Abstract
Direct reprogramming of human fibroblasts into functional neurons in vitro by defined factors provides an invaluable resource for regenerative medicine. However, clinical applications must consider the risk of immune rejection, thus patient-specific induced neuronal cells (iNCs) may serve as an ideal source for autologous cell replacement. In this study, we report a robust process for functional neuronal cells from the patients' scalp by lentiviral gene delivery of Ascl1, Myt1l, and Sox2. These three-factor iNCs are similar to human neuronal cells in morphology, surface antigens, gene expression, and electrophysiological characteristics. Our findings might provide a source of patient-specific functional neurons for cell therapy.
Introduction
Regenerative medicine aims to create functional cells of the same type to replace diseased or damaged tissues. Two major problems that need to be resolved urgently are the shortage of functional cells available for transplantation and the risk of immune rejection. Successful reprogramming of adult fibroblasts into induced pluripotent stem (iPS) cells using defined transcription factors gives a potential approach to generate functional cells for the patient himself [1–14]. Rather than switching the fate of differentiated cells going backward and then going forward, there might be a shortcut—directly converting one adult cell type into another without going through the pluripotent state. Recent pioneering works show that this method is feasible. For example, MyoD (also called Myod1), which is a protein with a key role in regulating muscle differentiation can convert cultured embryonic fibroblasts, chondroblasts, and retinal epithelial cells into contracting muscle cells [15]. CCAAT/enhancer-binding protein alpha (C/EBP-α), a critical regulator for early myeloid differentiation has been shown to convert mature B lymphocytes into macrophages or pre-B cells [16]. By Math1, inner ear support cells also can be reprogrammed to hair cells [17,18]. Similarly, a specific combination of three transcription factors [Ngn3 (also known as Neurog3], Pdx1, and Mafa) efficiently reprograms differentiated pancreatic exocrine cells of adult mice into cells that closely resemble β-cells in vivo [19]. These methods were only effective for fate switching within the three major lineages. In 2010, Vierbuchen et al. described, for the first time, the direct conversion of mouse fibroblasts into induced neuronal cells (iNCs) by overexpression of the neuronal transcription factors Ascl1, Brn2, and Myt1l, which established the interlineage transdifferentiation in vitro [20]. A few months later, the same group reported human fibroblasts have been directly converted into neuronal cells (human iNCs) by the addition of the NeuroD1 transcription factor to the reprogramming cocktail [21]. Then, Pfisterer et al. demonstrated that Ascl1, Brn2, Myt1l and Lmx1a, FoxA2 were sufficient to obtain the functional human dopaminergic neurons (iDAs) [22]. Contrary to this, Caiazzo et al. substituted FoxA2 for Nurr1/Nr4a2, while maintaining that Lmx1a and Ascl1 also generated iDA cells [23], as well as the minimal iNC-factor cocktail defined by the Wernig laboratory (BAM). Qiang et al. overexpressed Zic and/or Olig2 and used specific medium conditions to obtain iNCs that could be used for disease modeling studies [24]. Son et al. demonstrated that eight factors (Ascl1, Brn2, Myt1l, Lhx3, Ngn2, Isl1, Hb9, and NeuroD1) contributed to the generation of functional human induced motor neurons [25]. Different miRNAs have been shown to influence the outcome of human iNCs. The combination of miR124 and miR9/9*, followed by Ascl1, yt1l, and NeuroD2 increased human fetal fibroblast conversion efficiencies and the three-factor combination of miR-124, Brn2, and Myt1l without Ascl1, a common factor to all neuronal conversion cocktails, was sufficient to directly reprogram adult human dermal fibroblasts to functional neurons under defined conditions. Up to now, less than three transcription factors hardly induced the directed conversion of human fibroblasts into functional neurons [26,27]. Cells generated through direct reprogramming of somatic cells are probably not tumorigenic, and give new possibilities for disease modeling and cellular repair.
However, it remains to be seen whether adult human somatic cells from a skin biopsy or a surgery could be efficiently converted into functional neurons through similar means, if feasible, which may serve as an ideal source for generating patient/disease-specific neurons. In the present study, we directly reprogram human fibroblasts from patients undergoing neurosurgery into functional iNCs by using three transcription factors (Ascl1, Myt1l, and Sox2), which lead to a distinct neuronal subtype such as γ-aminobutyric acid (GABA)ergic and glutamatergic neurons. We also demonstrate that these iNCs can express human neuronal cell markers, and express the voltage-gated ion channels, generate spontaneous action potentials, express functional neurotransmitter receptors, form excitatory or inhibitory postsynaptic currents. Moreover, we have confirmed the safety of human iNCs in vivo by observing long term. Our findings not only represent a successful generation of patient-specific neurons from the somatic cells, but also open up a new ex vivo gene therapy approach that the skin cells could be cultured and used for gene transfer, and furthermore, the iNCs could be implanted into the brain to repair injury.
Materials and Methods
Ethics statement
Human fibroblasts were derived from the scalp samples of patients with traumatic brain injury (TBI), which were collected after obtaining informed consent for a protocol approved by the Institutional Review Board Committee of Huashan Hospital Shanghai.
All animal experiments were performed according to the guidelines that were approved by the Animal Ethics Committee of Fudan University in Shanghai.
Cell cultures of adult human fibroblasts from patient
Briefly, the skin tissue was harvested from the patient's scalp in neurosurgery following informed consent under protocols approved by the Institutional Review Board Committee of Huashan Hospital Shanghai. Each tissue was washed with sterile HBSS containing antibiotics (1% penicillin/streptomycin), then dissected blood vessels, hair follicles, and subcutaneous fat carefully under an anatomical microscope. Use eye scissors to cut the piece into strips as small as possible, and dissociate the minced tissue pieces with collagenase IV and trypsin (Sigma) in a Falcon tube. Subsequently, cells were filtered and plated in the DMEM (Gibco) supplemented with 10% fetal bovine serum (Hyclone), 1×GlutaMAX (Invitrogen), 0.1 mM nonessential amino acids (Gibco), and 0.5% penicillin and streptomycin (Gibco). The medium was changed every other day. Primary fibroblasts were expanded for three to five passages to remove other types of cells (such as keratinocytes) and retained fibroblasts only.
Lentivirus infection and iNC generation
Human cDNAs for Ascl1, Myt1l, and Sox2 were subcloned into the lentiviral vector pHRST-IRES-GFP. The 293FT cells (Invitrogen) were used to produce sufficient lentivirus for infection. One day before transduction, human fibroblasts were seeded at 5×105 cells/2 mL/well of a six-well gelatin-coated plate. Viruses supplemented with polybrene (5 mg mL−1) were added into the culture medium and incubated for 24 h. To improve the efficiency of transduction, two rounds of viral infection were performed 24 h apart or incubated cells with a virus for a subsequent 48 h. After the viral supernatants were removed, the medium was replaced with the N3 medium [DMEM/F2 (Invitrogen), 500 mg mL−1 transferrin (Sigma), 25 mg mL−1 insulin (Sigma), 30 nM sodium selenite (Sigma), 20 nM progesterone (Sigma), 100 nM putrescine (Sigma), and penicillin/streptomycin(Sigma)] supplemented with neurotrophic factors, including the brain-derived neurotrophic factor, glial cell-derived neurotrophic factor, neurotrophin-3, and ciliary neurotrophic factor. The culture medium was changed every other day.
Efficiency calculation
By following the method used by Vierbuchen et al. [20], the total number of GFP/Tuj1 double-positive cells with a neuronal morphology and absence of fibroblast-like morphology was quantified. Cell counting was performed in ten randomly selected 10× visual fields from three replicates. The conversion efficiency was determined by dividing the number of Tuj1/Map2 double-positive neurons formed by the number of fibroblasts plated before infection. Data are presented as mean±SD.
Western blotting
Cells were harvested and lysed in the RIPA lysis buffer [50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% Nonidet P-40 (NP-40), 0.5% sodium deoxycholate, and 0.1% SDS], supplemented with 1×phosphatase inhibitor cocktail (Pierce) and 1×protease inhibitor cocktail (Roche). Twenty-five to 100 μg cell lysate proteins were heated for 5 min at 65°C in 2×SDS sample buffer, and separated by electrophoresis on 5% or 10% polyacrylamide gel and transferred onto a polyvinylidene difluoride membrane (Millipore). Antibodies used for western blotting were anti-Ascl1 (1:200; Santa Cruz), anti-Sox2 (1:500; Abcam), anti-Myt1l (1:200; Abnova), anti-Map2 (1:500; Sigma), anti-NeuN (1:500; Millipore), anti-Synapsin (1:500; Millipore), and anti-p53 (1:500; Millipore).
Quantitative real-time polymerase chain reaction
Total RNA was isolated by using Trizol (Invitrogen), according to the manufacturer's instructions (TRIzol® Reagent). For each sample, 1–2 μg of RNA was used for reverse transcription performed with random primers and Super-Script III Reverse Transcriptase (Invitrogen). Each cDNA was diluted 1:20, and 2 μL was used for each real-time reaction. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed with SYBR Green in a two-step cycling protocol on 7700 Fast Real-Time PCR System (Applied Biosystems). All the quantitative PCRs were done in triplicate, and the expression data were averaged upon normalization to GAPDH expression. Data were quantified with the ΔΔCt method. The primer sequences are listed in previous publications [1,21].
Immunocytochemical analysis
Cells were fixed in 4% paraformaldehyde for 10 min at room temperature and washed three times with phosphate-buffered saline (PBS). Permeabilized cells with 0.25% Triton X-100 in PBS for 8 min at room temperature, then incubated the cells in 10% normal donkey or goat serum (Chemicon) in PBS with 0.25% Triton X-100 for 1 h at room temperature for blocking. After rinsed with PBS, cells were incubated with primary antibodies overnight at 4°C, and then labeled with secondary antibodies. Primary antibodies included Vimentin (1:200; Abcam), Nestin (1:200; Abcam), collagen I (1:200; Abcam), Pax6 (1:200; Abcam), Sox10(1:200; Abcam), Nanog (1:100; Santa Cruz),Tuj1 (1:200; Abcam), Map2 (1:100; Abcam), GABA (1:100; Millipore), vGluT1 (1:100; Millipore), glial fibrillary acidic protein (GFAP, 1:250; Abcam), and MBP(1:100; Abcam). Rinse the cells in PBS with serum three times for 10 min, and then incubate the cells in secondary antibodies. Secondary antibodies used were Alexa Fluor 555 donkey anti-mouse IgG (1:200; Invitrogen) and Alexa Fluor 647 goat anti-mouse IgG (1:200; Invitrogen). 4′,6-diamidino-2-phenylindole (DAPI) was used for nuclei counterstaining.
Electrophysiology
The electrophysiological recordings were performed from human iNCs in voltage-clamp and current-clamp mode at room temperature. The internal solution for voltage-clamp recordings contained (in mM) as follows: 140 CsCl, 10 NaCl, 0.1 CaCl2, 2 MgCl2, 10 HEPES, and 1 EGTA (pH 7.2, 310mOsm). The internal solution for current-clamp recordings contained (in mM) as follows: 140 KCl, 0.5 EGTA, 5 HEPES, and 3 Mg-ATP (pH 7.3, 300mOsm). The external bath solution contained (in mM) as follows: 142 NaCl, 3 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 HEPES (pH 7.3, 320mOsm). To prepare brain slices, recipient mice were anesthetized, decapitated on ice, and the brains were quickly transferred into an ice-cold dissection buffer containing (in mM) as follows: 254 sucrose, 10 D-glucose, 25 NaHCO3, 2 CaCl2, 3 KCl, 2 MgSO4, and 1.25 NaH2PO4 (pH 7.4 after application of 95% O2+5% CO2, ∼305mOsm). Coronal slices (300 μm thickness) were prepared with a vibratome (Leica) and placed on a holding chamber containing oxygenated artificial cerebrospinal fluid (ACSF, in mM) as follows: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 10 D-glucose, 2 MgSO4, and 1 CaCl2, incubating at 28°C–32°C for >1 h before use. Whole-cell recordings were made in current-clamp mode with a Multiclamp 700B amplifier (Axon instruments). Electrodes had resistances of 2–4 MΩ when filled with an intracellular solution (in mM) as follows: 150 K-gluconate, 10 HEPES, 0.2 EGTA, 4 KCl, 2 NaCl, 14 phosphocreatine, 2 Mg-ATP, and 0.3 Na-GTP (pH 7.35, ∼305mOsm). In voltage-clamp mode, cells were held at a membrane potential between −60 mV and −70 mV. For the initiation voltage-gated currents, we used voltage steps from −90 mV to +50 mV in 10 mV increments. In current-clamp mode, cells were recorded for up to 25 min to examine possible spontaneous firing. Data were filtered at 10 kHz, digitized at 20 kHz, and analyzed with Clampfit 9.2 (Axon instruments).
Results
Preparation of adult human fibroblasts from patients
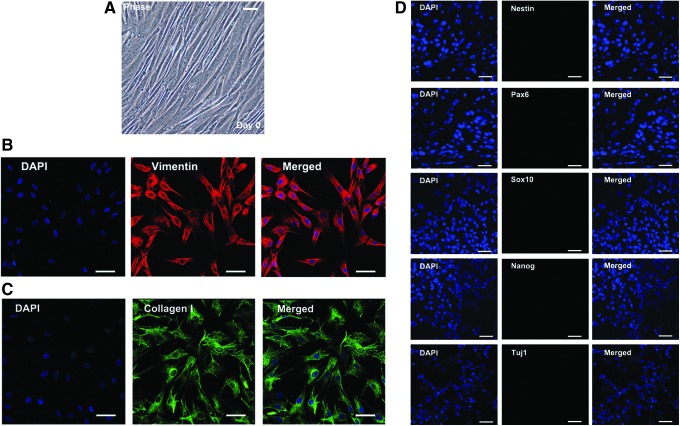
First, we collected the scalps of patients with TBI under informed consent, since TBI is one of leading causes of disability and death nowadays; our previous studies showed that autologous stem cell transplantation made a significant contribution to the recovery of TBI patients [28–30]. The scalp tissues were dissociated by enzymes and plated under standard fibroblast conditions (DMEM containing 10% FBS and 0.5% penicillin and streptomycin). After three passages, we checked the morphology and antigenic expression pattern of cultured cells, which exhibited a typical fibroblast-like morphology and a reliable fibroblast marker Vimentin. Although in some aspects Vimentin is a neural stem cell (NSC) marker, yet Nestin is a widely recognized protein marker for NSCs, and if the cells express Vimentin whereas not Nestin, we could consider they are indeed fibroblasts and not NSCs. Moreover, the cultured cells expressing collagen I without the neuroepithelial marker Pax6, the neural crest marker Sox10, and the stem cell marker Nanog, confirmed their fibroblast identity and not derived from neural crest derivatives or stem cells. Besides, the absence of neuronal markers Tuj1 showed that the starting cells contain no neurons (Fig. 1A–D).
FIG. 1.
Establishment of human adult scalp fibroblast culture. (A) Morphology of untransduced human adult scalp fibroblasts. (B) Human adult scalp fibroblasts exhibited a reliable fibroblast marker Vimentin; (C) positive for collagen I (D) but do not express Nestin, Pax6, Sox10, Nanog, and Tuj1. Scale bars: 50 μm.
Transcription factor screen and generation of human iNCs
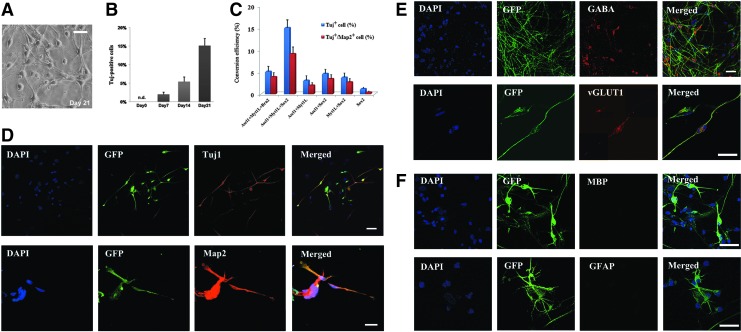
Inspired by recent findings, we surmised that the iNC reprogramming shared common mechanisms with iPS cells [31]. To test this hypothesis, we set a pool of transcription factors containing neural-specific transcription factors (Ascl1, Myt1l, and Brn2) and lineage-specific transcription factors (Oct4, Sox2, Klf4, and c-Myc). These genes were cloned into the lentiviral vectors harboring a GFP reporter, and then we virally transduced fibroblasts with different unique combinations of these vectors for the factor screen. Although most of our combinations can induce a neuron-like phenotype, including expression of Tuj1 and Map2, we observed that the substitution of Brn2 by Sox2, improved the conversion efficiency threefold than Ascl1 + Brn2 + Myt1l and Ascl1 + Myt1l, Ascl1 + Sox2, Myt1l + Sox2, or Sox2 alone resulted in a significant reduction in conversion efficiency. Thus, based on the number of cells and morphological criteria, the combination of Ascl1, Myt1l, and Sox2 was the most efficient for iNC induction, which gave the highest conversion efficiency 9.15%±1.71% (Fig. 2C).
FIG. 2.
Induction of human induced neuronal cells (iNCs) from patient-derived fibroblasts. (A) Ascl1, Myt1l, and Sox2 transduced fibroblasts exhibited a mature neuronal morphology resembling neurons at 21 days after transduction. (B) Quantification of GFP/Tuj1 double-positive human iNCs. (C) The percentage of Tuj1/Map2 double-positive iNCs reflects the human iNC conversion efficiency. (D) Immunocytochemistry of Tuj1 and Map2. (E) Human iNCs expressed neuronal subtype marker γ-aminobutyric acid (GABA) and vGluT1. (F) iNCs were negative for MBP and glial fibrillary acidic protein (GFAP). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bars: 50 μm.
After two rounds of viral infection, the cells were cultured in the N3 neural induction medium [32]. About 7 days later, transduced GFP+ cells presented simple neuronal morphology with a small cell body and mono- or bipolar neurite. Fourteen days after transduction, we can observe that those GFP+ cells exhibited more complex neuronal morphologies similar to that of human neuronal cells with long fine processes resembling neuronal axons. After 21 days, cells with more mature neuronal morphologies were positive for neuronal markers, including Tuj1 (also known as βIII-tubulin) and MAP2 (Fig. 2A, D). The GFP+/Tuj1+ neuronal cells were quantified at indicated time points after transduction, the number of double-positive cells peaked at 21 days (Fig. 2B).
In contrast, the cells cultured in neural induction media without viral infection were not Tuj1 positive although some morphological changes occurred, and the lentiviral expression of GFP alone in human fibroblasts was not capable for reprogramming in spite of treating identically. Both of the two controls confirmed that human fibroblasts lack spontaneous neuronal differentiation potential without the presence of transcription factors.
Human iNCs express human neural subtype markers
It would be of high interest if the presented combination leads to a distinct neuronal subtype. We sought to characterize the neurotransmitter phenotype of human iNCs. Been further cultured more than 30 days, we detected vGLUT1-positive puncta outlining GFP-positive cells, indicating the presence of excitatory, glutamatergic neurons. In addition, we found iNCs labeled with antibodies against GABA, the major inhibitory neurotransmitter in the brain (Fig. 2E). Although Vierbuchen et al. stated a low proportion of mouse iNCs expressed markers of GABAergic neurons [20], indeed, 5.3% of our AMS human iNCs expressed GABA, and 2.1% were vGLUT1 positive, whereas we were unable to detect tyrosine hydroxylase, choline acetyltransferase, or serotonin expression.
Nevertheless, there were no GFAP-positive or Myelin basic protein (MBP)-positive cells in iNC cultures, revealing that human fibroblasts could be not converted into astroglia or oligodendroglia under this culture condition (Fig. 2F).
Characterization of protein and gene expression in human iNCs
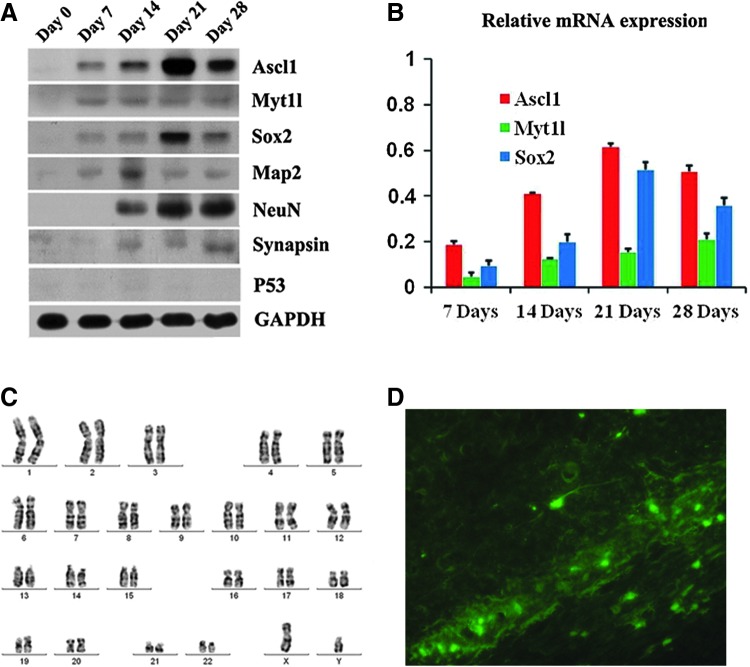
Moreover, we conducted western blot analysis of iNCs with the three transcripts and some markers of neural lineages. The results showed a tendency toward upregulation of Ascl1 and Sox2 from day 7 to 28, while Myt1l maintained at a relatively constant expression level. Nevertheless, at day 28, all the three transcripts had a lower expression level than before. So, transcription factors might play an initiating role in the reprogramming process. Protein levels of Map2, NeuN, and Synapsin with a disparity in expression time and quantity reflected a conversion of fibroblasts to neurons. We also analyzed the P53 protein expression level, its consistent low level showed a probable safety (Fig. 3A). qRT-PCR analysis corroborated these results (Fig. 3B).
FIG. 3.
Characterization of human iNCs. (A) Western blot analysis of lentiviral-mediated genes and neural marker genes. (B) Quantitative real-time polymerase chain reaction analysis of the three transcription factor expression iNCs harvested at multiple time points during the direct reprogramming process. (C) Normal karyotype observed with iNCs. (D) GFP+ cells were found integrated in the host brain.
Functional characterization of human iNCs
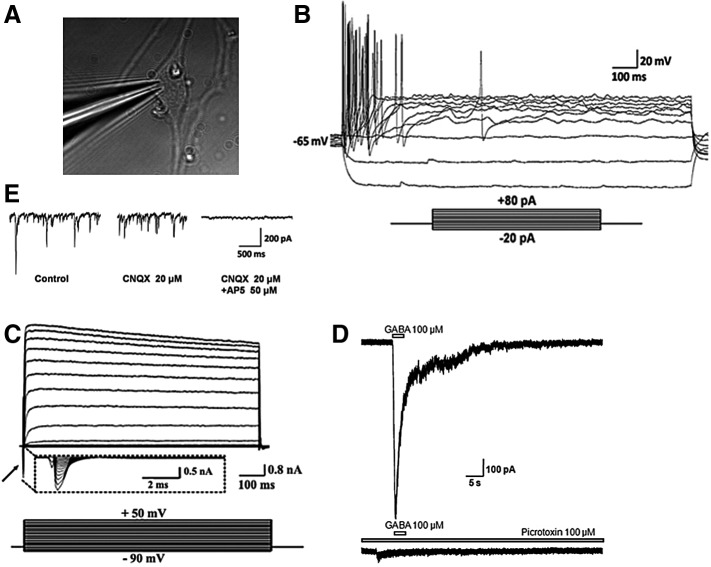
Next, we performed patch-clamp recordings to evaluate the electrophysiological phenotype of human iNCs after day 30 of initial induction. Whole-cell patch-clamp recordings confirmed that iNCs exhibited properties of functional mature neurons. About 80% (n=12 out of 15 cells recorded) of human iNCs fired mature action potentials in response to depolarizing current injection (Fig. 4A), and expressed the voltage-gated inward Na+ and outward K+ currents (Fig. 4B). We further evaluated the GABAergic ligand-gated ion channel activity in human iNCs. Human iNCs responded to exogenous puff application of GABA (100 μM), displaying typical hyperpolarizing currents, which means there existed GABA receptors (Fig. 4C). We also detected spontaneous excitatory postsynaptic currents, which could be reversibly blocked by receptor antagonists (Fig. 4D). Thus, human iNCs showed several major electrophysiological properties of mature neurons in terms of temporal parameters of action potentials, spontaneous spiking activity, and inhibition of cell firing through different receptor antagonists.
FIG. 4.
Membrane properties of human iNCs. (A) Picture of infrared DIC images of a cultured iNC after formation of a gigaseal. (B) Action potential firing characteristics of human iNCs. Ten repetitive traces are elicited in response to depolarizing current injections, increased from −20 to +80 pA in 10 pA increments. (C) In voltage-clamp mode, both voltage-gated sodium (INa) (arrow) and delayed outwardly rectifying potassium currents (Ik) were observed. (D) Human iNCs respond to exogenous application of GABA (100 μM). Lower panel showing that the GABA-induced current response could be blocked by application of picrotoxin (30 μM). (E) Human iNCs showed glutamate-mediated excitatory postsynaptic currents, which were partially blocked by the competitive AMPA/kainate receptor antagonist CNQX (20 μM), and the rest component was totally abolished by the NMDA receptor antagonist AP5 (50 μM).
Human iNCs were safe from tumorigenesis and cytogenetic abnormalities
In theory, one major advantage of skipping the pluripotent stage is the avoidance of tumor formation [33], so it is essential to confirm that the human iNCs were safe from tumorigenesis. We transplanted iNCs subcutaneously into NOD/SCID mice over both short (3 month) and long (6 month) term; no teratoma emerged (n=15 out of 15 mice recorded). We also injected iNCs into the brains of NOD/SCID mice to see whether tumorigenesis happens; no neural tumors occurred (n=10 out of 10 mice recorded) and 6 weeks after transplantation, GFP+ cells were found integrated in the host tissue showing a long-term survival of iNCs in mice (Fig. 3D). In addition, normal karyotype of human iNCs compared was confirmed (Fig. 3C).
Discussion
Our findings demonstrate the feasibility of scalp fibroblasts from patients directly reprogramming into functional neurons with three transcription factors without going through an intermediate pluripotent stage. Substitution of Brn2 by Sox2 in human research improved reprogramming efficiency probably because Sox2 is an essential factor involved in establishing early neural cell fate decisions. In a separate study, a combination of nine factors containing Sox2 reprogrammed sertoli cells into induced neural stem cells (iNSCs) [34]. It also has been reported that three factors, Sox2, Brn2, and FoxG1 can reprogram mouse fibroblasts to tripotent, self-renewing induced neural progenitor cells (iNPCs) [35]. Recently, two studies have shown that the combinations of Sox2, Klf4, and c-Myc or Sox2, Brn4, Klf4, c-Myc, and E47/Tcf3 can reprogram mouse fibroblasts into iNSCs [36,37]. Most recently, direct reprogramming of mouse and human fibroblasts into multipotent NSCs with only Sox2 had been reported [38], and nonviral generation of neural precursor-like cells from adult human fibroblasts also using Sox2 as one of the two transcription factors [39]. We hypothesized that Sox2 and Ascl1 are thought to establish a profile of homeodomain transcription factors that is necessary for the subsequent reprogramming of human cells [40]. It is speculated that the Sox2-Ascl1 complex regulates the structure of chromatin, thus allowing binding of Myt1l to their specific target sites and leading to full activation of the neuronal genesis program [41].
A major concern in using primary fibroblasts for reprogramming is that neural crest derivatives could be present in the starting cell population and they serve as the cellular origin of induced cells [42]. To exclude this trepidation, we carefully screened the cells with a pack of antibodies against neural progenitors, neuroepithelial cells, neural crest cells, and stem cells after three passages. The results demonstrated that the starting cell population does not contain any neural crest derivatives.
Recently, direct conversion of human fibroblasts to dopaminergic neurons opens new possibilities for regenerative therapies for neurodegenerative disease and related disorders [22,23]. Our results also demonstrated that additional fate specification of iNCs is possible, such as GABAergic neurons, which were considered to play a critical role in modulating neuropsychiatric behaviors and indicate that manipulations of this neuronal subtype may be a promising avenue for therapeutic interventions [43]. One of the next important steps will be to generate human iNCs of other specific neuronal subtypes.
A big concern in using iNCs is the tumor formation. Unlike those studies, which direct reprogramming of fibroblasts into iNSCs or iNPCs, our protocol generated only neurons without GFAP-positive astroglia or MBP-positive oligodendroglia, which means the iNCs were not tumorigenic. We also have confirmed the safety of human iNCs in vivo by observing long term. How human iNCs might impact on the overall functional aspects of the host brain will need to be tested further in long-term in vivo transplantation studies. An ultimate goal of iNCs will be using the direct reprogramming strategy for cell therapy. Our findings indicate that the patient-specific iNCs were similar to human neuronal cells in morphology, surface antigens, gene expression, and electrophysiological signals, such as expressing the voltage-gated ion channels, generating spontaneous action potentials, expressing functional neurotransmitter receptors, and forming excitatory or inhibitory postsynaptic currents. Even if it is a far way for us to advance this technology for potential regenerative medicine, the generation of patient-specific iNCs still provides a reasonable basis for future exploration of neural cell therapy.
Acknowledgments
This study was supported by grants (81271003, 2010CB945500, 2012CB966300, 2009CB941100, and 11JC140100) from the National Nature Science Foundation, Ministry of Science and Technology of China and Shanghai City Science Foundation. J Z. is a Cheung Kong Professorship Scholar of the Ministry of Education.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K. and Yamanaka S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- 2.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, et al. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- 3.Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, et al. (2008). Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321:1218–1221 [DOI] [PubMed] [Google Scholar]
- 4.Ebert AD, Yu J, Rose FF, Jr., Mattis VB, Lorson CL, Thomson JA. and Svendsen CN. (2009). Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457:277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K. and Daley GQ. (2008). Disease-specific induced pluripotent stem cells. Cell 134:877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soldner F, Hockemeyer D, Beard C, Gao Q, Bell GW, Cook EG, Hargus G, Blak A, Cooper O, et al. (2009). Parkinson's disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136:964–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL. and Melton DA. (2009). Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 106:15768–15773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye Z, Zhan H, Mali P, Dowey S, Williams DM, Jang YY, Dang CV, Spivak JL, Moliterno AR. and Cheng L. (2009). Human-induced pluripotent stem cells from blood cells of healthy donors and patients with acquired blood disorders. Blood 114:5473–5480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Cai J, Zhang Y, Wang X, Li W, Xu J, Li F, Guo X, Deng K, et al. (2010). Induced pluripotent stem cells can be used to model the genomic imprinting disorder Prader-Willi syndrome. J Biol Chem 285:40303–40311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee G, Papapetrou EP, Kim H, Chambers SM, Tomishima MJ, Fasano CA, Ganat YM, Menon J, Shimizu F, et al. (2009). Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461:402–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raya A, Rodríguez-Pizà I, Guenechea G, Vassena R, Navarro S, Barrero MJ, Consiglio A, Castellà M, Río P, et al. (2009). Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, et al. (2010). Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest 120:3127–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, et al. (2010). Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N Engl J Med 363:1397–1409 [DOI] [PubMed] [Google Scholar]
- 14.Ghodsizadeh A, Taei A, Totonchi M, Seifinejad A, Gourabi H, Pournasr B, Aghdami N, Malekzadeh R, Almadani N, Salekdeh GH. and Baharvand H. (2010). Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev 6:622–632 [DOI] [PubMed] [Google Scholar]
- 15.Choi J, Costa ML, Mermelstein CS, Chagas C, Holtzer S. and Holtzer H. (1990). MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A 87:7988–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie H, Ye M, Feng R. and Graf T. (2004). Stepwise reprogramming of B cells into macrophages. Cell 117:663–676 [DOI] [PubMed] [Google Scholar]
- 17.Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE. and Raphael Y. (2005). Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 11:271–276 [DOI] [PubMed] [Google Scholar]
- 18.Zheng JL. and Gao WQ. (2000). Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci 3:580–586 [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Brown J, Kanarek A, Rajagopal J. and Melton DA. (2008). In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC. and Wernig M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Südhof TC. and Wernig M. (2011). Induction of human neuronal cells by defined transcription factors. Nature 476:220–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfisterer U, Kirkeby A, Torper O, Wood J, Nelander J, Dufour A, Björklund A, Lindvall O, Jakobsson J. and Parmar M. (2011). Direct conversion of human fibroblasts to dopaminergic neurons. Proc Natl Acad Sci U S A 108:10343–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caiazzo M, Dell'Anno MT, Dvoretskova E, Lazarevic D, Taverna S, Leo D, Sotnikova TD, Menegon A, Roncaglia P, et al. (2011). Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476:224–227 [DOI] [PubMed] [Google Scholar]
- 24.Qiang L, Fujita R, Yamashita T, Angulo S, Rhinn H, Rhee D, Doege C, Chau L, Aubry L, et al. (2011). Directed conversion of Alzheimer's disease patient skin fibroblasts into functional neurons. Cell 146:359–371 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Son EY, Ichida JK, Wainger BJ, Toma JS, Rafuse VF, Woolf CJ. and Eggan K. (2011). Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW. and Crabtree GR. (2011). MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambasudhan R, Talantova M, Coleman R, Yuan X, Zhu S, Lipton SA. and Ding S. (2011). Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu J, Zhou L, Ge F. and Wu X. (2006). Tracking neural stem cells in brain trauma patients. N Engl J Med 355:2736–2738 [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Wu X. and Zhang HL. (2005). Adult neural stem cell therapy: expansion in vitro, tracking in vivo and clinical transplantation. Curr Drug Targets 6:97–110 [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Shen YW, Chen LP. and Zhang HL. (2008). Biology and therapy of adult human brain stem cells. Cell Res 18:s113 [Google Scholar]
- 31.Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K. and Ding S. (2011). Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci U S A 108:7838–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wernig M, Tucker KL, Gornik V, Schneiders A, Buschwald R, Wiestler OD, Barde YA. and Brüstle O. (2002). Tau EGFP embryonic stem cells: an efficient tool for neuronal lineage selection and transplantation. J Neurosci Res 69:918–924 [DOI] [PubMed] [Google Scholar]
- 33.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, et al. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27:743–745 [DOI] [PubMed] [Google Scholar]
- 34.Sheng C, Zheng Q, Wu J, Xu Z, Wang L, Li W, Zhang H, Zhao XY, Liu L, et al. (2012). Direct reprogramming of Sertoli cells into multipotent neural stem cells by defined factors. Cell Res 22:208–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lujan E, Chanda S, Ahlenius H, Sudhof TC. and Wernig M. (2012). Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proc Natl Acad Sci U S A 109:2527–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, et al. (2012). Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–479 [DOI] [PubMed] [Google Scholar]
- 37.Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, et al. (2012). Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10:465–472 [DOI] [PubMed] [Google Scholar]
- 38.Ring KL, Tong LM, Balestra ME, Javier R, Andrews-Zwilling Y, Li G, Walker D, Zhang WR, Kreitzer AC. and Huang Y. (2012). Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 11:100–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maucksch C, Firmin E, Butler-Munro C, Montgomery JM, Dottori M. and Connor B. (2012). Non-viral generation of neural precursor-like cells from adult human fibroblasts. J Stem Cells Regen Med 8:162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chew JL, Loh YH, Zhang W, Chen X, Tam WL, Yeap LS, Li P, Ang YS, Lim B, Robson P. and Ng HH. (2005). Reciprocal transcriptional regulation of Pou5f1 and Sox2 via the Oct4/Sox2 complex in embryonic stem cells. Mol Cell Biol 25:6031–6046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okumura-Nakanishi S, Saito M, Niwa H. and Ishikawa F. (2005). Oct-3/4 and Sox2 regulate Oct-3/4 gene in embryonic stem cells. J Biol Chem 280:5307–5317 [DOI] [PubMed] [Google Scholar]
- 42.Sareen D. and Svendsen CN. (2010). Stem cell biologists sure play a mean pinball. Nat Biotechnol 28:333–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, et al. (2009). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature 468:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]