that require small quantities of drug for normal growth in tissue culture 18. Overexpression of βV-tubulin in Chinese hamster ovary (CHO) cells has been shown to contribute to the dependence of these cells on Taxol for growth 19. Therefore, βV tubulin expression may be a potentially important marker for defective microtubule stabilization associated with cellular transformation, or drug resistance.
Herein we describe the generation and characterization of a human-specific βV-tubulin antibody and its expression by immunohistochemistry in normal and malignant tissue.
Materials and methods
Tubulin peptides and antibodies
The peptides CGEEAFEDEEEEIDG and CYEDDEEESEAQGPK corresponding to human βV- and βIII- tubulin C-terminal sequences, respectively, were custom synthesized by the Laboratory for Molecular Analysis at Einstein College. The cysteine residue at the N-terminus of each peptide was introduced for conjugation of peptides to maleimide-activated keyhole limpet hemocyanin (KLH), or maleimide-activated bovine serum albumin (BSA) (Pierce). Rabbits were immunized with βV-tubulin peptide-KLH conjugates by Covance Immunology Services to produce sera containing a rabbit polyclonal βV-specific antibody. Bleeds from naïve and immunized rabbits were analyzed by ELISA using βV- or βIII-tubulin peptide-BSA conjugates. Sera from the first bleed were used in all experiments. Other antibodies used were rodent βV-tubulin5, (SHM.12G11, a gift from Dr Luduena, UHSC, San Antonio), βIII-tubulin (TUJ1 antibody, SDL.3D10, Sigma), βI-tubulin (SAP.4G5, Sigma), βIV-tubulin (ONS.1A6, Sigma), total β-tubulin (DM1B, Sigma), Kα1-tubulin (4D1, Sigma), actin (AC-40, Sigma), insulin (Dako), glucagon (Dako) and GAPDH (Invitrogen).
Taxol pelleted microtubules and Immunoblotting
A549 human lung cancer and Hey human ovarian cancer cells from ten 100 mm tissue culture dishes (Corning), at approximately 80–90% confluency, were harvested and Taxol pelleted microtubules were prepared for 2D gel electrophoresis as described previously3. Microtubule pellets (containing approximately 100–200 μg of protein) were resuspended in 350 μL of solubilization buffer (7 M urea, 2 M thiourea, 4% CHAPS, 0.5% Triton X-100, 0.5% ampholyte-containing buffer pH 4.5–5.5, 20 mM DTT, and bromophenol blue), and loaded onto 24 cm IPG strips with a linear gradient of pH 4.5–5.5 (Amersham). The IPG strips were loaded onto Tris-HCl 10% acrylamidecriterion gels (Biorad) and run at 200 Volts.
Immunoblots were probed with βV-tubulin rabbit polyclonal antibody at 1:20,000 dilution in 3% milk Tris buffered saline containing 0.05% Tween 20.
Tissue Culture and Cell Lines
All cell lines were purchased from ATCC and grown in RPMI (Gibco) supplemented with 10% Australian FBS (Gibco) at 37°C, in a 5% humidified CO2 atmosphere. Mouse embryonic fibroblast cells were generated in our laboratory using standard protocols.
Immunohistochemistry of paraffin embedded sections
Asynchronous cells were fixed in 4% methanol-free formaldehyde, pelleted and resuspended in matrigel. The solidified matrigel cassettes were submerged in 10% neutral buffered formalin and paraffin embedded sections were prepared by the Pathology Core at Albert Einstein College of Medicine. Re-hydrated slides were blocked for 1 h at room temperature in 5% donkey serum, 2% BSA. Antibody against βV-tubulin was used at 1:1000 in blocking solution and incubated overnight at 4°C in a humidified chamber. Standard washing and labeling with secondary antibodies were performed. Slides were developed with DAB for 45 seconds, washed for 3 minutes with running tap water and counterstained with hematoxylin and mounted.
Human archived tissue that was formalin fixed and paraffin embedded was processed using the same protocol. These sections were left over from standard of care, and as such were exempt from institutional review board approval. The intensity and the percentage of cells positive for βV-tubulin expression were evaluated using the semi-quantitative H-score, which multiplies the percentage of cells staining positively by staining intensity (scored from 0 to 3).
Immunofluorescence
HeLa cells were seeded onto Poly-D-lysine coated chamber slides, and after overnight growth, fixed in 3.7% formaldehyde in 1x PBS for 5 min (Polysciences). Cells were permeabilized with 0.1% Triton X-100 in 1x PBS for 3 minutes and slides were blocked in 1% goat serum and 3% BSA in 1x PBS for 30 minutes at room temperature, followed by incubation with the βV-tubulin antibody at a 1:1000 dilution in blocking buffer for 1 h at room temperature. Slides were incubated with goat-anti-mouse alexa 555 and goat-anti-rabbit alexa 488 secondary antibodies (Molecular Probes) for 45 min, and mounted using Prolong Gold anti-fade with DAPI (Molecular Probes).
RESULTS
Specificity of a human βV-tubulin polyclonal antibody
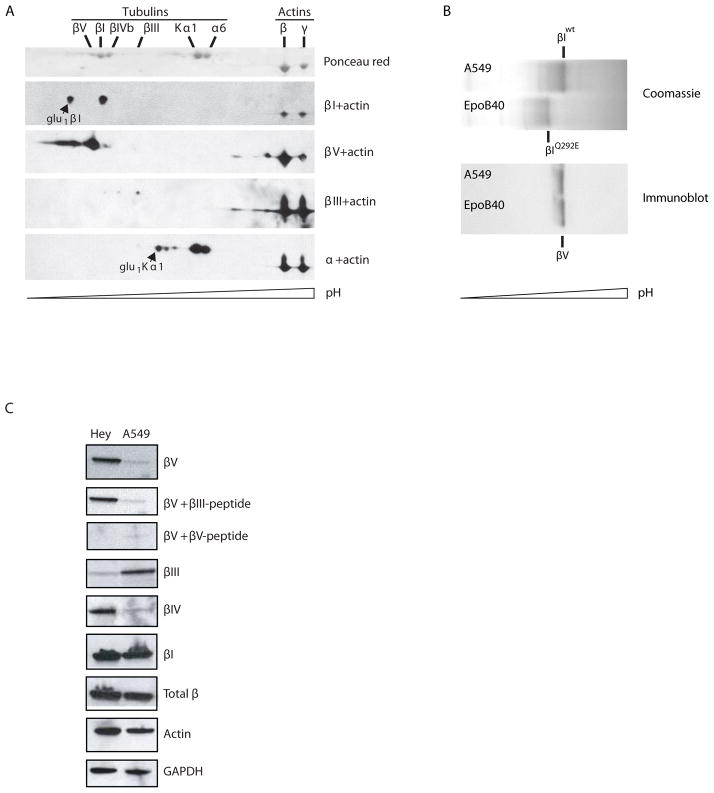
In previous studies we have demonstrated the ability of narrow-range isoelectric focusing to resolve tubulin isotypes differing in isoelectric point (pI) by as little as 0.014. Subsequent mass spectrometry of excised bands has validated the identity of these isotypes. In addition, SILAC, IEF and MS were combined to quantify relative differences in βIII- and βV-tubulin levels of expression in human cancer cell lines20–22. These previous studies indicated that βV-tubulin was approximately three to four-fold more abundant in Hey ovarian carcinoma cells compared to human A549 lung cancer cells. To test the specificity of the βV-tubulin antibody in this study, two-dimensional (2D) immunoblots of Taxol-pelleted microtubules were carried out. Microtubules from Hey and A549 cells were used as sources of high and low βV-tubulin content, respectively20. These 2D immunoblots were probed with antibodies specific for Kα1-, βI-, βIII-tubulins, and the βV-tubulin-specific antibody described in the present study. In order to clearly position βIII-tubulin spots, we used microtubules from A549 cells that expressed detectable levels of βIII-tubulin. Consistent with high expression in Hey cells, the anti βV-tubulin antibody strongly stained a major specific spot positioned at the expected calculated pI of 4.77 (Fig. 1A)20. Minor spots that were more acidic in pI were also observed. Both α-tubulin and β-tubulins undergo glutamylation and phosphorylation on their C-termini23 that could account for such additional spots. We previously observed monoglutamylation of Kα1 and βI-tubulin in cancer cell lines24 and more acidic spots corresponding to these isoforms are indicated.
Figure 1. Isotype-specific immunoreactivity of a human βV-tubulin antibody.
A) Taxol-pelleted microtubules from Hey cells were resolved by isoelectric focusing using IPG strips at pH 4.5–5.5, followed by SDS-PAGE, total protein staining and immunoblotting with isotype specific antibodies as indicated. B) Isoelectric focusing of wild type and mutant βI-tubulin in A549 and A549.EpoB40, respectively. IEF gels were either stained with Coomassie blue (top), or electrotransfered onto nitrocellulose for immunoblotting with anti human βV-tubulin antibody (bottom). C) Pre-incubation of antibodies with βIII- or βV-tubulin C-terminal peptides and subsequent immunoblotting using the βV-tubulin antibody on Hey and A549 cell lysates. Also shown are immunoblots with the indicated isotype specific antibodies for βV-, βIII-, βI-, and total β-tubulin.
We were able to exclude cross reactivity of the βV-tubulin antibody with βI-tubulin using a high resolution one-dimensional isoelectric focusing gel transferred onto nitrocellulose to analyze the expression of tubulin in microtubule pellets generated from an epothilone-B resistant cell line derived from A549 cells25. These cells have a Gln to Glu mutation at residue 292 of βI-tubulin, which causes a loss of 0.02 unit of pI for βI-tubulin. If the βV antibody also cross-reacted with βI-tubulin, two bands with different electrophoretic mobilities would be apparent, similar to the profile shown by the Coomassie blue stain. In fact, the βV antibody reacted with a protein focusing between wild-type (pI= 4.78) and mutant βI-tubulin (pI=4.76) (Fig. 1B). Thus, the βV-tubulin antibody does not cross-react with βI-tubulin. Next, to further test specificity, the βV-tubulin antibody was pre-incubated with βV- or βIII-tubulin C-terminal peptides prior to immunoblot analysis for βV-tubulin (Fig. 1C). Since the antibody-defining regions of BIII and BV are the most homologous among the tubulins, we used C-terminal peptides of BIII to confirm BV antibody specificity. As expected, pre-incubation of the βV-tubulin antibody with βV-tubulin C-terminal peptide but not with the βIII-tubulin C-terminal peptide prevented the detection of βV-tubulin. Lastly, immunoblots of Hey and A549 carcinoma cell lines were probed with βI-, βIII-, βIV-, and βV-tubulin antibodies and respective signals were normalized to those of total β-tubulin and GAPDH (Fig. 1C). The observed differences in expression levels were comparable to those revealed by mass spectrometry20.
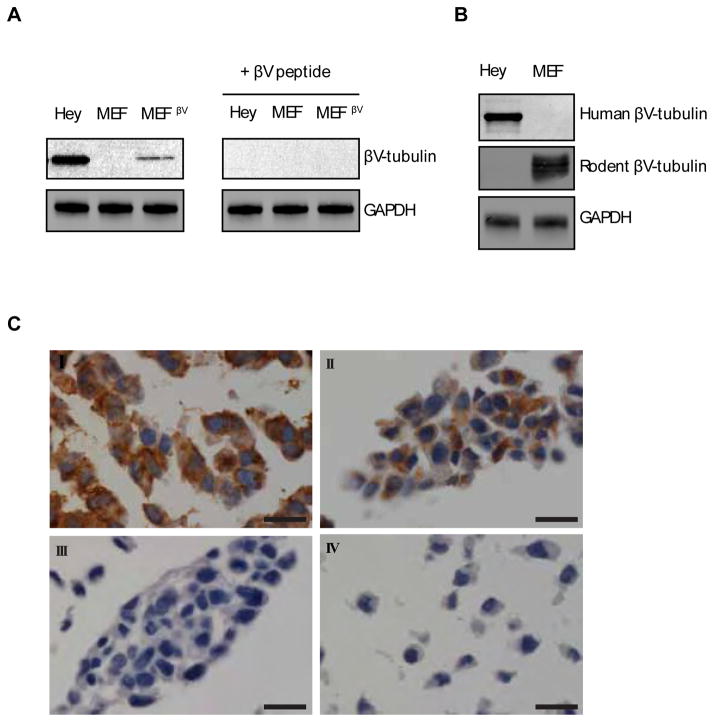
Human and mouse βV-tubulin have 97.7% overall amino acid identity, while the antigen-defining region has 79% homology (Fig. S1). To test whether the human βV-tubulin antibody that we developed would cross react with mouse βV-tubulin, mouse embryonic fibroblasts (MEFs) were transduced with full length human βV-tubulin, or vector alone. Subsequent immunoblotting indicated that the βV-tubulin antibody was only able to detect human βV-tubulin in the transduced MEFs and not in the endogenous mouse βV-tubulin protein (Fig. 2A). We detected the expression of endogenous mouse βV-tubulin in MEF lysates using a monoclonal antibody specific to rodent βV-tubulin (Fig. 2B). This result indicated that the βV-tubulin antibody we generated is specific for the human isotype. A BLAST protein sequence similarity search was performed with the C-terminus sequence of βV-tubulin. We found that this sequence was similar to an internal sequence (262EEDEEEEIDV271) of human N-Myc oncoprotein, a protein with a MW similar to tubulin (49 kDa). In order to eliminate the possibility of cross reactivity between the two, particularly for immunohistochemical applications, we performed immunoblot analysis using the βV-tubulin antibody against recombinant full-length N-Myc protein containing a 26 kDa N-terminal GST tag, which decreases its electrophoretic mobility to an approximate MW of 80kDa. Lysates from SH-SY5Y neuroblastoma cells that are known to overexpress N-Myc were used as a control. The βV-tubulin antibody was unable to detect N-Myc by immunoblotting, even with prolonged exposure (Fig. S2). Immunohistochemistry performed on N-myc rich neuroblastoma tissue was negative for expression of βV-tubulin (data not shown). Therefore no cross-reactivity occurs between βV-tubulin and N-myc. This result and the non cross-reactivity of our anti-human βV-tubulin antibody with the C-terminus of mouse βV-tubulin strongly suggest that the recognized epitope involves the last two amino acid residues and the C-terminal carboxyl.
Figure 2. Specificity of human βV-tubulin immunoreactivity in mouse embryonic fibroblasts (MEFs) expressing exogenous human βV-tubulin.
A) Mouse embryonic fibroblasts transfected with empty vector or full length, human βV-tubulin (MEFβV) were immunoblotted with the βV-tubulin antibody in the absence and presence of βV-tubulin peptides. B) Immunoblot analysis of MEF and Hey cells with a rodent specific βV-tubulin monoclonal antibody. C) Immunohistochemical analysis of βV-tubulin expression in formalin-fixed paraffin-embedded cells (I) Hey cells, positive control for high expression of endogenous βV-tubulin, (II) MEFs expressing exogenous human βV-tubulin, (III) MEFs transfected with empty vector, and (IV) Hey cells incubated with secondary antibody only. All slides were counterstained with hematoxylin. Scale bars, 20μm.
Previously published data utilizing a rodent βV-tubulin monoclonal antibody indicated that βV-tubulin is concentrated in the inner ear, localizing specifically to pillar cells and deiter cells in gerbil cochlea5. Due to difficulties obtaining equivalent human specimens, we were unable to confirm these results with the human antibody. To test the capability of the βV-tubulin antibody for immunohistochemistry, we prepared formalin-fixed, paraffin-embedded MEF cell pellets sections from MEFs overexpressing βV-tubulin or tranduced with the empty vector. The immunohistochemical staining pattern for βV-tubulin antibody was cytoplasmic with a clean background. βV-tubulin was strongly expressed in stably-transfected MEFs and negative in vector-only tranfected MEFs (Fig 2C).
Localization of human βV-tubulin in cultured cells
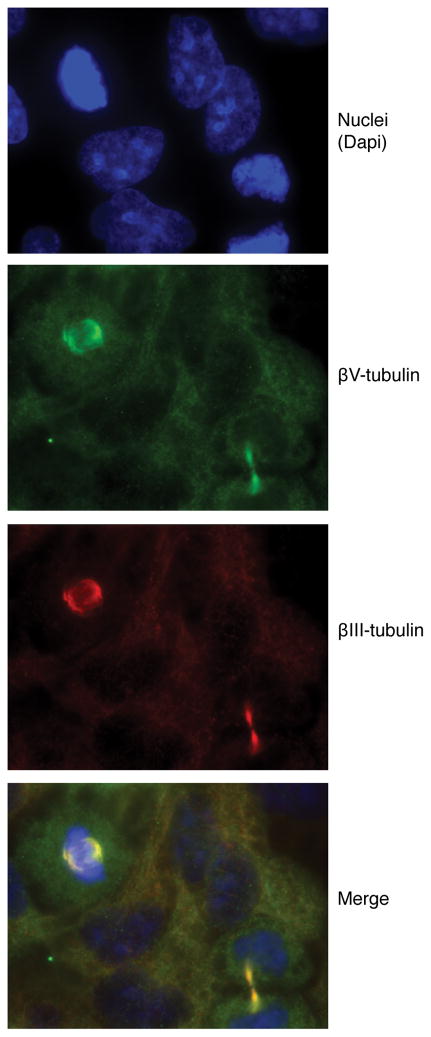
The human βV-tubulin antibody we generated was also evaluated by immunofluorescence in HeLa cells. Localization was contrasted with βIII-tubulin. Both βV- and βIII-tubulin had diffuse staining that localized throughout the cytoplasm of interphase cells, with intense staining of mitotic cell spindles and of the intercellular bridge in late telophase (Fig. 3). Immunofluorescence using the murine βV-tubulin antibody in NIH3T3 cells also demonstrated that βV-tubulin is concentrated at microtubule spindles and is present in the microtubule network of interphase cells 5. There may be a subtle difference in the distribution of βIII versus βV in the spindles of HeLa cells. Cytoplasmic βV-tubulin immunostaining was also observed in Hey cells, similar to the distribution observed by immunohistochemistry.
Figure 3. Immunofluorescence of endogenous βV- and βIII-tubulin in HeLa cells.
Field includes interphase and mitotic cells. From top: dapi staining of nuclei, alexa 488 labeling of βV-tubulin (green), alexa 555 labeling of βIII-tubulin (red), and merged image.
Immunohistochemical analysis of βV-tubulin in human tissue
The distribution of βV-tubulin protein in human tissue has never been determined due to the unavailability of a specific antibody. Using our human specific βV tubulin antibody, we determined βV expression in a variety of human normal tissue types (Table 1). As indicated by immunofluorescence, βV-tubulin showed a cytoplasmic pattern of staining by immunohistochemistry, as shown in Figure 4.
Table 1.
Distribution of βV-tubulin in Normal Tissues by Immunohistochemistry
| Tissue | Intensity | |
|---|---|---|
| Skin | Sebaceous glands | +++ |
| Squamous epithelium | + (variable) | |
| Basal cells | + | |
| Muscle | Smooth muscle | +++ |
| Skeletal muscle | ++ | |
| Cardiac Muscle | ++ | |
| Blood vessel | Endothelial cells | +++ |
| smooth muscle | ++ | |
| GI Tract ¶ | Epithelium | − |
| Liver | Hepatocytes | − |
| Bile ducts | ++ | |
| Pancreas | Ducts | + |
| Islets | ++ | |
| Acini | − | |
| Kidney | Renal tubules | − to ++ (variable) |
| glomeruli | ++ | |
| Testis | Seminiferous tubules | − |
| Mature germ cells | − | |
| Immature germ cells | ++ | |
| Sertoli cells | − | |
| Prostate | Glandular cells | ++ (variable) |
| Basal cells | − | |
| Mammary gland | Luminal cells | − |
| Lactating secretory cells | ++ | |
| Myoepithelial cells | +++ | |
| Ovary | Surface epithelium | − |
| Stromal cells | + | |
| Fallopian tube | Epithelium | − |
| Lung | Pneumocytes | − |
| Respiratory epithelium | − | |
| Thyroid | Follicular cells | − to ++ (variable) |
| Adrenal gland | Cortex and medulla | − |
| Salivary Gland | Acini | + (variable) |
| Ducts | ++ | |
| Nerve | Neuronal Cells | − |
Abbreviations: (−) no staining, (+) weak staining, (++) moderate staining, (+++) strong staining
(Stomach, Small Intestine, Appendix, Colon, Gall bladder)
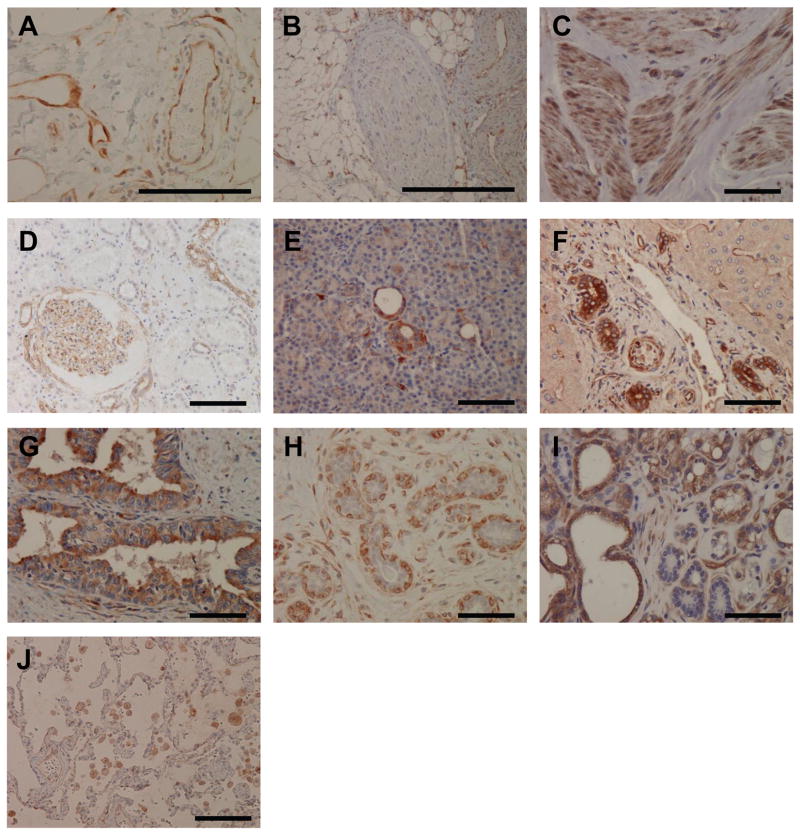
Figure 4. Immunohistochemistry of βV-tubulin in normal human tissue.
A) endothelial cells of vessels and capillaries, B) nerve fiber, C) smooth muscle, D) glomerulus and renal tubules, E) pancreatic ducts, F) bile ducts of liver; G) prostate glands, H) normal breast ducts/lobules; I) lactating breast, J) normal lung. Scale bars, 100 μm.
In general, βV-tubulin was consistently expressed in the endothelial cells of the blood vessels (Fig 4A), myocytes and cells with muscle differentiation. βV-tubulin was expressed in muscles of all three types with particularly strong expression in smooth muscle. Specialized cells such as myoepithelial cells of the breast and myoid cells of the testis showed strong βV-tubulin staining, consistent with their muscle differentiation. The glomeruli of the kidney have specialized tuft-like vascular structure composed of fenestrated endothelium, podocytes and mesangial cells. βV-tubulin showed an interesting mosaic pattern outlining the glomeruli. This pattern was correlated with the positive expression in endothelial cells and mesangial cells, a specialized cell type with muscle differentiation. βIII-tubulin is known to be highly expressed in neuronal cells, and is currently used as a marker of neuronal differentiation26. βV-tubulin, unlike βIII-tubulin, was not expressed in nerves (Fig. 4B). To confirm our findings by immunohistochemistry, immunoblot analysis of a human microvascular endothelial cell line (HMEC-1) was used (Fig. S2). βV-tubulin, but not βIII was expressed minimally in these cells.
Moderate staining was consistently observed in cells with secretory/transport function such as renal tubules, bile ducts of the liver, pancreatic exocrine ducts and ducts of salivary glands (Table 1 and Figure. 4). These cells transport ions and water and have small molecule exchange/secretion/absorption functions during transport processes, suggesting the possibility that βV-tubulin participates in transport function, and certain ion or small molecule exchange. βV-tubulin was also strongly expressed in sebaceous glands of the skin and variably expressed in prostate glandular epithelium and thyroid follicular cells, which are epithelial tissues with secretary funtions. In contrast, βV staining was not detected in the epithelium of the GI tract (stomach, duodenum, gall bladder, small and large intestine), hepatocytes, ovarian surface epithelium and pneumocytes. In normal breast tissue, βV-tubulin was absent in luminal epithelial cells of the ducts/lobules, however, it was strongly expressed in the luminal cells of the breast during lactation (Fig 4H–I). We hypothesize that this specific expression of βV is acquired to meet the demanding secretory needs of the breast during lactation. In addition, macrophages in various tissues showed positive staining for βV-tubulin. Macrophages are inflammatory mediators with secretory function, therefore βV-tubulin expression in macrophages is consistent with the likely secretory-related functions of this tubulin isotype.
Expression of βV-tubulin in pancreatic islets and testis
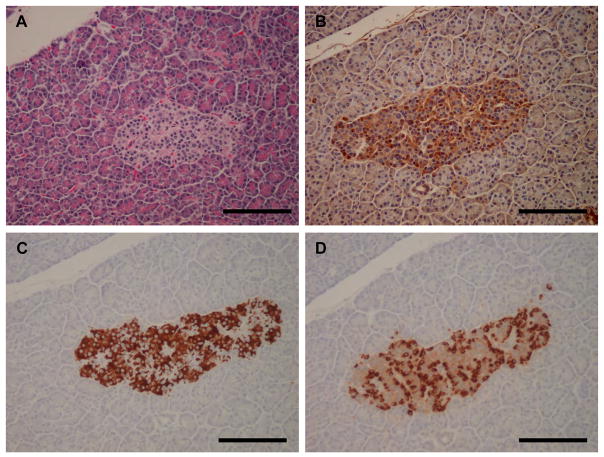
Interestingly, while βV was largely negative in pancreatic parenchyma (exocrine glands), it was positively expressed in pancreatic islets (Fig. 4E and Fig. 5). Pancreastic islets are endocrine components of the pancreas that are composed of alpha and beta cells that are responsible for the secretion of glucagon and insulin, respectively. We were able to compare the immunostaining pattern of βV-tubulin with that of glucagon and insulin using specific antibodies on serially sectioned pancreatic tissue (Fig. 5). BV-positive cells were localized around the periphery of the islets in a region that qualitatively correlates with the localization of glucagon-stained alpha cells (Fig. 5D). Thus, these data suggest that the glucagon-producing alpha cells may require βV-tubulin for a specific function.
Figure 5. Immunohistochemistry of βV-tubulin in pancreatic islets.
A) Hematoxylin and eosin, B) βV-tubulin, C) insulin, D) glucagon. Scale bars, 100 μm.
βV-tubulin also exhibited an interesting pattern in testis. A testicular biopsy from a child with cryptochidism showed βV-tubulin positivity highlighting spermatogonia in the seminiferous tubules (Figure 6). In tissue samples of intratubular germ cell neoplasia (ITGCN), surprisingly specific strong expression of βV-tubulin was observed in the neoplastic germ cells, while in contrast, normal seminiferous tubules were negative. Although the number of cases tested was limited (3/3), we believe that βV-tubulin may be a potential diagnostic tool for identifying ITGCN. To date, there is no diagnostic immunohistochemical marker of this entity, and the biology underlying the association of βV-tubulin expression with germ cell neoplasia will require investigation.
Figure 6. Immunohistochemistry βV-tubulin in the germ cells of immature testis.
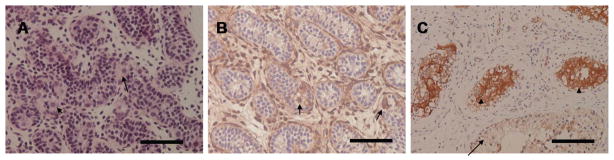
A) Hematoxylin and eosin staining of a tissue section from a one year old child with testis cryptochidism, B) βV-tubulin was expressed in the germ cells- spermatogonia in the seminiferous tubules (arrows). Sertoli cells were negative. The surrounding outer myoid cells were positive staining for βV-tubulin due to their muscle differentiation C) An adult testis with intratubular germ cells neoplasia (ITGCN). βV-tubulin was strongly expressed in ITGCN (arrowheads). Normal seminiferous tubules in the background were negative for βV-tubulin (arrow). Scale bars, 100 μm.
Expression of βV-tubulin in human cancer
Since tumorigenesis often causes altered differentiation of affected cells, as is the case with the neuronal-specific βIII-tubulin isotype that is expressed in numerous malignancies, we evaluated the expression of βV-tubulin in a small cohort of carcinomas including breast, lung, prostate and ovary. We found that, in contrast to the negative staining in their normal counterparts, lung, breast and ovarian cancers (the latter not shown), had aberrant βV-tubulin expression. Conversely, while normal prostate glands showed variable βV-tubulin staining, expression was completely lost in prostate cancer (Fig. 7).
Figure 7. Immunohistochemistry of βV-tubulin in human malignancy.
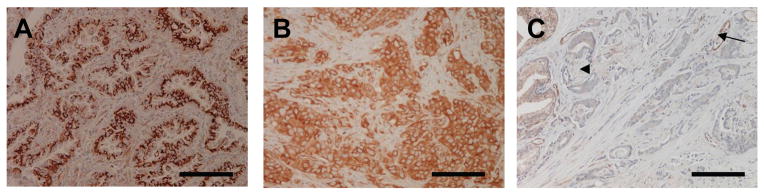
βV-tubulin was strongly expressed in A) lung adenocarcinoma and B) invasive breast cancer. C) loss of βV-tubulin in infiltrating prostate adenocarcinoma and perineural invasion of the adenocarcinoma. A nerve fiber that is negative for βV-tubulin is indicated by the arrowhead. Variable expression of βV-tubulin in normal prostate glands is present in the upper left. Endothelial cells lining blood vessels positive for βV-tubulin are indicated (arrow), Scale bars, 100 μm.
Immunobloting for βV- tubulin was also performed in a series of lung (NSCLC), ovarian, and breast cancer cell lines to determine if there was a histological association with its expression (Figure S3). βV-tubulin was expressed in all but one cell line, although at highly variable levels, supporting previous studies suggesting that it is ubiquitously expressed in cancer cell lines27. Thus these data are consistent with the immunohistochemical data obtained for lung, ovarian and breast cancer.
We conducted a study of βV-tubulin expression in 32 cases of breast cancers with various differentiation states. βV-tubulin was aberrantly expressed in ten cases (30%), of which nine were poorly differentiated, suggesting that expression is associated with maligant transformation and high tumor grade. Thus, our preliminary data indicate that βV-tubulin is abberrantly expressed in lung, ovarian and breast cancer and collectively imply that like βIII-tubulin, βV-tubulin has irregular expression in transformed cells, though additional studies to confirm and extend these observations are needed.
Discussion
Several studies have established that mammalian microtubules will incorporate all available tubulin isotypes as evidenced by immunofluorescence using isotype specific antibodies and secondly, by transfection of non-mammalian isotypes into mammalian cells 28–30. βV-tubulin is a minor tubulin isotype, composing less than 10% of the total tubulin in most tissues in the chicken, 31, less than 20% in mouse embryonic fibroblasts, and less than approximately 7% of total tubulin in Chinese hamster ovary cells 28, 30, 31.
Numerous studies have documented overexpression of βIII-tubulin in different malignancies, and its utility as a marker of poor survival and, in some cases response to therapy7,9. This has prompted widespread interest in characterizing the expression of other tubulin isoforms in human malignancies. In the absence of a commercially available specific βV-tubulin antibody, many of the studies to date have employed overexpression or RNA-based knockdown techniques to modulate βV in numerous cell lines, without a real appreciation of the tissue-specific expression of this isotype in normal tissue. A previous qRT-PCR study reported ubiquitous expression of βV-tubulin in human tissue 15, however we did not find this by immunohistochemistry. It is possible that the levels of βV-tubulin protein in the RT-PCR positive tissues are extremely low, or that the ubiquitous expression was an artefact due to contamination with a βV-tubulin positive cell type, such as endothelial cells or macrophages, since laser-capture microdissection was not performed.
To circumvent this inherent problem with mRNA expression profiling, we sought to generate a human-specific βV-tubulin antibody that could be used to delineate the repetoir of expression in normal and malignant tissue, and as such provide evidence about the potential function of this specific tubulin isoform.
Unlike βIII tubulin that is normally expressed only in neuronal tissue and cells, βV was expressed in a wide range of human non-neuronal cell and tissue types. The observation that βV-tubulin was preferentially expressed in cells with a secretory function, such as pancreatic ductal cells, was unexpected. One of the functions of microtubules is to move secreted proteins within cells. Colchicine, a microtubule destabilizing agent that interacts with the tubulin dimer, has been shown to inhibit the secretion of several proteins from secretory tissues. Specifically, colchicine inhibits the secretion of thyroid hormones from thyroid epithelial cells, lipoproteins from liver cells, insulin from pancreatic islet cells, HCO3 from pancreatic ducts, and apolipoprotein E from macrophages 32–35. Interestingly, cell types that constitute the pancreastic islet, salivary ducts and bile duct showed positive staining for βV-tubulin, reinforcing the view that there are isotype-specific functions for tubulins. Future studies to modulate the intrinsic expression of βV tubulin in these cell types will be required to fully determine whether this isotype actually participates in the transport of protein cargo as part of the secretory process, or is an authentic marker of a secretory cell type.
Although not a major focus of this study, the data from tumor tissue indicate potentially interesting alterations in the expression of βV in normal, versus malignant breast, lung, ovary, and prostate tissue. Specifically, βV was undetectable in epithelial cells, yet was aberrantly expressed in approximately one-third of invasive breast carcinomas analyzed. Similarly, βV-tubulin was not expressed in normal lung pneumocytes or ovarian epithelium, however, some lung and ovarian cancers were positive. Conversely, while variably expressed in normal prostate glands, βV-tubulin expression was lost in prostate cancer.
Therefore, similar to the expression of βIII that occurs in malignant cells versus normal cells, there also appears to be a trend toward reversal of βV-tubulin expression during malignancy. Thus, it is plausible that the altered expression in malignant cells reflects an inherent change in the differentiation status of the normal tissue, such that βV-tubulin transcripts that are normally not expressed in a given cell type become expressed; while in tissues that normally expresses βV-tubulin, transcripts are silenced.
Overexpression of mouse βV-tubulin, like βIII, has been demonstrated to confer resistance to Taxol in cells, 11, 16, 19 although βV- tubulin was thought to confer more deleterious effects on cell division 36. In contrast, the overexpression of βI- βII-, βIV, or βVI- tubulin had no effect on microtubule assembly or drug resistance when overexpressed in cells 16, 19. Such studies make it tempting to speculate that βV-tubulin, like βIII, may be implicated in poor prognosis for some malignancies and possibly poor response to therapy. However we caution against overstating the significance of cell based studies until more extensive immunohistochemical analyses have been performed. Furthermore, human βV-tubulin has a different sequence in the Taxol binding site relative to mouse βV-tubulin20, thus conclusions about the role βV-tubulin obtained from mouse cell models may not be applicable to human βV-tubulin. The availability of this human-specific βV-tubulin antibody will enable mechanistic studies to shed light on the biological function of this tubulin isoform, and its potential involvement in drug resistance.
Moreover, the unique profile of βV-tubulin expression that we have characterized in several malignancies lends support to our hypothesis that altered expression of this tubulin isoform may be associated with tumorigenesis. These initial studies in breast and lung cancers will form the basis of extended analyses that will probe the relationship between βV-tubulin, differentiation, stage, survival and response to therapy. Immunohistochemical-based analyses of βV-tubulin expression may be a useful diagnostic tool and a potential prognostic marker.
Supplementary Material
Acknowledgments
The authors thank Drs. J. Locker and C. Ferlini for helpful discussions, and Dr. R. Luduena for providing the mouse βV-tubulin antibody.
References
- 1.Joshi HC, Yen TJ, Cleveland DW. In vivo coassembly of a divergent beta-tubulin subunit (c beta 6) into microtubules of different function. J Cell Biol. 1987;105:2179–2190. doi: 10.1083/jcb.105.5.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luduena RF. Multiple forms of tubulin: different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- 3.Miller LM, Xiao H, Burd B, Horwitz SB, Angeletti RH, Verdier-Pinard P. Methods in tubulin proteomics. Methods Cell Biol. 2010;95:105–126. doi: 10.1016/S0091-679X(10)95007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdier-Pinard P, Pasquier E, Xiao H, Burd B, Villard C, Lafitte D, Miller LM, Angeletti RH, Horwitz SB, Braguer D. Tubulin proteomics: towards breaking the code. Anal Biochem. 2009;384:197–206. doi: 10.1016/j.ab.2008.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee A, Jensen-Smith H, Lazzell A, Prasad V, Elguezabal G, Hallworth R, Luduena RF. Localization of betav tubulin in the cochlea and cultured cells with a novel monoclonal antibody. Cell Motil Cytoskeleton. 2008;65:505–514. doi: 10.1002/cm.20280. [DOI] [PubMed] [Google Scholar]
- 6.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kavallaris M. Microtubules and resistance to tubulin-binding agents. Nat Rev Cancer. 2010;10:194–204. doi: 10.1038/nrc2803. [DOI] [PubMed] [Google Scholar]
- 8.Seve P, Dumontet C. Class III beta tubulin expression in nonsmall cell lung cancer. Rev Mal Respir. 2010;27:383–386. doi: 10.1016/j.rmr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Ferrandina G, Zannoni GF, Martinelli E, Paglia A, Gallotta V, Mozzetti S, Scambia G, Ferlini C. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin Cancer Res. 2006;12:2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 10.Seve P, Dumontet C. Is class III beta-tubulin a predictive factor in patients receiving tubulin-binding agents? Lancet Oncol. 2008;9:168–175. doi: 10.1016/S1470-2045(08)70029-9. [DOI] [PubMed] [Google Scholar]
- 11.Kavallaris M, Kuo DY, Burkhart CA, Regl DL, Norris MD, Haber M, Horwitz SB. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J Clin Invest. 1997;100:1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seve P, Reiman T, Lai R, Hanson J, Santos C, Johnson L, Dabbagh L, Sawyer M, Dumontet C, Mackey JR. Class III beta-tubulin is a marker of paclitaxel resistance in carcinomas of unknown primary site. Cancer Chemother Pharmacol. 2007;60:27–34. doi: 10.1007/s00280-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 13.McCarroll JA, Gan PP, Liu M, Kavallaris M. betaIII-tubulin is a multifunctional protein involved in drug sensitivity and tumorigenesis in non-small cell lung cancer. Cancer Res. 2010;70:4995–5003. doi: 10.1158/0008-5472.CAN-09-4487. [DOI] [PubMed] [Google Scholar]
- 14.Lee KM, Cao D, Itami A, Pour PM, Hruban RH, Maitra A, Ouellette MM. Class III beta-tubulin, a marker of resistance to paclitaxel, is overexpressed in pancreatic ductal adenocarcinoma and intraepithelial neoplasia. Histopathology. 2007;51:539–546. doi: 10.1111/j.1365-2559.2007.02792.x. [DOI] [PubMed] [Google Scholar]
- 15.Leandro-Garcia LJ, Leskela S, Landa I, Montero-Conde C, Lopez-Jimenez E, Leton R, Cascon A, Robledo M, Rodriguez-Antona C. Tumoral and tissue-specific expression of the major human beta-tubulin isotypes. Cytoskeleton (Hoboken) 2010;67:214–223. doi: 10.1002/cm.20436. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya R, Cabral F. Molecular basis for class V beta-tubulin effects on microtubule assembly and paclitaxel resistance. J Biol Chem. 2009;284:13023–13032. doi: 10.1074/jbc.M900167200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganguly A, Yang H, Cabral F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol Cancer Ther. 2010;9:2914–2923. doi: 10.1158/1535-7163.MCT-10-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martello LA, Verdier-Pinard P, Shen HJ, He L, Torres K, Orr GA, Horwitz SB. Elevated levels of microtubule destabilizing factors in a Taxol-resistant/dependent A549 cell line with an alpha-tubulin mutation. Cancer Res. 2003;63:1207–1213. [PubMed] [Google Scholar]
- 19.Bhattacharya R, Cabral F. A ubiquitous beta-tubulin disrupts microtubule assembly and inhibits cell proliferation. Mol Biol Cell. 2004;15:3123–3131. doi: 10.1091/mbc.E04-01-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verdier-Pinard P, Shahabi S, Wang F, Burd B, Xiao H, Goldberg GL, Orr GA, Horwitz SB. Detection of human betaV-tubulin expression in epithelial cancer cell lines by tubulin proteomics. Biochemistry. 2005;44:15858–15870. doi: 10.1021/bi051004p. [DOI] [PubMed] [Google Scholar]
- 21.Verdier-Pinard P, Wang F, Martello L, Burd B, Orr GA, Horwitz SB. Analysis of tubulin isotypes and mutations from taxol-resistant cells by combined isoelectrofocusing and mass spectrometry. Biochemistry. 2003;42:5349–5357. doi: 10.1021/bi027293o. [DOI] [PubMed] [Google Scholar]
- 22.Miller LM, Menthena A, Chatterjee C, Verdier-Pinard P, Novikoff PM, Horwitz SB, Angeletti RH. Increased levels of a unique post-translationally modified betaIVb-tubulin isotype in liver cancer. Biochemistry. 2008;47:7572–7582. doi: 10.1021/bi8005225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–2160. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 24.Verdier-Pinard P, Wang F, Burd B, Angeletti RH, Horwitz SB, Orr GA. Direct analysis of tubulin expression in cancer cell lines by electrospray ionization mass spectrometry. Biochemistry. 2003;42:12019–12027. doi: 10.1021/bi0350147. [DOI] [PubMed] [Google Scholar]
- 25.Yang CP, Verdier-Pinard P, Wang F, Lippaine-Horvath E, He L, Li D, Hofle G, Ojima I, Orr GA, Horwitz SB. A highly epothilone B-resistant A549 cell line with mutations in tubulin that confer drug dependence. Mol Cancer Ther. 2005;4:987–995. doi: 10.1158/1535-7163.MCT-05-0024. [DOI] [PubMed] [Google Scholar]
- 26.Lee MK, Tuttle JB, Rebhun LI, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific beta-tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 27.Bhattacharya R, Frankfurter A, Cabral F. A minor beta-tubulin essential for mammalian cell proliferation. Cell Motil Cytoskeleton. 2008;65:708–720. doi: 10.1002/cm.20292. [DOI] [PubMed] [Google Scholar]
- 28.Lopata MA, Cleveland DW. In vivo microtubules are copolymers of available beta-tubulin isotypes: localization of each of six vertebrate beta-tubulin isotypes using polyclonal antibodies elicited by synthetic peptide antigens. J Cell Biol. 1987;105:1707–1720. doi: 10.1083/jcb.105.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis SA, Gu W, Cowan NJ. Free intermingling of mammalian beta-tubulin isotypes among functionally distinct microtubules. Cell. 1987;49:539–548. doi: 10.1016/0092-8674(87)90456-9. [DOI] [PubMed] [Google Scholar]
- 30.Sawada T, Cabral F. Expression and function of beta-tubulin isotypes in Chinese hamster ovary cells. J Biol Chem. 1989;264:3013–3020. [PubMed] [Google Scholar]
- 31.Sullivan KF, Havercroft JC, Machlin PS, Cleveland DW. Sequence and expression of the chicken beta 5- and beta 4-tubulin genes define a pair of divergent beta-tubulins with complementary patterns of expression. Mol Cell Biol. 1986;6:4409–4418. doi: 10.1128/mcb.6.12.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kockx M, Guo DL, Huby T, Lesnik P, Kay J, Sabaretnam T, Jary E, Hill M, Gaus K, Chapman J, Stow JL, Jessup W, Kritharides L. Secretion of apolipoprotein E from macrophages occurs via a protein kinase A and calcium-dependent pathway along the microtubule network. Circ Res. 2007;101:607–616. doi: 10.1161/CIRCRESAHA.107.157198. [DOI] [PubMed] [Google Scholar]
- 33.Williams JA, Wolff J. Colchicine-binding protein and the secretion of thyroid hormone. J Cell Biol. 1972;54:157–165. doi: 10.1083/jcb.54.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montague W, Howell SL, Green IC. Insulin release and the microtubular system of the islets of Langerhans. Identification and characterization of tubulin-like protein. Biochem J. 1975;148:237–243. doi: 10.1042/bj1480237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stein O, Sanger L, Stein Y. Colchicine-induced inhibition of lipoprotein and protein secretion into the serum and lack of interference with secretion of biliary phospholipids and cholesterol by rat liver in vivo. J Cell Biol. 1974;62:90–103. doi: 10.1083/jcb.62.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhattacharya R, Yang H, Cabral F. Class V beta-tubulin alters dynamic instability and stimulates microtubule detachment from centrosomes. Mol Biol Cell. 2011;22:1025–1034. doi: 10.1091/mbc.E10-10-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.