Abstract
Epidermal stem cells (EpSCs) hold great expectations in a regenerative medicine context, but innovative methods that permit to obtain a significant yield of EpSCs or stem-like epidermal cells are still required. We propose a two-step strategy to obtain a superior epidermal stem-like cell fraction among primary keratinocytes (KCs) isolated from adult human skin. The approach is based on the combination of rapid adherence to collagen IV with the rock-associated kinase inhibitor (ROCKi) treatment, and the subsequent immunomagnetic separation of the α6high/CD71dim cell subset. The combined collagen IV and ROCKi treatment showed not only to enhance cells clonogenic capacity, but also to induce an early epidermal phenotypic signature, along with the diminished expression of late differentiation-associated markers. More importantly, collagen IV and the ROCKi efficiently promoted a synergized effect over α6high/CD71dim expression, boosting the number of highly proliferative KCs stem-like cells as demonstrated by the expression of ki67. This cell fraction showed a superior ability to generate a 3D stratified epithelium formed by cells with successive differentiation phenotypes. Overall, this strategy indulged the possibility to uncover, among adult KCs, a superior epidermal cell population with stem-like proliferation capacity and early differentiation degree to be used in numerous skin regeneration approaches.
Introduction
Skin keratinocytes (KCs) are accessible cells that, when applied as epidermal sheets or as a part of tissue engineering constructs, have proved useful in the healing of burns, chronic wounds, and ulcers. Nonetheless, primary KCs have limited lifespan in culture, which reduces their proliferative potential and success in clinics [1]. As suggested by Watt et al. [2], better approaches for establishing primary KCs cultures or for increasing epidermal stem cells (EpSCs) numbers in those cultures are required to improve cell-based therapies and to shorten the time to treat skin-wounded patients.
The interfollicular epidermis, located between the orifices of hair follicles, regenerates throughout adult life and is known to ensure the continuous replacement of the outermost layer of terminally differentiated KCs that are continuously shed from skin surface [3,4]. This seems to indicate that, under normal homeostatic conditions, bulge stem cells do not contribute to the regeneration of interfollicular epidermis [5], which relies on its resident stem cell population within the basal layer, the EpSCs.
The need for specific and exclusive EpSCs markers has been, and still remains, a shortcoming of the field. Many works have been addressing this issue and nonexclusive markers, including α6-integrin [6,7], β1-integrin [8], Keratin 15, as well as Keratin 19 [9] related to EpSCs. Moreover, elevated expression of E-cadherin in adherens junctions and reduced levels of desmosomes [10] have also been associated with EpSCs, stressing the importance of both extracellular and intercellular cues, but confirming the lack of specificity of these markers. EpSCs are distinguished from other basal cells by the high expression of the adhesion molecule α6 and low levels of CD71; thus, cells with this combined phenotype were elected to represent the stem cell subpopulation within basal cells [6]. However, the fraction of isolated skin KCs capable as behaving as EpSCs, that is, to undergo continuous cell-renewal and expansion on demand and lead regeneration, is limited in in vitro cultures. In fact, the estimated keratinocytes stem cells (KSCs) frequency in human epidermis ranges from 1% to 10% [6], and its success is highly dependent on the isolation and culture methodologies [11].
The attempts to overcome this problem started by proposing rapidly adhering epidermal cells to skin basement membrane proteins, such as collagen IV, as EpSCs due to their very high proliferative capacity [12]. Nonetheless, further data demonstrated that this method was not exclusive, as both EpSCs and transit amplifying (TA) cells adhered rapidly to this matrix, both of which exhibited high β1-integrin levels [13]. Thus, these data demonstrated that a complementary approach is needed to select EpSCs. ROCK kinase, central mediator of the signals from Ras homolog gene family, member A (RhoA), appears to play a critical role in the regulation of the balance between human KCs (hKCs) proliferation and differentiation [14]. It has been reported that blocking ROCK signaling in the initial 6 days of primary culture of epithelial cells from different sources, with the pharmacological inhibitor Y-27632, leads to an increased number of KCs with higher clonogenic capacity, in a concentration-dependent manner [15].
Considering the need for a superior epidermal cell fraction with enhanced proliferation capacity at an early maturation stage for further use in skin regeneration purposes, we propose a two-step approach that reunites the use of collagen IV for the selection of rapid adherent cells in combination with Rock-associated kinase inhibitor (ROCKi) treatment, and the subsequent separation of α6high/CD71dim cell subset. We hypothesized that the joined effect of collagen IV and ROCKi would boost the number of cells with KC stem-like cell behavior among freshly isolated adult human skin KCs, which when selected would allow obtaining a fraction that would exhibit higher proliferation capacity, early differentiation marker expression, lack of late KCs associated markers, and ideally holding the capacity to form a 3D stratified epithelia in vitro. Evidence that the proposed approach permitted to obtain a high number of cells with superior characteristics to explore in the skin regenerative medicine context is demonstrated here.
Materials and Methods
KCs isolation from human skin
Healthy skin was obtained from consenting adult female patients who underwent abdominoplasty surgical procedures in Hospital da Prelada, Porto, Portugal, under a collaboration protocol with the 3B's Research Group approved by the ethics committees of the involved institutions. Briefly, skin specimens (ranging between 20 and 75 cm2) were incubated in Dispase (2.4 U/mL) (BD Biosciences), epidermis was peeled off, and dermis was used to isolate human dermal fibroblasts (hDFb) (see Supplementary Materials and Methods; Supplementary Data are available online at www.liebertpub.com/scd) for the organotypic culture. Epidermis was then enzymatically digested by Trypsin-EDTA (0.05%) (Invitrogen) and resuspended in KC serum-free medium (KSFM) (Invitrogen) with 1% Antibiotic/Antimycotic (Invitrogen).
Cell culture
Freshly isolated hKCs were cultured in KSFM at a density of 2×104 cells/cm2 in polysterene culture plates (BD Biosciences) at 37°C and 5% CO2 until cultures reached 80% confluence. The ROCKi cultures were obtained by adding Y-27632 (Sigma) to KSFM at a final concentration of 10 μM. Cell culture flasks (BD Biosciences), coated with collagen IV from human placenta (5 mg/cm2) (Sigma) for 1 h at 37°C, were used to obtain the rapidly adherent cells from freshly isolated adult skin KCs suspensions by removing nonadherent cells 30 min after plating. The combined effect of collagen IV and ROCKi was obtained by adding KSFM with Y-27632 at a final concentration of 10 μM to RACs. Media were changed every 3 days in control and collagen IV conditions as well as in the ROCKi-containing cultures to maintain its activity (Fig. 1).
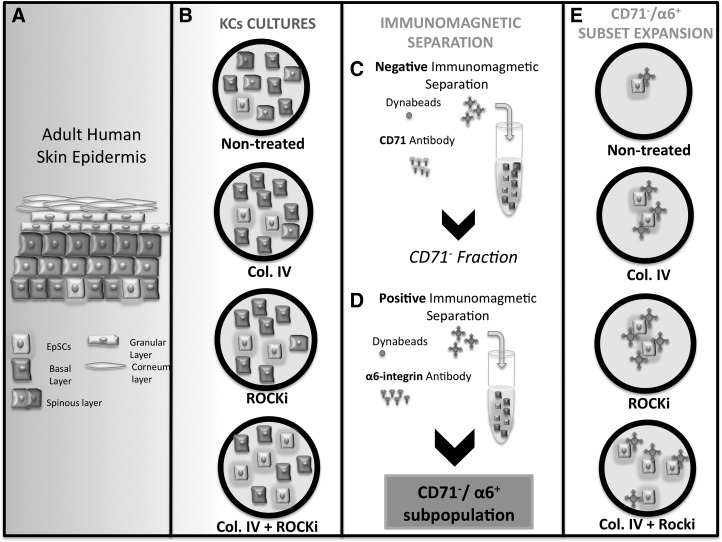
FIG. 1.
Schematic representation of the overall workflow. (A) Illustration of the different cell types present in the adult human skin stratified epidermis. (B) Keratinocytes (KCs) freshly isolated from human skin were cultured for approximately 80% confluence in standard (nontreated) and collagen IV-coated (Col. IV) tissue culture plates, and, respectively, in the presence of rock-associated kinase inhibitor (ROCKi) and Col. IV+ROCKi, as KC stem-like cells enrichment strategies. (C) After culture under the different conditions, the CD71− cell fraction was separated by negative selection using CD71 antibody-coated magnetic beads. (D) The CD71−/α6+ subpopulation was then obtained from the CD71− population by positive selection with α6 integrin antibody-coated magnetic beads, and cultured for approximately 80% confluence for (E) expansion under the initial defined culture conditions.
Immunomagnetic selection
The separation of CD71−/α6+ fraction was carried out using immunomagnetic Dynal M-450 Epoxy beads (Dynabeads) (Invitrogen), according to the manufacturer's instructions. Supernatant containing the unbound cells (CD71− fraction) was plated in KSFM, and cells were expanded until reaching 80% confluence (P2). Protocol was repeated for the selection of CD49f+ cells from the CD71− fraction, and the obtained population was plated for further analysis (P3). A fraction of the original cell population, without the immunomagnetic selection, was kept as a control.
Colony-forming unit assay
To determine the clonogenic capacity of cells obtained after collagen IV, ROCKi and combined treatment in comparison to the untreated cells, an in vitro colony-forming units (CFUs) assay originally described by Rheinwald and Green [16] was performed. Cells were plated at densities ranging from 52 cells/cm2 (5×102 cells) to 520 cells/cm2 (5×103 cells) in six-well plates (Falcon, BD Biosciences). After 5 days of culture, plates were fixed with 3.7% formalin (Sigma) and stained with crystal violet (Sigma). The number and diameter of CFUs when 5×102 cells were plated were determined by analyzing the results for four independent samples with Image J software (Wayne, Rasband, NIH).
Flow cytometry
For surface marker analysis, cells were incubated with CD71-PE (0.4 μg/μL; BD Biosciences) and CD49f-APC (0.4 μg/μL; eBioscience) antibodies. For intracellular staining, Fix and Perm Cell Fixation & Cell Permeabilization Kit (Invitrogen) was used, and manufacturer's indications were followed. Cells were first fixed and permeabilized with reagent B before incubation with Keratin 14–FITC (1:50; AbD Serotec) or Cytokeratin 19 AF488 (1:20; ExBio) antibodies. For keratin 5 (1:1,000; Covance), intracellular indirect staining was performed with Alexa Fluor 488 donkey anti-Rabbit secondary antibody (1:500; Invitrogen). Samples were analyzed with an FACSCalibur flow cytometer (BD Biosciences) and Cell Quest Pro version 4.0.2 (BD Biosciences) software.
Organotypic culture
Organotypic cultures were prepared as previously described [17], with minor modifications. Briefly, 5×105 hDFb were incorporated in a 4 mg/mL rat tail collagen I (Invitrogen) matrix that was cast into polycarbonate transwells (BD Biosciences). The different cell populations, treated and untreated whole and CD71−/α6+ fractions (1×106 cells/gel), were then seeded on the top of collagen I gel and cultured in KSFM. After 7 days, constructs rose to air-liquid interface in 3D prime medium CnT-02-3DP1 (CELLnTEC). Cultures were allowed to stratify for 14 days and were then fixed in 3.7% formalin overnight. Samples were paraffin embedded and sectioned for further analysis.
Immunocytochemical staining
The different cell populations and samples from the organotypic culture were characterized by fluorescent immunocytochemistry. Primary antibodies were used as follows: keratin 5 (1:1,000), Keratin 10 (1:1,000; Covance), p63 (1:100; Biolegend), β1-integrin (1:30; BD Biosciences), involucrin (1:100; Sigma), and ki67 (1:50; Abcam). AlexaFluor 488/594 (Invitrogen) secondary antibodies (1:500) were used. All fluorescence microscopy images were captured in AXIOIMAGER Z1M (Zeiss), using the Axiovision software (Zeiss). Image J software was used to determine the total number of cells (number of nuclei stained with DAPI) and the percentage of positive cells for ivolucrin, p63, and ki67. Three random images were analyzed for each one of the four independent samples.
Statistical analysis
Human skin samples from four different donors (n=4) were used in each group for a specific time point. Standard deviation is reported as a measure of sample deviation. Statistical analysis of the flow cytometry data of non-treated and treated keratinocytes was performed using two-way ANOVA with Bonferroni post-tests. CFUs data and p63, ki67 and involucrin expression results were evaluated by one-way ANOVA with Tukey's post-tests (GraphPad Prism 4.02). Significance levels between groups, determined using post-tests, were set for p≤0.05.
Results
Treated KCs exhibit typical epidermal basal cells morphology and higher clonogenic ability
The cells obtained from the fresh KCs isolated from human adult skin after culture under the distinct treatment conditions were characterized, in relation to nontreated cells, with regard to morphology and proliferative capacity. The KCs selected by rapid adherence to collagen IV, cultured in the presence of ROCKi and in both collagen IV and ROCKi (P0), were actively dividing, reaching confluence at 6 days after isolation (Supplementary Fig. S1A–C). In contrast, nontreated cultures, at the same culture time, exhibited a few small cells colonies (Supplementary Fig. S1D) and only reached confluence between day 10 and 15. Furthermore, the morphology of the cells treated with collagen IV (Supplementary Fig. S1A), ROCKi (Supplementary Fig. S1B), or Col IV+ROCKi (Supplementary Fig. S1C) was more homogenous, exhibiting a small and cuboidal appearance, characteristic of epidermal basal cells.
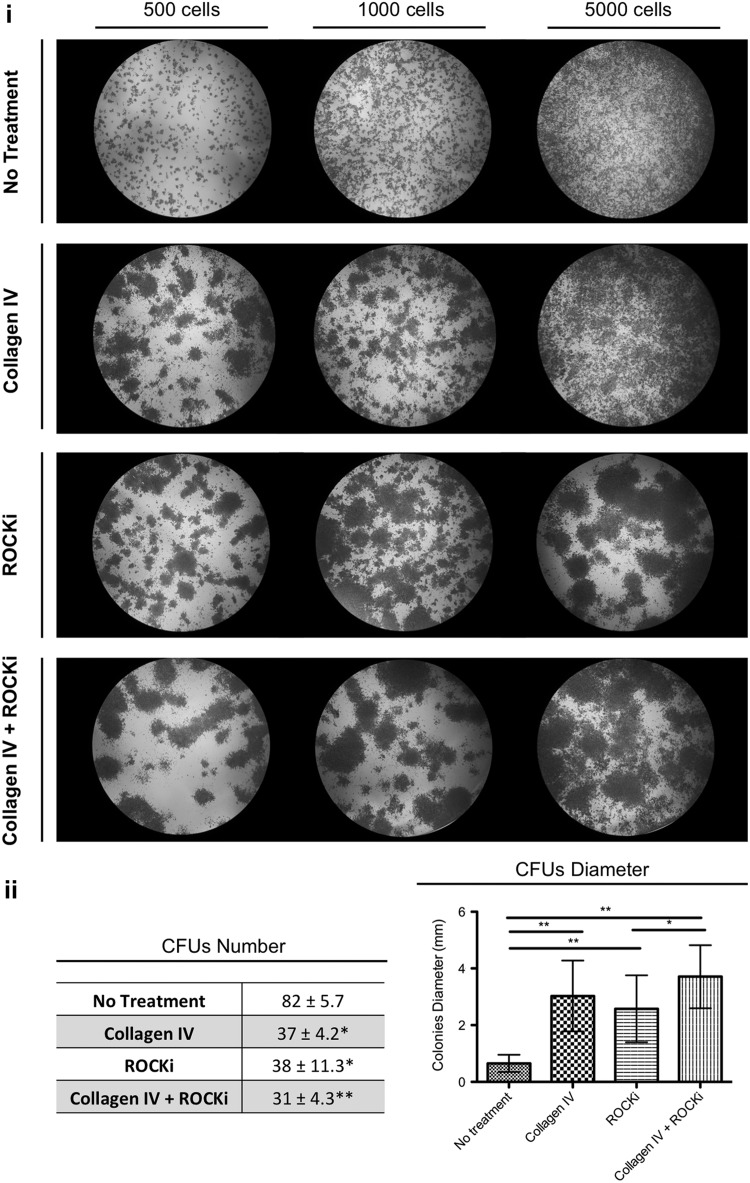
The influence of the different treatments in the clonogenic capacity of the respective cells was analyzed for approximately passage 3 in order to confirm the suitability of the culture conditions along the passages (Fig. 2i). The number of colonies generated by the treated and nontreated hKCs was determined for the plating density of 500 cells/well (Fig. 2ii), as for the other densities the edges were difficult to define due to the overlap with neighboring colonies. Nontreated hKCs formed a significantly (P<0.05) higher number of colonies than the treated ones. However, treated hKCs led to significantly larger (P<0.05) colonies than untreated ones. Moreover, ROCKi+Col IV-treated cells proliferated at higher rates than those treated with ROCKi, giving rise to significantly higher (P<0.05) diameter colonies (Fig. 2ii).
FIG. 2.
(i) Representative photographs of colony-forming units (CFUs) from passage 3 keratinocytes, nontreated and previously treated with collagen IV, ROCKi, and with both collagen IV and ROCKi, seeded at 500, 1,000, and 5,000 cells/well and after 5 days in culture in keratinocyte serum-free medium. (ii) Quantification of the CFUs number and diameter when cells were plated at a density of 500 cells/well. Results are presented as mean±standard deviation. In the CFUs quantification, the indicated statistical difference is in relation to the no-treatment condition. *P<0.05; **P<0.01, n=4.
KCs simultaneously treated with collagen IV and ROCKi display an early-associated phenotypic profile and diminished expression of terminal differentiation markers
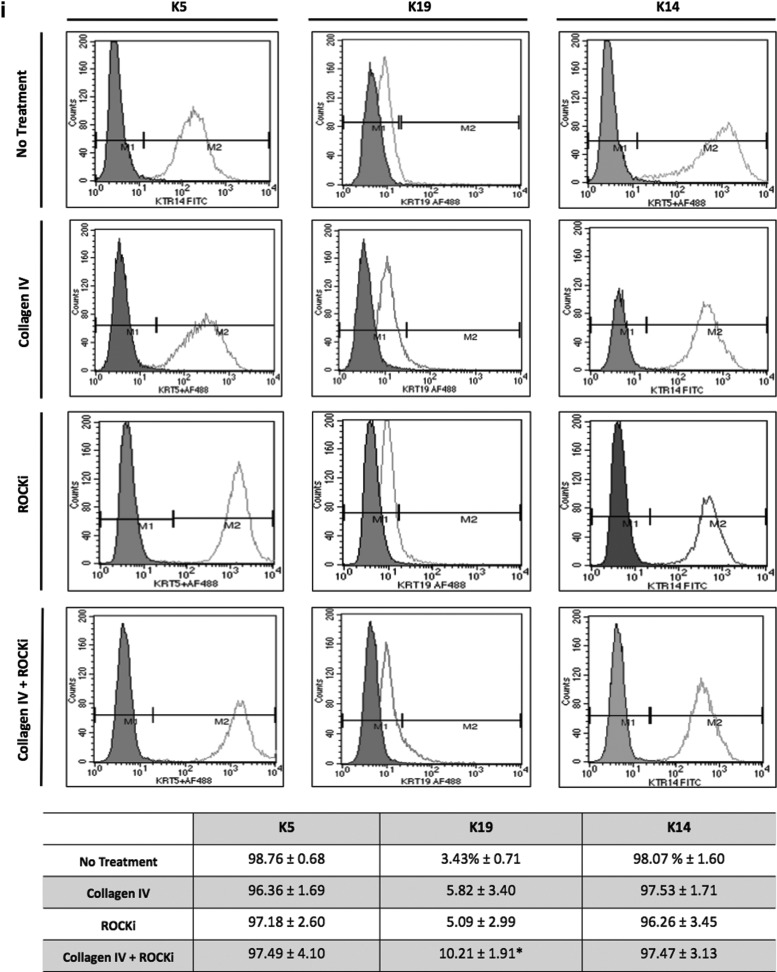
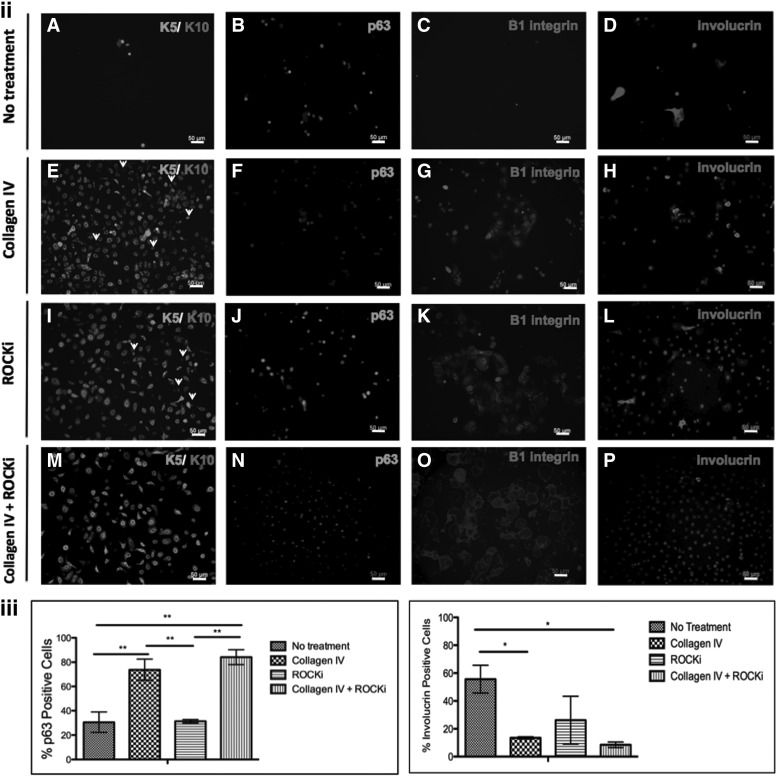
In addition to the distinct clonogenic properties, the phenotype of the cultures obtained after different treatments is of major significance with regard to cells' ultimate performance. The profile of early (K5, K14, p63, β1-integrin, and K19) and late (K10, involucrin)-associated epidermal differentiation/maturation markers was, therefore, analyzed (Fig. 3). The expression of early markers, in general, was higher in the Col IV+ROCKi-treated cultures, although K14/K5 expression, in particular, did not vary with the treatments, being expressed by the majority of the cells in all the conditions tested (Fig. 3i, ii). A significantly higher (P<0.05) percentage of cells expressing p63 (Fig. 3ii, iii), in comparison to no treatment, was detected for the collagen IV and ROCKi+Col IV conditions. Similarly, the combined Col IV+ROCKi treatment also resulted in a significantly higher (P<0.05) percentage of cells expressing K19 in comparison to untreated cells (Fig. 3i). In what concerns the expression β1-integrin, a higher percentage of cells expressing this marker was identified after the treatments but at the levels observed for the untreated cells. (Fig. 3ii)
FIG. 3.
Phenotypic profile of nontreated and treated KCs, at passage 3, assessed by (i) flow cytometry and (ii, iii) immunocytochemistry. (i) Representative flow cytometry histograms and data regarding the percentage of cells positive for K5, K14, and K19 confirming the high expression of early differentiation associated markers K5, K14 by all of the groups, and a (*) significantly higher expression of K19 after treatment in comparison to the nontreated cells. Filled areas in the flow cytometry histograms represent the negative population for the respective marker. (ii) (A–P) Immunofluorescence micrographs illustrating the higher expression of early differentiation-associated markers K5 (E, I, M), p63 (F, J, N), and β1-integrin (G, K, O) in treated KCs, when compared with nontreated ones (A–C). Conversely, late differentiation-associated marker involucrin was clearly expressed in nontreated cells (D) and collagen IV (H) and ROCKi independently treated KCs (L), but almost absent in the collagen IV and ROCKi combined treated KCs (P). A few k10 positive cells (arrowheads) were observed in collagen IV (E) and ROCKi (I) independently treated KCs and none in the nontreated cells (A) and in the collagen IV and ROCKi combined treated KCs (M). Scale bar corresponds to 50 μm. DAPI was used as nuclear staining. (iii) Graphical representations of p63 and involucrin positive cells quantified after immunocytochemistry. *P<0.05, **P<0.01, n=4.
In what concerns late differentiation-associated markers expression, a few k10 positive cells were observed in collagen IV (Fig. 3ii, E) and ROCKi (Fig. 3ii, I) independently treated KCs. Moreover, K10 expression was not detected in the nontreated cells (Fig. 3ii, A) and in the Col IV+ROCKi-treated KCs (Fig. 3ii, M). In opposition, a significantly higher (P<0.05) number of cells expressing involucrin, in comparison to the collagen IV and Col IV+ROCKi treatments, were identified in the untreated condition (Fig. 3ii, iii).
The reduced number of cells, observed between the different culture conditions in the immunocytochemistry analysis (Fig 3ii, A–P), and in particular in the no treatment condition (Fig. 3ii, A–D), might be related with the distinct type of colonies formed (Fig. 2).
Collagen IV and ROCKi synergize to boost the expression of α6high/CD71dim subset, which is enriched in early associated markers
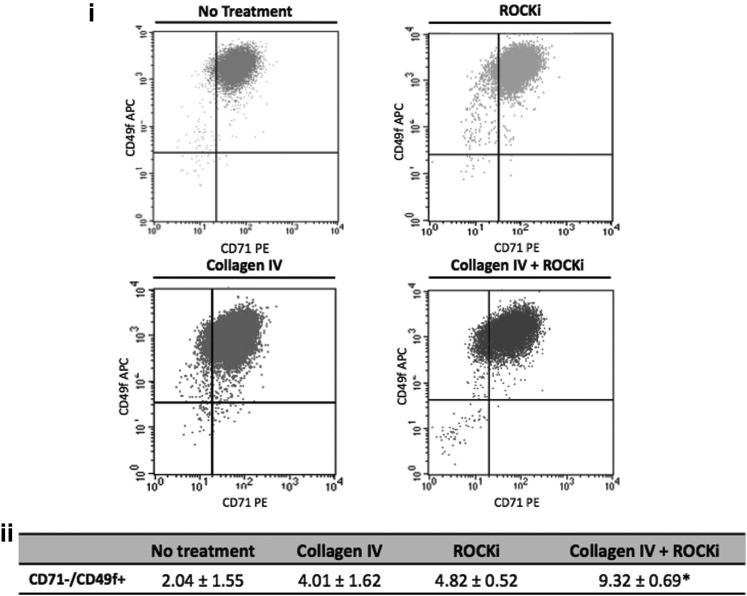
Considering that the balance between the expression of the adhesion molecule α6 and CD71 permits to distinguish a stem-like cell subpopulation within basal cells, the influence of collagen IV, ROCKi, and the combined treatment over α6high/CD71dim subpopulation incidence was analyzed by flow cytometry (Fig. 4). The occurrence of this α6high/CD71dim subset was affected by the proposed treatments (Fig. 4ii). An average of 2.04% of the nontreated KCs represented the referred subpopulation. In the collagen IV and ROCKi-treated cultures, the percentage of α6high/CD71dim cells, respectively, increased to an average of 4.01% and 4.82%. While these values were not statistically different, the percentage of α6high/CD71dim cells (9.32%) identified in the Col IV+ROCKi-treated cultures was significantly higher (P<0.05) than the 2.04% observed in the untreated cells.
FIG. 4.
(i) Representative flow cytometry dot plots of nontreated and treated keratinocytes, from four independent samples, highlighting the distinct potential of the treatments on the enrichment of 71dimα6high subset. (ii) The 71dimα6high subpopulation of interest (upper left region in the graphs) represents around 2% of the nontreated culture, respectively, increasing to 4% and 5% in the collagen IV and in the ROCKi independently treated cultures and to 9% in the combined collagen IV and ROCKi-treated cells. *represents statistical difference (P<0.05) to no treatment condition. Data are presented as mean±standard deviation, n=4.
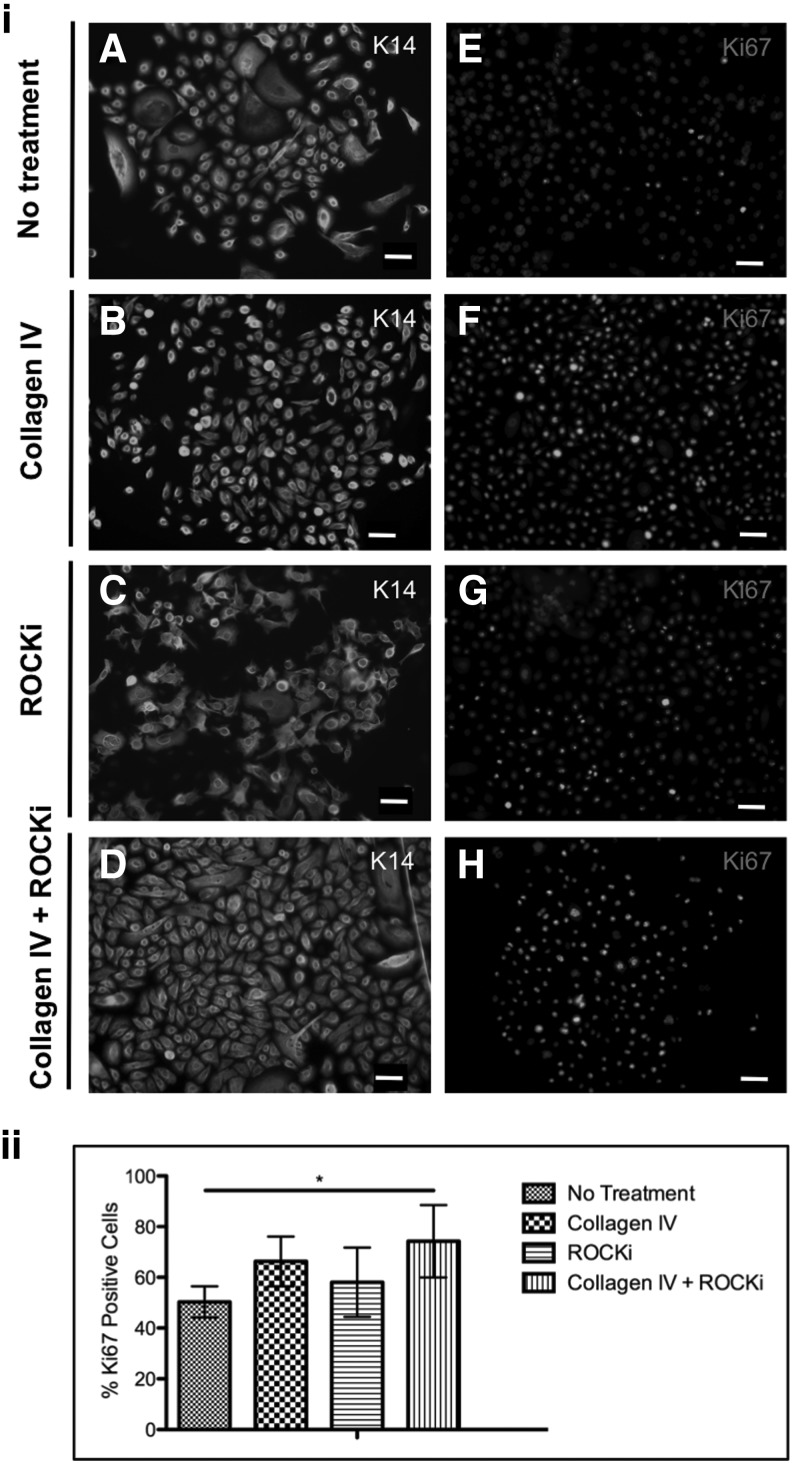
The selected α6high/CD71dim subpopulation was then analyzed regarding the expression of the early keratin 14 and the proliferative-associated marker ki67 (Fig. 5). Keratin 14 was expressed by the majority of the population, as before the separation (Fig. 5i). However, the percentage of cells expressing Ki67 was significantly higher (P<0.05), when compared with the subset isolated from the untreated cells, in the α6high/CD71dim fraction obtained from the Col IV+ROCKi treatment (Fig. 5ii).
FIG. 5.
(i) Immunohistochemical analysis of the expression of (A–D) keratin 14 and (E–H) proliferation-associated marker ki67 in α6+/71− cell fractions obtained from (A, E) nontreated, (B, F) collagen IV, (C, G) ROCKi and (D, H) combined collagen IV and ROCKi-treated keratinocytes, respectively, showing the early keratin profile expression and the proliferative nature of this 71−α6+ subset. Scale bar represents 50 μm. DAPI was used as nuclear staining. (ii) Graphical representation of the number of cells expressing Ki67 confirming the higher proliferative character of the combined collagen IV and ROCKi treated cells. *P<0.05, n=4.
Selected α6high/CD71dim subpopulations have the ability to form 3D stratified epithelia
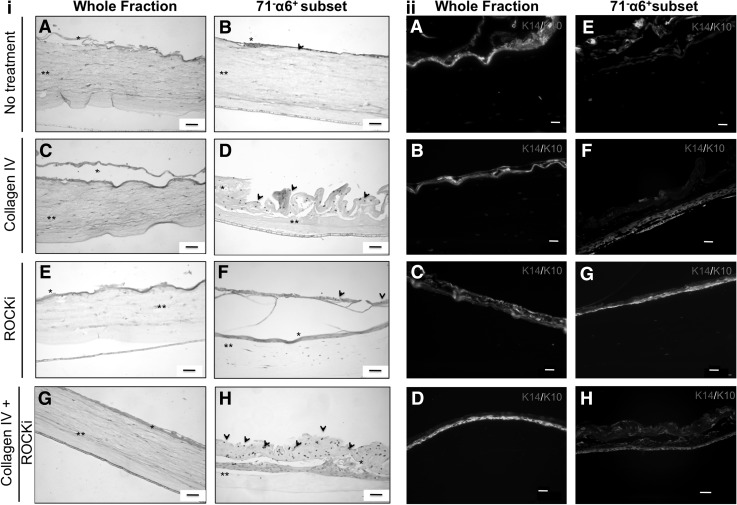
The retention of the differentiation/maturation capacity of treated and nontreated KCs, and of KC stem-like cells obtained by immunomagnetic selection of the respective α6+/CD71− subsets, was assessed based on their capacity to generate a stratified epithelium. Independently of the condition, all the populations were able to generate a uniform stratified epithelium (Fig. 6). The whole KCs fraction, independently of the culture conditions, formed poorly organized epithelia (Fig. 6ii) A-D). In contrast, the α6+/CD71− subsets selected from the treated populations gave rise to more defined structures (Fig. 6ii, F–H). These organized 3D epithelia with K14 positive cells in the basal, and the differentiated K10 positive cells in the suprabasal layers, were more evident for the α6+/CD71− subsets obtained from the collagen IV (Fig. 6ii, F) and Col IV+ROCKi-treated populations (Fig. 6ii, H). Interestingly, dynabeads were distinguished in the outermost layers of the stratified epithelia generated by the nontreated and treated cells KC stem-like cells, the α6+/CD71− subsets (Fig. 6i, B, D, F, H). This confirmed not only the differentiation/maturation of the selected cells but also the distinct effect of the test conditions denoted by the observed diversity of patterns.
FIG. 6.
(i) H&E stained histological transversal sections of the 21 days 3D organotypic co-cultures of human dermal fibroblasts with the (A, C, E, G) whole fraction and (B, D, F, H) the respective α6+/71− subset of nontreated (A, B) and (C, D) collagen IV, (E, F) ROCKi and (G, H) combined collagen IV and ROCKi treated keratinocytes, at passage 3. Dynabeads (arrowheads) are visible in the mature part of the generated epithelia. * represents stratified areas and ** collagen I gels. (ii) Immunohistochemical analysis of the 3D stratified epithelia generated by (A–D) whole keratinocytes fraction and by (E–H) stem-like cells (α6+/71− subset) (A, E) nontreated, and subjected to (B, F) collagenIV, (C, G) ROCKi and (D, H) combined collagen IV, and ROCKi treatments, revealing an organized structure with K14-positive cells in the basal, and the differentiated K10-positive cells in the suprabasal differentiated layers. DAPI was used as nuclear staining. Scale bar corresponds to 50 μm.
Discussion
Purified EpSCs offer the opportunity to develop next-generation products with enhanced features, overcoming issues such as typical terminal differentiation of epidermal cells caused by extensive culture that results in poor re-epithelialization on transplantation [18]. However, to propose innovative methodologies to obtain purified stem-like epidermal cells for further use in skin regeneration purposes has been a continuous challenge and a few works [7,12,15] have addressed this need. The current work envisioned to achieve a superior purified epidermal cell fraction by boosting KC stem-like cells among freshly isolated human skin KCs having as rationale the available knowledge regarding the EpSCs interfollicular basal niche. The combination of rapid adherence to collagen IV with the effect of the pharmacological inhibitor of rho-associated protein kinases, ROCKi, followed by the immunomagnetic separation of α6+/CD71− cell subset was hypothesized as a reliable method to enable the establishment of purified hKC stem-like cell cultures.
In accordance to previous works [12,13,19] which demonstrated that both EpSCs and TA cells comprise rapidly adhering cells to collagen IV, we were able to obtain cells with typical basal cell morphology, independently of using collagen IV and ROCKi as individual or combined treatments. It is also known that KCs expressing high levels of β1-integrin, collagen IV ligand, give rise to holoclones, whereas KCs with low levels of β1-integrin produce small colonies [12]. In fact, larger holoclone-like colonies formed by more proliferative cells were generated by the collagen IV and Col IV+ROCKi-treated cells, despite the higher number of CFUs formed by the nontreated cells. Moreover, cells treated only with ROCKi also gave rise to significantly larger colonies than in the control, although smaller than those formed in the ROCKi+Col IV treatment. ROCKi has a safety history in preclinical studies [1], and several reports [15,20,21] have described the involvement of ROCK signaling in KCs differentiation process and in prolonging KC lifespan. Thus, it seems that the combination of collagen IV and ROCKi is effective in the selection of epidermal basal cells. In fact, the phenotypic analysis of the obtained populations showed that Col IV+ROCKi-treated KCs not only maintain the expression of early associated epidermal markers, such as K14 and K5, but also depicted increased expression of K19 and p63, which is usually observed in interfollicular adult epidermis [22,23]. In addition, the proposed Col IV+ROCKi treatment diminished the expression of involucrin, a late-stage epidermal marker [24], in a more effective way than the independent ROCKi treatment. This reinforces the effectiveness of the ROCKi role in inhibiting epidermal differentiation when the initial culture contains more KCs stem-like cells selected by rapid adherence. This assumption was, in fact, confirmed based on the premise that the α6high/CD71dim subset represents the stem-like cells within the treated KCs populations, significantly enhanced by the Col IV+ROCKi treatment. Overall, and considering that estimated KSCs frequency ranges from 1% to 10% [6] and only 0.1%–1% of adult freshly isolated epidermal cells can act as KSCs [16,25,26], we were able to significantly increase those numbers probably by enhancing the frequency of the symmetric division of stem-like cells resultant from the highest clonogenic nature of the Col IV+ROCKi treatment. The expected expression of the early epidermal differentiation-associated marker keratin 14 was demonstrated and displayed by hKC stem-like cells obtained from every treatment, and also from nontreated hKC, as shown before the separation. Nonetheless, further epidermal basal cells features were demonstrated after selection and subsequent culture, by the expression profile of the ki67 marker, typically expressed in a small percentage of epidermal cells in the basal layer and in the hair follicles. This marker was differently expressed in the KCs stem-like cells obtained from distinctively treated cells and in a significantly higher percentage by the cells of the α6high/CD71dim fraction obtained from the Col IV+ROCKi treatment.
Based on the properties of the treated KCs cultures before and after α6high/CD71dim subset selection, the question of whether the obtained cells were capable of entering the KCs differentiation program in which stratification occurs was raised [27]. Cells from the whole fraction generated poorly organized 3D-stratified epithelia in organotypic cultures. In contrast, selected hKC stem-like cells, the α6high/CD71dim subsets, formed more organized 3D stratified structures. In fact, the magnetic beads used to select the α6+ cells were found in the outermost cell layers of the generated epithelium, thus confirming that the purified cells were able to give rise to daughter cells, cells without magnetic beads in basal layer, and to go into the normal differentiation program reaching the epithelium surface layer. Nonetheless, it seems that epithelia generated by the α6+/CD71− subsets obtained from the collagen IV and ROCKi+Col IV-treated cells presented a pattern of successively higher differentiation degree than those from the ROCKi-treated condition. Despite the known rock signaling effect in epidermal stratification [14], it appears that rapid adherence to collagen IV affect the capacity of the α6+/CD71− cells to differentiate under the defined conditions.
Hence, the proposed methodology allowed rescuing the cells of interest, actively proliferating, exhibiting an early differentiation phenotype, and capable to generate a fully differentiated and stratified epithelium. A two-step methodology for the boost and isolation of a superior epidermal stem-like cell subset, from freshly isolated adult human skin KCs, is successfully anticipated here. Rapid adhesion to collagen IV and ROCKi administration led to increased KCs stem-like numbers, which demonstrated a superior functional behavior by generating a fully stratified epithelium.
Supplementary Material
Acknowledgments
The authors would like to thank Hospital da Prelada (Porto), in particular Paulo Costa for human skin collection. Financial support by Skingineering (PTDC/SAU-OSM/099422/2008), Portuguese Foundation for Science and Technology (FCT) funded project, is also acknowledged.
Part of this work has been presented at XXXVIII Congress of the European Society for Artificial Organs (ESAO 2011) and IV Biennial Congress of the International Federation on Artificial Organs (IFAO 2011) held in Porto, Portugal, October 2011, and 7th International Meeting of the Portuguese Society for Stem Cells and Cell Therapies (SPCE-TC), held in Porto Portugal, May 2012, as oral communications.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.van den Bogaard EH, Rodijk-Olthuis D, Jansen PA, van Vlijmen-Willems IM, van Erp PE, Joosten I, Zeeuwen PL. and Schalkwijk J. (2012). Rho kinase inhibitor Y-27632 prolongs the life span of adult human keratinocytes, enhances skin equivalent development, and facilitates lentiviral transduction. Tissue Eng Part A 18:1827–1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watt FM, Lo Celso C. and Silva-Vargas V. (2006). Epidermal stem cells: an update. Curr Opin Genet Dev 16:518–524 [DOI] [PubMed] [Google Scholar]
- 3.Kaur P. (2006). Interfollicular epidermal stem cells: identification, challenges, potential. J Invest Dermatol 126:1450–1458 [DOI] [PubMed] [Google Scholar]
- 4.Fuchs E. (2007). Scratching the surface of skin development. Nature 445:834–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy V, Lindon C, Harfe BD. and Morgan BA. (2005). Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell 9:855–861 [DOI] [PubMed] [Google Scholar]
- 6.Li A, Simmons PJ. and Kaur P. (1998). Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 95:3902–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tani H, Morris RJ. and Kaur P. (2000). Enrichment for murine keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci U S A 97:10960–10965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wan H, Stone MG, Simpson C, Reynolds LE, Marshall JF, Hart IR, Hodivala-Dilke KM. and Eady RA. (2003). Desmosomal proteins, including desmoglein 3, serve as novel negative markers for epidermal stem cell-containing population of keratinocytes. J Cell Sci 116:4239–4248 [DOI] [PubMed] [Google Scholar]
- 9.Michel M, Torok N, Godbout MJ, Lussier M, Gaudreau P, Royal A. and Germain L. (1996). Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci 109 (Pt 5):1017–1028 [DOI] [PubMed] [Google Scholar]
- 10.Jamora C, DasGupta R, Kocieniewski P. and Fuchs E. (2003). Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422:317–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris RJ. and Potten CS. (1994). Slowly cycling (label-retaining) epidermal cells behave like clonogenic stem cells in vitro. Cell Prolif 27:279–289 [DOI] [PubMed] [Google Scholar]
- 12.Jones PH. and Watt FM. (1993). Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73:713–724 [DOI] [PubMed] [Google Scholar]
- 13.Kaur P. and Li A. (2000). Adhesive properties of human basal epidermal cells: an analysis of keratinocyte stem cells, transit amplifying cells, and postmitotic differentiating cells. J Invest Dermatol 114:413–420 [DOI] [PubMed] [Google Scholar]
- 14.Liebig T, Erasmus J, Kalaji R, Davies D, Loirand G, Ridley A. and Braga VM. (2009). RhoE Is required for keratinocyte differentiation and stratification. Mol Biol Cell 20:452–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terunuma A, Limgala RP, Park CJ, Choudhary I. and Vogel JC. (2010). Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A 16:1363–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rheinwald JG. and Green H. (1975). Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell 6:331–343 [DOI] [PubMed] [Google Scholar]
- 17.Gangatirkar P, Paquet-Fifield S, Li A, Rossi R. and Kaur P. (2007). Establishment of 3D organotypic cultures using human neonatal epidermal cells. Nat Protoc 2:178–186 [DOI] [PubMed] [Google Scholar]
- 18.Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT. and Warden GD. (1995). Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg 222:743–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones PH, Harper S. and Watt FM. (1995). Stem cell patterning and fate in human epidermis. Cell 80:83–93 [DOI] [PubMed] [Google Scholar]
- 20.McMullan R, Lax S, Robertson VH, Radford DJ, Broad S, Watt FM, Rowles A, Croft DR, Olson MF. and Hotchin NA. (2003). Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol 13:2185–2189 [DOI] [PubMed] [Google Scholar]
- 21.Chapman S, Liu X, Meyers C, Schlegel R. and McBride AA. (2010). Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest 120:2619–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pontiggia L, Biedermann T, Meuli M, Widmer D, Bottcher-Haberzeth S, Schiestl C, Schneider J, Braziulis E, Montano I, Meuli-Simmen C. and Reichmann E. (2009). Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J Invest Dermatol 129:480–490 [DOI] [PubMed] [Google Scholar]
- 23.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F. and De Luca M. (2001). p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A 98:3156–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watt FM. and Green H. (1981). Involucrin synthesis is correlated with cell size in human epidermal cultures. J Cell Biol 90:738–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terunuma A, Shaya MB, Udey MC. and Vogel JC. (2004). An in vivo competitive repopulation assay system to evaluate human keratinocyte stem cells. J Invest Dermatol 123:993–995 [DOI] [PubMed] [Google Scholar]
- 26.Terunuma A, Kapoor V, Yee C, Telford WG, Udey MC. and Vogel JC. (2007). Stem cell activity of human side population and alpha6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells 25:664–669 [DOI] [PubMed] [Google Scholar]
- 27.Hodivala KJ. and Watt FM. (1994). Evidence that cadherins play a role in the downregulation of integrin expression that occurs during keratinocyte terminal differentiation. J Cell Biol 124:589–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.