Abstract
Studies in model organisms constitute the basis of our understanding of the principal molecular mechanisms of cell fate determination in the developing central nervous system. Considering the emergent applications in stem cell-based regenerative medicine, it is important to demonstrate conservation of subtype specific gene expression programs in human as compared to model vertebrates. We have examined the expression patterns of key regulatory genes in neural progenitor cells and their neuronal and glial descendants in the developing human spinal cord, hindbrain, and midbrain, and compared these with developing mouse and chicken embryos. As anticipated, gene expression patterns are highly conserved between these vertebrate species, but there are also features that appear unique to human development. In particular, we find that neither tyrosine hydroxylase nor Nurr1 are specific markers for mesencephalic dopamine neurons, as these genes also are expressed in other neuronal subtypes in the human ventral midbrain and in human embryonic stem cell cultures directed to differentiate towards a ventral mesencephalic identity. Moreover, somatic motor neurons in the ventral spinal cord appear to be produced by two molecularly distinct ventral progenitor populations in the human, raising the possibility that the acquisition of unique ventral progenitor identities may have contributed to the emergence of neural subtypes in higher vertebrates.
Introduction
Numerous studies have demonstrated that multipotent stem cells can be directed to form clinically relevant cell types, such as oligodendrocytes, motor neurons (MN), and mesencephalic dopamine neurons (mesDN), which is promising for future cell-based treatments or modeling of diseases, including multiple sclerosis (MS), amylotrophic lateral sclerosis (ALS), and Parkinson's disease [1–3]. The safety and efficacy in such efforts depend on efficient in vitro differentiation protocols and reliable identification criteria of specific cellular subtypes. To date, developmental studies in model organisms have been instrumental in providing both neuronal subtype specific markers and an understanding of how cell intrinsic and extrinsic factors can be utilized in directing neuronal cell fate in vitro [4–6].
Studies of neural tube development have revealed principal mechanisms for cell fate determination in central nervous system (CNS) development. A crucial initial step is the establishment of morphogen gradients along the dorsoventral (D-V) and anterioposterior (A-P) axes, forming a grid-like set of regional cues in the developing neural tube. By controlling the expression of distinct sets of transcription factors, positional identities (patterning) are provided to progenitor cells depending on their location in the neural tube [7]. These discrete expression patterns are further refined by specific microRNAs and repressive interactions between specific sets of transcription factors, ultimately resulting in the establishment of progenitor domains, each defined by a unique molecular expression profile [8,9]. As progenitor cells within such domains exit the cell cycle, these transcription factor codes are deciphered into subtypes of specific differentiation programs [7] and distinct subtypes of neurons arise.
The regional specification of neuronal subtypes is largely conserved among vertebrates, although minor mechanistic variations have been observed [10]. In view of the emergent applications of developmental studies in human regenerative medicine, it is important to determine human expression patterns of regulatory genes at a cellular resolution during early stages of fate specification, as well as in the subsequent differentiation and maturation. In recent years, there are a growing number of online public resources [eg, Allen Brain Atlas (www.brain-map.org); HUDSEN (www.hudsen.org)] providing human brain region specific transcriptome profiling and/or in situ hybridization of the human CNS, which doubtless will advance the field. However, combinatorial gene expression studies with cellular resolution in human at stages when neuronal subtype specification takes place (3–7 weeks) are still very few [11–13].
In this study, we provide a detailed analysis of the early human gene expression pattern in progenitor cells and their neuronal and glial descendants in regions that are well characterized in mouse and chicken, including spinal cord, hindbrain, and midbrain. In particular, we have focused on mapping the human expression pattern of key progenitor transcription factors and postmitotic markers for clinically relevant CNS cells, such as mesDNs, serotonin neurons (5HTNs), MNs, and oligodendrocytes. Although the majority of the expression patterns analyzed were conserved between chick, mouse, and human, we also identified a number of human-specific features. These novel findings might not only serve as guidelines for the identification criteria of human cell types in stem cell differentiations but may also help to develop more efficient cell differentiation protocols aided toward novel treatments of human neurological disorders.
Materials and Methods
Maintenance and differentiation of embryonic stem cells
Mouse embryonic stem cells (mESCs) (E14.1) were maintained as described [14]. Differentiation was performed in monolayer culture in N2B27 differentiation medium [14,15] supplemented with 0.1 μM all-trans retinoic acid (RA) (Sigma) and 100 nM Sonic Hedgehog (Shh) agonist (Hh-Ag1.3) (Curis) for 5 days.
Human embryonic stem cell (hESC) lines (HS401, HS293, and HS351) were maintained as previously described [16]. To induce differentiation, hESCs were mechanically dissociated into small fragments and grown as embryonic bodies (EBs) in DMEM/F12:neurobasal medium supplemented with 1× B27 and 1× N2 for 5 days. EBs were plated on poly-ornithine/laminin (POL) (Sigma) coated dishes in DMEM/F12:Neurobasal medium supplemented with 0.5×B27, 0.5×N2, 10 ng/mL bFGF, and grown for 7 days until early neuroepithelial rosettes were visible (all cell culture media were from Invitrogen). After 2 days of adherent culture, the medium was supplemented with 70 nM Hh-Ag1.3 and 100 ng/mL FGF8 (R&D) for midbrain-like differentiation or with 1 μM RA and 200 nM Hh-Ag1.3 for ventral spinal cord-like differentiation. After 1 week, neuroepithelial rosettes were mechanically isolated and plated on fresh POL coated dishes in Neurobasal medium supplemented with 1×N2. The three different hESC lines gave very similar results. See Supplementary Fig. S1 (Supplementary Data are available online at www.liebertpub.com/scd) and S5 for schematic drawings of the differentiation protocols.
Tissue and ESC preparation
Human embryos (4–10 weeks of gestation) were collected after elective routine abortions with consent given by the pregnant woman and approval from the Regional Human Ethics Committee, Stockholm. Tissue was fixed in 4% paraformaldehyde (PFA) for 1–2 h on ice, washed in phosphate-buffered saline (PBS), and transferred to 30% sucrose in PBS over night. Embryos were subsequently mounted in Tissue-tek and cryosectioned. Mouse and chicken embryonic tissue were prepared the same way as described above. hESCs and mESCs were fixed at room temperature in 4% PFA for 10 min before analysis by immunocytochemistry.
Immunohistochemistry and in situ hybridization
Immunohistochemical localization of proteins and in situ hybridization were performed as described [17]. A slightly different in situ hybridization protocol was used [18] when applying the Sim1 probe, which was detected using Fast Red (Roche).
The antibodies used were, mouse Lmx1b, LIM1/2, LIM3, Ascl1, Nkx2.2, FoxA2, Shh, En1, Evx1/2, Hb9, Pax7, Pax3, Msx1/2, Hoxb4 (Developmental Studies Hybridoma Bank), PDGFRα (Pharmingen), Brn3a, tyrosine hydroxylase (TH) (Chemicon), GATA3 (Santa Cruz), bTuj1 (Nordic Biosite); rabbit Lbx1, Gsh1/2, Tlx3 (M. Goulding), Dbx1, Dbx2 (A. Pierani), Irx3, Sox9, Sox10 (J. Briscoe), Pax2, Pax6, Hoxb1 (Covance), 5-HT, Ptf1a (H. Edlund), Lmx1a (M. German), Phox2b (J.F. Brunet), Nurr1 (Santa Cruz), Olig2 (Chemicon), Chx10, Nkx6.1, Nkx2.2 (T. Jessell), Pet1 (J. Ericson), Brn3a; guinea pig Olig2 (chicken and mouse specific) (B. Novitch), Pitx3 (J. Ericson), Lmx1b, Isl1/2, Nkx6.1 (T. Jessell), Tlx3 (C. Birchmeier); goat Olig2 (R&D system), Bhlh5 (Santa Cruz), rat Olig1 (B. Novitch), and Olig3 (C. Birchmeier).
In situ hybridization was carried out using probes for human BMP4, BMP7, Ngn1, Sim1 (Invitrogen) GDF4, GDF7, Wnt1, Wnt3, BMP1-3, BMP5, 6, ActivinB and Atoh1 (Geneservice).
Results
Ventral spinal cord
Patterning of the ventral spinal cord has been extensively characterized in many vertebrate species, including mouse and chicken, and therefore, provides a solid basis for a comprehensive comparison to patterning processes in humans [7,19,20]. The signaling molecule Shh, initially secreted from axial mesoderm and later by the floor plate cells at the ventral midline of the spinal cord, acts in a graded manner to control the spatial expression pattern of homeodomain or basic helix-loop-helix containing transcription factors in progenitor cells. Based on the nested expression of these transcription factors, five major molecularly defined ventral progenitor domains are established, and the integrity of each domain is manifested by the formation of cross-repressive transcription factor pairs [9,21]. From each progenitor domain, upon cell cycle exit and differentiation, distinct types of interneurons (INs) or MNs are generated which are defined by subtype specific marker expression.
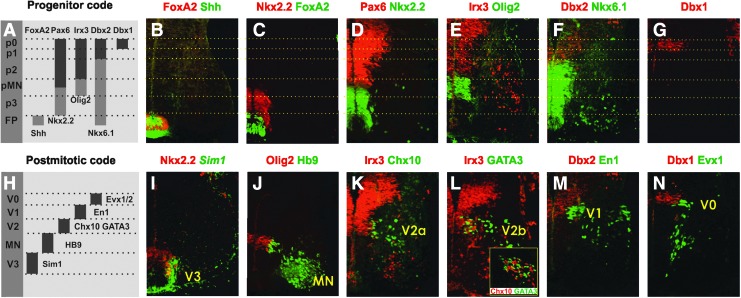
The expression pattern of key ventral regulatory genes found in model organisms was examined in the human ventral spinal cord at neurogenic stages (4–5.5 weeks) by immunohistochemistry (Fig. 1B–G). At the ventral midline, the human floor plate was distinctively marked by both Shh and FoxA2 (Fig. 1B) as described in other vertebrates [22]. Dorsal to the floor plate, the expected five main progenitor domains (p3, pMN, p2, p1, and p0) characterized by the nested expression patterns of Olig2, Nkx6.1, Pax6, Irx3, Dbx1, and Dbx2 (Fig. 1A) were also identified in humans (Fig. 1C–G) [23]. Furthermore, mutually exclusive expression patterns were observed for Pax6:Nkx2.2, Irx3:Olig2, and Dbx2:Nkx6.1, indicating cross-repression between these pairs as has been demonstrated in model organisms (Fig. 1D–G). However, in contrast to the mouse and chicken in which Nkx2.2 and Olig2 are expressed in a largely nonoverlapping fashion and abut each other at the p3/pMN boundary at neurogenic stages [7] (Fig. 2I, J), we noticed that the expression of Nkx2.2 expression (albeit weaker) encroached into the Olig2 expression domain in the human spinal cord (Fig. 1D, E). Colabeling analysis at the single cell level showed an extensive overlap; approximately one-third of the Olig2+ cells coexpressed Nkx2.2 (Fig. 2A, B, E).
FIG. 1.
Gene expression in progenitor domains and neuronal subtypes in the human ventral spinal cord. (A) Schematic drawing of the domain specific expression profile of ventral progenitor cells. (B–G) Expression of regulatory genes defining the major progenitor domains (p0–p3, pMN) and the FP (5.5 weeks). Note the mutually exclusive expression of repressor pairs in (D), (E), and (F). (H) Schematic drawing of marker gene expression in ventral neuronal subtypes. (I–N) Correlation between the generation of neuronal subtypes (V0–V3, MN) and progenitor domains, each marked by representative marker genes (5–5.5 weeks). Note that Chx10 and GATA3 were expressed in distinct cell populations (Fig. 1L infold). Dotted lines indicate borders between the progenitor domains. Pictures of two consecutive immunohistochemical stained sections were merged in (K). MN, motor neuron.
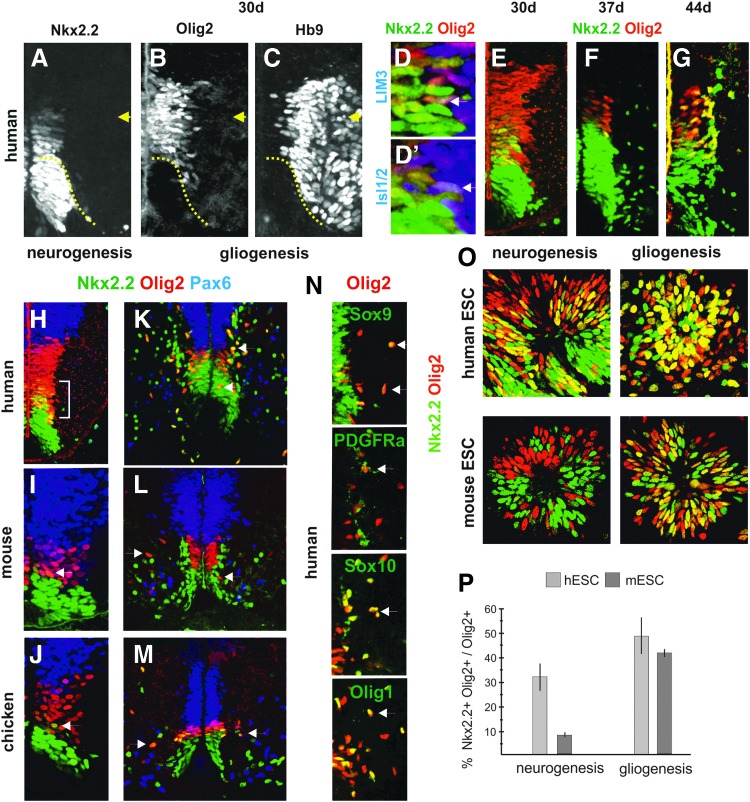
FIG. 2.
Sequential generation of MNs and OLPs from ventral spinal cord progenitor cells and ESCs. (A–C) The Nkx2.2+Olig2+ domain (between arrow and dotted line) correlates to Hb9 positive cells in the human neural tube at the D-V level (30 days). Single Nkx2.2+Olig2+ cells coexpressed LIM3 (D) or Isl1/2 (D’) (arrows) at the border between the progenitor and postmitotic zones. See Supplementary Fig. S1A for separate pictures of each staining. (E–G) Dynamic temporal expression of Nkx2.2 relative Olig2 in human embryos (30–44d). (H–J) Expression of Nkx2.2, Olig2, and Pax6 in human (4.5 weeks), mouse (E10.5), and chicken (day 4) at neurogenic stages. Bracket and arrows indicate Nkx2.2+Olig2+ cells. (K–M) Expression of progenitor and OLP markers in human (9 weeks), mouse (E12.5), and chicken (day 7.5) at gliogenic stages. Many early migratory OLPs expressed both Nkx2.2 and Olig2 in chicken and human (Fig. 2K, M, arrows) but only Olig2 in mouse (L, arrows). (N) Expression of OLP markers (arrows) in human (9 weeks). (O) Coexpression of Nkx2.2 and Olig2 in differentiating hESCs and mESCs at neurogenic stages (20 DDC) and 5 DDC, respectively) and gliogenic stages (45 DDC and 8 DDC, respectively). (P) Diagram showing the percentage of Olig2+ cells that coexpress Nkx2.2 in O. n=3, mean±SD. Immunohistochemistry was used in all pictures. D-V, dorsoventral; mESC, mouse embryonic stem cell; hESC, human embryonic stem cell; OLP, oligodendrocyte precursor cell; DDC, days of differentiation culture.
To address whether the relation between the progenitor domains and their neuronal descendants is conserved in humans, we used antibodies (or in situ RNA probe for Sim1) recognizing distinct progenitor determinants and specific markers for neuronal subtypes (Fig. 1I–N). Human expression patterns (at 5–5.5 weeks) were identical to those in chicken and mouse in the three dorsal-most domains (Fig. 1H) [24]. V0 INs, assessed by the expression of Evx1/2, appeared to be generated from the p0 (Dbx1+) domain and V1 INs, expressing Evx1/2 seemed to form from the p1 domain (ventral Dbx2+) (Fig. 1M, N). V2a and V2b INs, marked by Chx10 and GATA3 respectively, appeared to be originating from the p2 domain (ventral Irx3+ cells) (Fig. 1K, L). To reveal the neuronal descendants of the ventral-most domains, we analyzed the expression of Olig2 and Nkx2.2 in relation to postmitotic MN markers HB9, Isl1/2, and LIM3 or the V3 marker Sim1. We observed that Hb9, Isl1/2, and LIM3 positive postmitotic cells correlated well with the Olig2+ Nkx2.2− and the Olig2+Nkx2.2+ domains at the D-V level (Figs. 1J and 2A–C, data not shown). Furthermore, single Olig2+Nkx2.2+ cells coexpressing either Isl1/2, Hb9, or LIM3 were identified at the border between the progenitor and postmitotic zones (Fig. 2D, D’, data not shown and Supplementary Fig. S1A). These data strongly suggest that Olig2+Nkx2.2+ progenitor cells give rise to MNs in the human neural tube, which is in contrast to mice in which MNs only are derived from Olig2+Nkx2.2− progenitors [25]. Similar to other vertebrates though, Sim1+ V3 INs appeared to be produced from the Nkx2.2+Olig2− domain (Fig. 1I). Accordingly, while most of the established transcription factor codes defining the major ventral progenitor domains and their neuronal descendants are largely conserved in the human spinal cord (Fig. 1A, H), the pMN appears to encompass two molecularly distinct sub-domains: a dorsal Olig2+Nkx2.2− and a ventral Olig2+Nkx2.2+ domain.
Sequential generation of neurons and oligodendrocytes in the ventral spinal cord
Subsequent to the initial phase of neurogenesis, oligodendrocyte precursor cells (OLPs) are produced from discrete domains along the D-V axis of the progenitor zone [26]. The majority of OLPs in the spinal cord originates from the pMN subsequent to MN generation in both chicken and mouse [27]. Although the pMN is Nkx2.2− at neurogenic stages in chicken, an expansion of the Nkx2.2 expression into the Olig2+ domain occurs just before the commencement of oligodendrogenesis [10,25] (Fig. 2M). In mouse, the pMN domain remains Olig2+ Nkx2.2− at early gliogenic stages, but may upregulate Nkx2.2+ at later stages [25,27,28] (Fig. 2L).
To compare the expression pattern in the ventral spinal cord at neurogenic and gliogenic stages in human, mouse, and chicken, we used antibodies recognizing Olig2, Nkx2.2, and Pax6. In human embryos, the initial broad Nkx2.2+ Olig2+ expression domain (Fig. 2E, H) was successively reduced, encompassing a few cell layers at the end of neurogenic stages (Fig. 2F). At the beginning of the expected gliogenic stage at around 6 weeks (through comparison with mouse) OLPs (laterally migrating Olig2+Nkx2.2+ cells) appeared to emerge from the pMN domain, which then mostly consisted of Olig2+Nkx2.2+ cells (Fig. 2G). At subsequent gliogenic stages (9 weeks), OLPs seemed to be derived both from the Olig2+Nkx2.2+ and the Nkx2.2+ domain (Fig. 2K, arrows). We verified that those Olig2+ cells in the marginal and lateral zones expressed other known OLP markers [29,30], such as Sox9, PDGFRα, Sox10, and Olig1 (Fig. 2N). In contrast to human embryos, very few cells expressed both Olig2 and Nkx2.2 in mouse and chicken at neurogenic stages as previously described (Fig. 2I, J, arrows) [25]. At early gliogenic stages, Olig2+Nkx2.2+ OLPs appeared to emerge from the Olig2+Nkx2.2+ pMN domain in chicken, while mouse OLPs expressed Olig2 but not Nkx2.2 and seemed to arise from Olig2+ pMN progenitors (Fig. 2M, L, arrows). Taken together, these data indicate that human OLPs originate both from the Olig2+Nkx2.2+ pMN sub-domain and Nkx2.2+ p3 domain in the ventral spinal cord. However, in contrast to chicken and mouse, an Nkx2.2+Olig2+ pMN sub-domain is established at neurogenic stages.
The unique coexpression of Nkx2.2 and Olig2 at early neurogenic stages in human embryos prompted us to test whether this feature is recapitulated in vitro during hESC differentiation. To promote a ventral spinal cord-like character of neural progenitors, hESCs, and mESCs were cultivated in neural induction media supplemented with Hh-Ag1.3 and RA (Supplementary Fig. S1B, C). A substantial proportion of neural progenitors in both cultures expressed Olig2 and the HoxB4/C4 indicating a spinal cord-like identity (data not shown). Neurogenesis, in particular, MN generation (as assessed by presence of Tuj1+Isl1/2+ cells) was observed from 18 days of differentiation culture (DDC) in hESCs and 3 DDC in mESCs, while gliogenesis (as assessed by presence of Olig2+Sox10+ cells) was detected from 38 DDC in hESCs and from 8 DDC in mESCs (Supplementary Fig. S1B–E). At representative neurogenic and gliogenic stages, we analyzed the percentage of Olig2+ cells that were also positive for Nkx2.2. In hESC cultures, high percentages of Olig2+Nkx2.2+ cells were found at both stages (32% and 49%, respectively) (Fig. 2O, P). In contrast, differentiating mESCs showed a low percentage of Olig2+ cells, which coexpressed Nk2.2 at neurogenic stages (8%), while the overlap was extensive at gliogenic stages (42%) (Fig. 1O, P). The correlation between a significant population of Nkx2.2+ Olig2+ progenitors and neurogenesis in differentiating hESCs but not mESCs cultures indicate that the specific feature of the human pMN domain may be reproduced in vitro.
Dorsal spinal cord
The patterning and proliferation of the dorsal spinal cord is controlled by members of the Wnt and bone morphogenetic protein (BMP) families, which are initially secreted from epidermal ectoderm and subsequently from the roof plate at the dorsal midline [31–33]. Based on the combinatorial gene expression pattern of transcription factors, six major progenitor domains (dP1–dP6), which generate eight dorsal IN subtypes (dI1–dI6; dILa, b) have been defined [33]. Human dorsal spinal cord (5–5.5 weeks) was analyzed with regards to the expression pattern in the roof plate, progenitor domains, and INs descendants using immunohistochemistry or in situ hybridization.
In the human roof plate, we detected expression of BMP12 (GDF7), Wnt1, and Wnt3 (Supplementary Fig. S2A–C), while BMP2, 4, 5, 6, 7, 9, and ActivinB were not specifically expressed in the roof plate but had a more general expression in the spinal cord (data not shown). In addition, the transcription factor Lmx1a but not Lmx1b was detected in the human roof plate, similar to in mouse (Supplementary Fig. S2D, E) [34], but in contrast to chicken where both Lmx1a and Lmx1b are expressed [35] (Supplementary Fig. S2F).
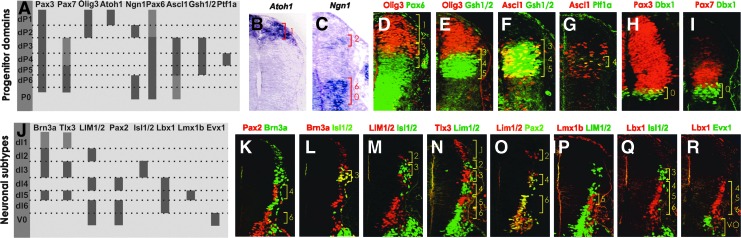
Progenitor zone patterning was analyzed in the human with respect to the previously well-studied transcription factors: Atoh1, Ngn1, Olig3, Pax6, Gsh1/2, Ascl1, Ptf1a, Pax3, Pax7, and Dbx1 [33,36–39]. We found that the expression pattern in human was reminiscent of that described in mouse (Fig. 3A–I). The expression of Pax7, however, spanned from dP1 to dP6 in chicken, but was limited to dP3–dP6 in human and mouse (Fig. 3I and Supplementary Fig. S2G, H).
FIG. 3.
Gene expression in progenitor domains and neuronal subtypes in the human dorsal spinal cord. (A) Schematic drawing of the domain specific gene expression profile in dorsal progenitor cells. (B–I) Nested expression of transcription factors in six dorsal progenitor domains (dP1–dP6) (5 weeks). (J) Schematic drawing of the subtype-specific expression in postmitotic neurons. (K–R) Combinatorial expression of marker genes defining six major dorsal neuronal subtypes (dI1–dI6) (5 weeks). Light gray indicates weak expression in A and J. Brackets indicate progenitor (dP) or neuronal (dI) domains. In (B) and (C) in situ hybridization was used, and in (D–I); (K–R) immunohistochemistry was applied.
Dorsal INs have been identified and discriminated from one another based on their combinatorial expression profile of marker genes, rather than from exclusively expressed transcription factors. Expression analysis of commonly used subtype specific markers for the dorsal INs included Pax2, Brn3a, Isl1/2, LIM1/2, Tlx3, Lmx1b, and Lbx1 [33,40]. Our analysis showed that the nested expression of all markers in mouse, and almost all in chicken, are applicable to human dorsal neuronal subtype development (Fig. 3J–R). The expression of Tlx3 has been reported to be confined to dI3 and dI5 in mouse [40], but in our analysis we detected low levels of protein also in dI1 neurons (Brn3a+) in mouse and human but not in chicken (Supplementary Fig. S2I–K).
In addition to pMN-derived OLPs, a dorsal source of OLPs has been found in the mouse [41–43]. To unravel whether human dorsal spinal cord generates OLPs, we used antibodies to Olig2 together with Pax7 or Pax3, which are exclusive dorsal marker genes (Fig. 3H, I). Olig2+Pax7+ and Olig2+Pax3+ cells emanating from dorsal positions were observed in 10-week human fetuses (Supplementary Fig. S2L; data not shown), suggesting that the human dorsal spinal cord gives rise to OLPs. In keeping with previous findings, we observed dorsal OLPs in embryonic day (E) 15.5 mouse embryos (Supplementary Fig. S2M) and also in 10 days old chick embryos (Supplementary Fig. S2N).
Ventral hindbrain
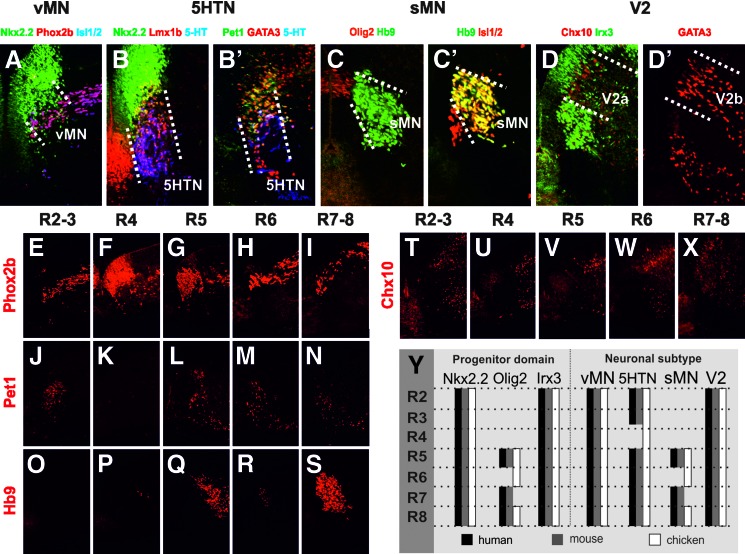
The hindbrain is compartmentalized into rhombomeres (r), which on the A-P axis primarily are patterned by Hox genes. The set of different neuronal subtypes generated along the D-V axis, thus, depend on rhombomere identity [44,45]. The human hindbrain (r2–8, 5.5 weeks) was analyzed with respect to the three ventral-most progenitor domains (Nkx2.2, Olig2 or Irx3-expressing), which in mouse and chicken produce five neuronal subtypes: visceral MN (vMNs), serotonergic cells (5HTN), somatic MN (sMNs), V2a and V2b INs, in a rhombomere-specific manner [44–47].
To define the human rhombomeres, we applied the landmarks reported in previous studies of human or mouse embryos, including Hoxb4 expression in r7–8 (Supplementary Fig. S3A–E) [48], presence of the otic vesicle around r5–6 [49] (data not shown) and distribution of Phox2b+ dorsal cell populations (pers. communication A. Pattyn) (data not shown). The ventral-most progenitor domain, marked by Nkx2.2 expression, appeared to give rise to vMNs as assessed by Phox2b and Isl1/2 (Fig. 4A) at all rhombomeric levels (Fig. 4E–I, Y). We also noted that the vMN determinant, Phox2b, was expressed in the progenitor domain selectively in r4 (Fig. 4F). 5HTNs, identified by Lmx1b, 5-HT, Pet1, and GATA3, (Fig. 4B, B′, J–N, Y) also seemed to emerge from the Nkx2.2 domain at all levels except for in r4 (Fig. 4K, Y). Dorsal to the Nkx2.2 domain in r5 and r7–8, Olig2+ progenitors appeared to give rise to sMNs expressing Hb9 and Isl1/2 ((Fig. 4C, C′, O–S). Furthermore, at all A-P levels, Irx3+ progenitors seemed to generate Chx10+ V2a and GATA3+ V2b INs (Fig. 4D, D’, T–X; data not shown). In more detail, we also determined the positioning of vMN, sMN, and V2a neuronal subtypes relative each other on the D-V and A-P axes of the hindbrain (Supplementary Fig. S3F–J).
FIG. 4.
Generation of four ventral neuronal subtypes from three progenitor domains along the A-P axis of human hindbrain. Expression of specific marker proteins for vMN (A), 5HTN (B, B’), sMN (C, C’), and V2 IN (D, D’) in relation to respective progenitor domain expression (5.5 weeks). Dotted lines indicate the borders of neuronal subtypes emerging from the characteristic progenitor domains. Generation of specific neuronal subtypes along the A-P axis using specific marker expressions for vMN (E–I), 5HTN (J–N), sMN (O–S), and V2a IN (T–X) (5.5 weeks). (Y) Schematic drawing comparing the progenitor gene expression and neuronal distribution along the A-P axis of human (black), mouse (gray), and chicken (white). Immunohistochemistry was used in all pictures. A-P, anterioposterior; IN, interneuron; vMN, visceral motor neuron; sMN, somatic motor neuron; 5HTN, serotonin neuron.
Taken together, based on the expression pattern of the selected regulatory genes, the human patterning and neuronal distribution along the D-V and A-P axes of the ventral hindbrain was highly reminiscent of that reported in mouse (Fig. 4Y). Chicken hindbrain development is also very similar but there are a few noticeable differences; 5HTNs are generated along the whole AP axis, including in r4 (unpublished observation by J.Dias) [50] and Olig2+ progenitors generate sMNs in r5, 6, and r8 [51] (Fig. 4Y).
Ventral midbrain
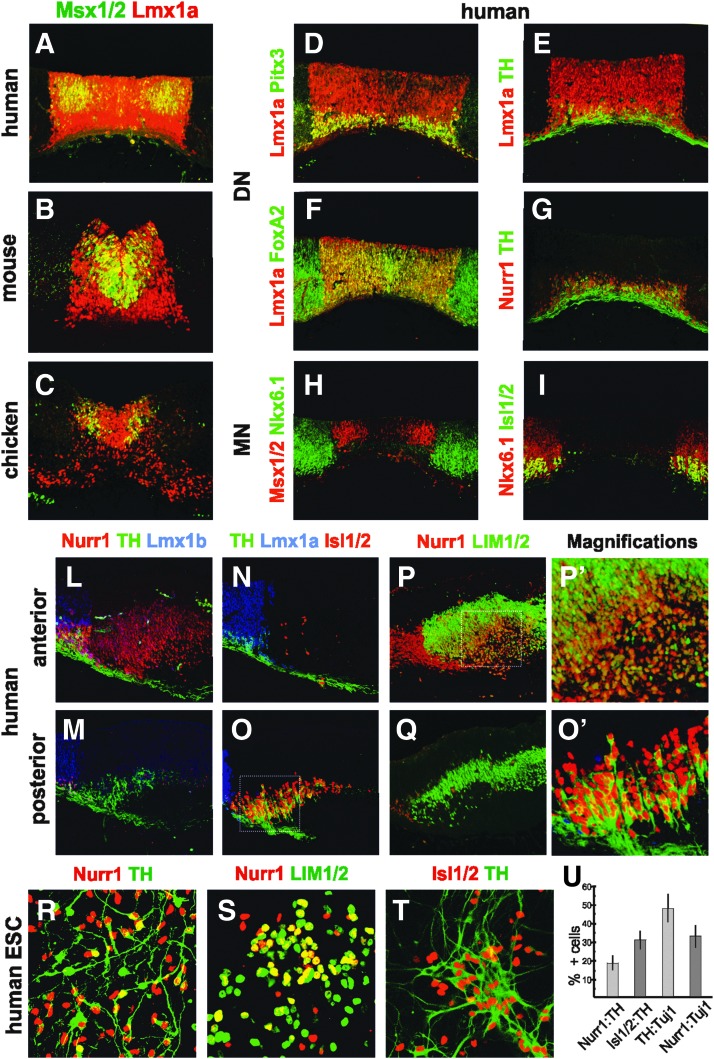
Parkinson's disease is a movement disorder associated with the degeneration of ventral mesDN in the substantia nigra (SN). There is therefore, great interest in developing protocols for the directed differentiation of human mesDN in vitro, which can be used in regenerative medicine or disease modeling [1,16,52]. The strategies and molecular markers utilized in such efforts are largely derived from developmental studies of model organisms showing that mesDNs arise at the ventral midline from a progenitor domain expressing Lmx1a, Msx1/2, Lmx1b, and FoxA2 (Fig. 5B, C and Supplementary Fig. S4B, C, E, F, H, I) [53]. As progenitor cells differentiate into mesDN precursors they continue to express Lmx1a, Lmx1b, and FoxA2 but also initiate the expression of Nurr1, TH, and Pitx3 [53]. To validate these observations for human, we have focused on determining the expression profile of commonly used mesDN regulatory genes in the early (5–5.5 weeks) developing human ventral midbrain.
FIG. 5.
Gene expression in the ventral midbrain and differentiated hESCs. (A–C) Expression of mesDN progenitor marker genes in the human (5.5 weeks), mouse (E12.5) and chicken (day 5). (D–G) Lmx1a expression demarcates the mesDN progenitor domain, generating mesDNs as assessed by specific marker expression in human (5.5 weeks). (H, I) MN (Isl1/2+) generation from Nkx6.1+ progenitor cells, abutting the Msx1/2+ domain. (L, N, P) A large anterior population of Nurr1+ cells, devoid of mesDN (Lmx1a, Lmx1b, TH) or MN (Isl1/2) markers coexpressed the IN marker LIM1/2. (M, O, Q) MNs (Isl1/2+LIM1/2−) coexpressed TH but not Lmx1b, Nurr1, or LIM1/2. Magnifications of Nurr1+LIM1/2+ cells (P’) indicated in box (P), and of TH+ Isl1/2+ cells (O’) indicated in box (O). (R–T) Significant populations of Nurr1+TH+, Nurr1+ LIM1/2+, and Isl1/2+TH+ neurons were found in differentiating hESCs at 40 DDC. (U) Diagram shows Nurr1+ out of TH+ (Nurr:TH), Isl1/2+ out of TH+ (Isl1/2:TH), TH+ out of Tuj1+ (TH:Tuj1), or Nurr1+ out of Tuj1+ (Nurr1:Tuj1). n=3 mean±SD. Immunohistochemistry was used in all pictures. mesDN, mesencephalic dopamine neuron; TH, tyrosine hydroxylase.
Progenitor Lmx1a expression specifically defines the mesDN domain and has been found to be crucial for mesDN specification both in vivo and in vitro [14]. By correlating Lmx1a expression to postmitotic mesDN markers Nurr1, TH, Pitx3, and FoxA2 [53], we could conclude that the mesDN domain in human corresponded well with Lmx1a expression (Fig. 5A, D–G) [13]. Msx1/2 expression spans the entire mesDN progenitor domain in mouse, while it in chicken forms two stripes leaving a gap at the ventral midline (Fig. 5B, C and Supplementary Fig. S4B, C) [14]. Similar to chicken, the ventral midline in human showed only sparse scattered Msx1/2+ expressing cells but was bordered by stronger expressing cells at more lateral positions (Fig. 5A and Supplementary Fig. S5A). Lmx1b was expressed at low levels in the human mesDN progenitor domain as in the mouse but in contrast to the chicken, where the expression is relatively strong (Supplementary Fig. S4G–I) [13,14]. FoxA2 was expressed in the human ventral midline but was broader than the mesDN domain, which is in agreement with previous observations in chicken and mouse (Fig. 5F; data not shown) [13,54].
The Lmx1a+ Msx1/2+ mesDN domain in mouse and chicken is flanked by progenitor cells expressing Nkx6.1, which are destined to become other neuronal subtypes, including ocular MNs [14,55,56]. Also in human we found that Msx1/2 and Nkx6.1 formed a boundary in the midbrain and that ocular MNs, assessed by Isl1/2, appeared to be generated from the Nkx6.1+ domain (Fig. 5H, I).
Correct identification of mesDNs is crucial for the development of safe and efficient cell-based therapies [1]. We, therefore, sought to validate the specificity of factors commonly used as mesDN markers in more detail. In the posterior human ventral midbrain lateral to the mesDNs we observed that a large subset of the MNs (Isl1/2) coexpressed TH (Fig. 5O, O′) but not other mesDN markers, such as Nurr1 or Lmx1b (Fig. 5M, Q). Transient TH expression has been described in human ocular MN [57], but we did not observe this in chicken or mouse (Supplementary Fig. S5J, K) [58]. Moreover, at anterior levels of the midbrain we detected a large ventral Nurr1+ cell population, which also expressed LIM1/2 (Fig. 5P, P’) but not Chx10, Lmx1a, Lmx1b, TH, or Isl1/2 (Fig. 5L, N; data not shown). A small lateral group of LIM1/2+Nurr1+ cells was detected in mouse and chicken, but the majority of the LIM1/2+ cells were Nurr1−, and most Nurr1+ cells appeared to define mesDNs (TH+ Nurr1+) (Supplementary Fig. S4L, M; data not shown). Together, patterning of the mesDN progenitor domain and the molecular marker distribution in mesDNs were largely conserved between the three species. However, TH and Nurr1, which are commonly used as specific markers for mesDNs, were also expressed in other neuronal subtypes in the human ventral midbrain.
Next we wanted to test whether the observed human-specific expression profile of TH and Nurr1 also is recapitulated in differentiating hESC cultures. To derive ventral midbrain-like neurons in vitro, hESCs were cultivated in neural inducing media supplemented with Hh-Ag1.3 and FGF8 (Supplementary Fig. S4N). At 40 DDC we found that approximately 70% of all cells expressed the neuronal marker Tuj1 (data not shown) and that around 50% and 35% of these neurons expressed TH or Nurr1 respectively (Fig. 5U). We next assessed the frequency of TH or Nurr1-expressing cells, which were not mesDN neurons. Although about 20% of the TH+ neurons coexpressed Nurr1 (Fig. 5R, U), a significant proportion of the Nurr1+ neurons coexpressed LIM1/2+ (Fig. 5S) and roughly 30% of the TH+ neurons displayed Isl1/2 expression (Fig. 5T, U). Hence, a greater proportion of the TH+ neurons coexpressed Isl1/2 than Nurr1 (Fig. 5U), indicating a MN rather than a mesDN identity. Thus, in the hESC-derived midbrain cultures a large fraction of the differentiated neurons expressing TH or Nurr1 were not mesDNs, but more likely MNs or INs, respectively.
Discussion
The extensive progress within the field of cell engineering provides great promise for future applications in disease modeling or regenerative therapy. However, before such efforts can be safely translated to the clinic, protocols need to be refined to produce the correct functional subtypes in sufficient yields, and in appropriate maturity stages. It is therefore, imperative to establish the human expression patterns of regulatory genes at a cellular level during fate specification and early differentiation. Here we provide a detailed human CNS expression pattern map of proteins implicated in the control of neuronal subtype diversification and oligodendrogenesis in model organisms, at early human developmental stages (4.5–10 weeks). The clear majority of expression patterns of regulatory genes in the developing spinal cord, ventral hindbrain and ventral midbrain were similar in human, mouse and chicken, which is likely to reflect the high conservation of gene regulatory networks and the cis-regulatory non-coding genomic sequences of patterning genes in these species [21,59]. We also found mammalian specific aspects of developmental programs, where mouse and human show conservation but not chicken, such as the lack of expression of Lmx1b in the roof plate and ventral hindbrain patterning. In addition, by combinatorial expression pattern analysis we identified a few unique aspects in the human, most importantly in the ventral spinal cord and ventral midbrain where clinically relevant cell types, such as oligodendrocytes, somatic MNs and mesDN are produced (discussed below).
Both MNs and oligodendrocytes are derived from two distinct progenitor domains in the ventral human spinal cord
Spinal MNs and oligodendrocytes have been derived from human pluripotent stem cells and may have potential uses in cell-replacement therapy or modeling of diseases, including ALS and MS [2,3]. The development of these rather successful protocols is based on studies in mouse embryos, demonstrating that MN and ventral OLPs are sequentially generated from a common Olig2+ Nkx2.2−domain in the ventral spinal cord (Supplementary Fig. S5) [25]. In the human neural tube, however, MNs appear to be generated from two molecularly distinct pMN sub-domains: an Olig2+ Nkx2.2− progenitor population as in other vertebrates, but also a more ventral population in which Olig2 and Nkx2.2 are coexpressed (Fig. 2A–D, D’ and Supplementary Fig. S5). In vertebrate models, the pMN domain produces several distinct MN subtypes [60] and the subtype identity has been linked to the D-V origin of the progenitor cells [61], raising the possibility that the sub-patterning of the human pMN domain into Nkx2.2+ and Nkx2.2− compartments could contribute to the diversification of MN subtypes.
Consistent with studies in mouse and chicken, OLPs seem to be sequentially generated from the human pMN domain. However, while oligodendrogenesis in mouse is associated with the Olig2+ Nkx2.2− pMN domain, OLPs appear to be generated both from the Nkx2.2+Olig2+ pMN and the Nkx2.2+Olig− p3 domain in human and chicken (Supplementary Fig. S5) [25,62]. The finding that both spinal MN and ventral OLPs are generated from two distinct ventral progenitor domains in the human may provide new premises as how to refine current protocols aimed to selectively generate specific MN subtypes or oligodendrocytes.
Generation of dopamine cells in the human ventral midbrain
Pioneering studies on the use of human fetal dopamine neurons for the treatment of Parkinson's disease [63] has encouraged the interest in developing cell replacement strategies utilizing renewable sources of human mesDNs. One promising alternative source is human pluripotent stem cells differentiated in vitro to mesDN progenitors or postmitotic mesDN [1,16, 64]. In the course of verification of bona fide mesDNs in such experiments, markers, such as TH, Nurr1, Lmx1a, Lmx1b and FoxA2 are often deployed. Our study shows that the most commonly used of these markers (TH and Nurr1) are actually not exclusively expressed in mesDNs in the human ventral midbrain. TH was expressed in ocular MNs (Isl1/2+) at the same time as mesDN cells are generated [57], and Nurr1 expression was observed in a large population of INs (LIM1/2+) lateral to the mesDNs. We furthermore demonstrated that both Isl1/2+TH+ and Nurr1+LIM1/2+ neurons were generated in large quantities in hESCs-derived ventral midbrain-like cultures. Thus, as individual markers, TH and Nurr1 are not faithful indicators of mesDNs in the human ventral midbrain nor in hESC differentiation cultures, emphasizing the importance of performing combinatorial marker analysis and functional assays in attempts to produce stem cell-derived human mesDNs.
The mesDNs can be subdivided into two functionally distinct subgroups, of which only the SN subtype is degenerated in Parkinson's disease, while the ventral tegmental area (VTA) type is related to reward and cognition [65]. Previous studies have suggested that the mesDN progenitor domain consists of functionally different subdomains, but their relation to SN and VTA postmitotic descendants has not been clarified [55,66,67]. In human, we found that the Msx1/2 expression was not evenly distributed in the mesDN progenitor domain but rather concentrated to the lateral edges, possibly reflecting the heterogeneity within this domain. We cannot ascertain the functional relevance for this sub-patterning, but it is possible that Msx1/2 could be utilized as a marker to enrich specific mesDN subtype progenitor populations in future cell engineering efforts.
Supplementary Material
Acknowledgments
We thank C. Birchmeier, J. Briscoe, H. Edlund, M. German, M. Goulding, T. Jessell, B. Novitch, and A. Pierani for supplying us with reagents. We thank A. Pattyn and J. Dias for sharing unpublished data. This work was supported by the Swedish Research Council (DBRM; 33X-06555, 521-2012-1676), the Royal Swedish Academy of Sciences by donation from the Wallenberg Foundation, Swedish Foundation for Strategic research (CEDB; SRL10-0030), seventh framework program under the grant agreements NeuroStemCell (grant 222943), the Knut and Alice Wallenberg Foundation (KAW2008.0123, KAW2011.0161 and KAW2012.01.01), Craig Hospital Foundation, and the research funds of Karolinska Institutet.
Author Disclosure Statement
No competing financial interest exists.
References
- 1.Arenas E. (2010). Towards stem cell replacement therapies for Parkinson's disease. Biochem Biophys Res Commun 396:152–156 [DOI] [PubMed] [Google Scholar]
- 2.Chipman PH, Toma JS. and Rafuse VF. (2012). Generation of motor neurons from pluripotent stem cells. Prog Brain Res 201:313–331 [DOI] [PubMed] [Google Scholar]
- 3.Patani R. and Chandran S. (2012). Experimental and therapeutic opportunities for stem cells in multiple sclerosis. Int J Mol Sci 13:14470–14491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu H. and Zhang SC. (2011). Specification of neuronal and glial subtypes from human pluripotent stem cells. Cell Mol Life Sci 68:3995–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panman L, Andersson E, Alekseenko Z, Hedlund E, Kee N, Mong J, Uhde CW, Deng Q, Sandberg R, et al. (2011). Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell 8:663–675 [DOI] [PubMed] [Google Scholar]
- 6.Wichterle H, Lieberam I, Porter JA. and Jessell TM. (2002). Directed differentiation of embryonic stem cells into motor neurons. Cell 110:385–397 [DOI] [PubMed] [Google Scholar]
- 7.Jessell TM. (2000). Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet 1:20–29 [DOI] [PubMed] [Google Scholar]
- 8.Chen JA, Huang YP, Mazzoni EO, Tan GC, Zavadil J. and Wichterle H. (2011). Mir-17-3p controls spinal neural progenitor patterning by regulating Olig2/Irx3 cross-repressive loop. Neuron 69:721–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhr J, Andersson E, Persson M, Jessell TM. and Ericson J. (2001). Groucho-mediated transcriptional repression establishes progenitor cell pattern and neuronal fate in the ventral neural tube. Cell 104:861–873 [DOI] [PubMed] [Google Scholar]
- 10.Zhou Q, Choi G. and Anderson DJ. (2001). The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron 31:791–807 [DOI] [PubMed] [Google Scholar]
- 11.Jakovcevski I, Mayer N. and Zecevic N. (2011). Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex 21:1771–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsay S, Sarma S, Martinez-de-la-Torre M, Kerwin J, Scott M, Luis Ferran J, Baldock R. and Puelles L. (2005). Anatomical and gene expression mapping of the ventral pallium in a three-dimensional model of developing human brain. Neuroscience 136:625–632 [DOI] [PubMed] [Google Scholar]
- 13.Nelander J, Hebsgaard JB. and Parmar M. (2009). Organization of the human embryonic ventral mesencephalon. Gene Expr Patterns 9:555–561 [DOI] [PubMed] [Google Scholar]
- 14.Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T. and Ericson J. (2006). Identification of intrinsic determinants of midbrain dopamine neurons. Cell 124:393–405 [DOI] [PubMed] [Google Scholar]
- 15.Ying QL, Stavridis M, Griffiths D, Li M. and Smith A. (2003). Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol 21:183–186 [DOI] [PubMed] [Google Scholar]
- 16.Friling S, Andersson E, Thompson LH, Jonsson ME, Hebsgaard JB, Nanou E, Alekseenko Z, Marklund U, Kjellander S, et al. (2009). Efficient production of mesencephalic dopamine neurons by Lmx1a expression in embryonic stem cells. Proc Natl Acad Sci U S A 106:7613–7618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briscoe J, Pierani A, Jessell TM. and Ericson J. (2000). A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101:435–445 [DOI] [PubMed] [Google Scholar]
- 18.Hjerling-Leffler J, Marmigere F, Heglind M, Cederberg A, Koltzenburg M, Enerback S. and Ernfors P. (2005). The boundary cap: a source of neural crest stem cells that generate multiple sensory neuron subtypes. Development 132:2623–2632 [DOI] [PubMed] [Google Scholar]
- 19.Dessaud E, McMahon AP. and Briscoe J. (2008). Pattern formation in the vertebrate neural tube: a sonic hedgehog morphogen-regulated transcriptional network. Development 135:2489–2503 [DOI] [PubMed] [Google Scholar]
- 20.Lupo G, Harris WA. and Lewis KE. (2006). Mechanisms of ventral patterning in the vertebrate nervous system. Nat Rev Neurosci 7:103–114 [DOI] [PubMed] [Google Scholar]
- 21.Oosterveen T, Kurdija S, Alekseenko Z, Uhde CW, Bergsland M, Sandberg M, Andersson E, Dias JM, Muhr J. and Ericson J. (2012). Mechanistic differences in the transcriptional interpretation of local and long-range shh morphogen signaling. Dev Cell 23:1006–1019 [DOI] [PubMed] [Google Scholar]
- 22.Placzek M. and Briscoe J. (2005). The floor plate: multiple cells, multiple signals. Nat Rev Neurosci 6:230–240 [DOI] [PubMed] [Google Scholar]
- 23.Jakovcevski I. and Zecevic N. (2005). Olig transcription factors are expressed in oligodendrocyte and neuronal cells in human fetal CNS. J Neurosci 25:10064–10073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poh A, Karunaratne A, Kolle G, Huang N, Smith E, Starkey J, Wen D, Wilson I, Yamada T. and Hargrave M. (2002). Patterning of the vertebrate ventral spinal cord. Int J Dev Biol 46:597–608 [PubMed] [Google Scholar]
- 25.Fu H, Qi Y, Tan M, Cai J, Takebayashi H, Nakafuku M, Richardson W. and Qiu M. (2002). Dual origin of spinal oligodendrocyte progenitors and evidence for the cooperative role of Olig2 and Nkx2.2 in the control of oligodendrocyte differentiation. Development 129:681–693 [DOI] [PubMed] [Google Scholar]
- 26.Richardson WD, Kessaris N. and Pringle N. (2006). Oligodendrocyte wars. Nat Rev Neurosci 7:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowitch DH. (2004). Glial specification in the vertebrate neural tube. Nat Rev Neurosci 5:409–419 [DOI] [PubMed] [Google Scholar]
- 28.Qi Y, Cai J, Wu Y, Wu R, Lee J, Fu H, Rao M, Sussel L, Rubenstein J. and Qiu M. (2001). Control of oligodendrocyte differentiation by the Nkx2.2 homeodomain transcription factor. Development 128:2723–2733 [DOI] [PubMed] [Google Scholar]
- 29.Li H. and Richardson WD. (2008). The evolution of Olig genes and their roles in myelination. Neuron Glia Biol 4:129–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolt CC, Lommes P, Sock E, Chaboissier MC, Schedl A. and Wegner M. (2003). The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17:1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesnutt C, Burrus LW, Brown AM. and Niswander L. (2004). Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol 274:334–347 [DOI] [PubMed] [Google Scholar]
- 32.Chizhikov VV. and Millen KJ. (2005). Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol 277:287–295 [DOI] [PubMed] [Google Scholar]
- 33.Helms AW. and Johnson JE. (2003). Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol 13:42–49 [DOI] [PubMed] [Google Scholar]
- 34.Millonig JH, Millen KJ. and Hatten ME. (2000). The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature 403:764–769 [DOI] [PubMed] [Google Scholar]
- 35.Chizhikov VV. and Millen KJ. (2004). Control of roof plate development and signaling by Lmx1b in the caudal vertebrate CNS. J Neurosci 24:5694–5703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glasgow SM, Henke RM, Macdonald RJ, Wright CV. and Johnson JE. (2005). Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development 132:5461–5469 [DOI] [PubMed] [Google Scholar]
- 37.Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F. and Johnson JE. (2005). Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development 132:2709–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q. and Goulding M. (2006). Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci 9:770–778 [DOI] [PubMed] [Google Scholar]
- 39.Muller T, Anlag K, Wildner H, Britsch S, Treier M. and Birchmeier C. (2005). The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev 19:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian Y, Shirasawa S, Chen CL, Cheng L. and Ma Q. (2002). Proper development of relay somatic sensory neurons and D2/D4 interneurons requires homeobox genes Rnx/Tlx-3 and Tlx-1. Genes Dev 16:1220–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai J, Qi Y, Hu X, Tan M, Liu Z, Zhang J, Li Q, Sander M. and Qiu M. (2005). Generation of oligodendrocyte precursor cells from mouse dorsal spinal cord independent of Nkx6 regulation and Shh signaling. Neuron 45:41–53 [DOI] [PubMed] [Google Scholar]
- 42.Fogarty M, Richardson WD. and Kessaris N. (2005). A subset of oligodendrocytes generated from radial glia in the dorsal spinal cord. Development 132:1951–1959 [DOI] [PubMed] [Google Scholar]
- 43.Vallstedt A, Klos JM. and Ericson J. (2005). Multiple dorsoventral origins of oligodendrocyte generation in the spinal cord and hindbrain. Neuron 45:55–67 [DOI] [PubMed] [Google Scholar]
- 44.Cordes SP. (2001). Molecular genetics of cranial nerve development in mouse. Nat Rev Neurosci 2:611–623 [DOI] [PubMed] [Google Scholar]
- 45.Kiyasova V. and Gaspar P. (2011). Development of raphe serotonin neurons from specification to guidance. Eur J Neurosci 34:1553–1562 [DOI] [PubMed] [Google Scholar]
- 46.Guidato S, Prin F. and Guthrie S. (2003). Somatic motoneurone specification in the hindbrain: the influence of somite-derived signals, retinoic acid and Hoxa3. Development 130:2981–2996 [DOI] [PubMed] [Google Scholar]
- 47.Pattyn A, Vallstedt A, Dias JM, Samad OA, Krumlauf R, Rijli FM, Brunet JF. and Ericson J. (2003). Coordinated temporal and spatial control of motor neuron and serotonergic neuron generation from a common pool of CNS progenitors. Genes Dev 17:729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkinson DG, Bhatt S, Cook M, Boncinelli E. and Krumlauf R. (1989). Segmental expression of Hox-2 homoeobox-containing genes in the developing mouse hindbrain. Nature 341:405–409 [DOI] [PubMed] [Google Scholar]
- 49.Muller F. and O'Rahilly R. (2003). Segmentation in staged human embryos: the occipitocervical region revisited. J Anat 203:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Craven SE, Lim KC, Ye W, Engel JD, de Sauvage F. and Rosenthal A. (2004). Gata2 specifies serotonergic neurons downstream of sonic hedgehog. Development 131:1165–1173 [DOI] [PubMed] [Google Scholar]
- 51.Chandrasekhar A. (2004). Turning heads: development of vertebrate branchiomotor neurons. Dev Dyn 229:143–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, et al. (2011). Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gale E. and Li M. (2008). Midbrain dopaminergic neuron fate specification: of mice and embryonic stem cells. Mol Brain 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferri AL, Lin W, Mavromatakis YE, Wang JC, Sasaki H, Whitsett JA. and Ang SL. (2007). Foxa1 and Foxa2 regulate multiple phases of midbrain dopaminergic neuron development in a dosage-dependent manner. Development 134:2761–2769 [DOI] [PubMed] [Google Scholar]
- 55.Deng Q, Andersson E, Hedlund E, Alekseenko Z, Coppola E, Panman L, Millonig JH, Brunet JF, Ericson J. and Perlmann T. (2011). Specific and integrated roles of Lmx1a, Lmx1b and Phox2a in ventral midbrain development. Development 138:3399–3408 [DOI] [PubMed] [Google Scholar]
- 56.Prakash N, Puelles E, Freude K, Trumbach D, Omodei D, Di Salvio M, Sussel L, Ericson J, Sander M, Simeone A. and Wurst W. (2009). Nkx6–1 controls the identity and fate of red nucleus and oculomotor neurons in the mouse midbrain. Development 136:2545–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Puelles L. and Verney C. (1998). Early neuromeric distribution of tyrosine-hydroxylase-immunoreactive neurons in human embryos. J Comp Neurol 394:283–308 [DOI] [PubMed] [Google Scholar]
- 58.Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S. and Ebendal T. (2004). Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis 40:67–73 [DOI] [PubMed] [Google Scholar]
- 59.Oosterveen T, Kurdija S, Enstero M, Uhde CW, Bergsland M, Sandberg M, Sandberg R, Muhr J. and Ericson J. (2013). SoxB1-driven transcriptional network underlies neural-specific interpretation of morphogen signals. Proc Natl Acad Sci U S A 110:7330–7335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kanning KC, Kaplan A. and Henderson CE. (2010). Motor neuron diversity in development and disease. Annu Rev Neurosci 33:409–440 [DOI] [PubMed] [Google Scholar]
- 61.Agalliu D, Takada S, Agalliu I, McMahon AP. and Jessell TM. (2009). Motor neurons with axial muscle projections specified by Wnt4/5 signaling. Neuron 61:708–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Soula C, Danesin C, Kan P, Grob M, Poncet C. and Cochard P. (2001). Distinct sites of origin of oligodendrocytes and somatic motoneurons in the chick spinal cord: oligodendrocytes arise from Nkx2.2-expressing progenitors by a Shh-dependent mechanism. Development 128:1369–1379 [DOI] [PubMed] [Google Scholar]
- 63.Lindvall O. and Bjorklund A. (2004). Cell therapy in Parkinson's disease. NeuroRx 1:382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Studer L. (2012). Derivation of dopaminergic neurons from pluripotent stem cells. Prog Brain Res 200:243–263 [DOI] [PubMed] [Google Scholar]
- 65.Bayer SA, Wills KV, Triarhou LC. and Ghetti B. (1995). Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res 105:191–199 [DOI] [PubMed] [Google Scholar]
- 66.Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, McKay R. and Awatramani R. (2009). Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci U S A 106:19185–19190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, Arenas E. and Ang SL. (2006). Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development 133:495–505 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.