Abstract
Recovery of motor function after stroke involves relearning motor skills and is mediated by neuroplasticity. Recent research has focused on developing rehabilitation strategies that facilitate such neuroplasticity to maximize functional outcome poststroke. Although many molecular signaling pathways are involved, brain-derived neurotrophic factor (BDNF) has emerged as a key facilitator of neuroplasticity involved in motor learning and rehabilitation after stroke. Thus, rehabilitation strategies that optimize BDNF effects on neuroplasticity may be especially effective for improving motor function poststroke. Two potential poststroke rehabilitation strategies that consider the importance of BDNF are the use of aerobic exercise to enhance brain function and the incorporation of genetic information to individualize therapy. Converging evidence demonstrates that aerobic exercise increases BDNF production and consequently enhances learning and memory processes. Nevertheless, a common genetic variant reduces activity-dependent secretion of the BDNF protein. Thus, BDNF gene variation may affect response to motor rehabilitation training and potentially modulate the effects of aerobic exercise on neuroplasticity. This perspective article discusses evidence that aerobic exercise promotes neuroplasticity by increasing BDNF production and considers how aerobic exercise may facilitate the acquisition and retention of motor skills for poststroke rehabilitation. Next, the impact of the BDNF gene val66met polymorphism on motor learning and response to rehabilitation is explored. It is concluded that the effects of aerobic exercise on BDNF and motor learning may be better exploited if aerobic exercise is paired more closely in time with motor training. Additionally, information about BDNF genotype could provide insight into the type and magnitude of effects that aerobic exercise may have across individuals and potentially help guide an individualized prescription of aerobic exercise to enhance motor rehabilitation poststroke.
Stroke is the leading cause of long-term disability in North America.1 Deficits in motor function are common following stroke; up to 75% of stroke survivors experience upper extremity impairments that persist into the chronic stage.1 Over the first 6 months after stroke onset, some spontaneous motor recovery occurs,2 but further advances in motor function rely on motor rehabilitation training. The process of motor rehabilitation is a form of motor learning,3 which refers to a relatively permanent change in motor behavior evoked by practice or experience.4 As such, individuals with stroke engage in motor rehabilitation training in an effort to relearn motor skills that were lost due to injury.
Consistent with motor learning in adults who are healthy, this relearning process is mediated by neuroplasticity,3 which is defined as the ability of the central nervous system (CNS) to undergo structural and functional change in response to new experiences.5 This neuroplasticity is detected in humans with a number of experimental techniques, including noninvasive brain stimulation (to measure shifts in size, location, and excitability of motor cortical maps) and functional magnetic resonance imaging (fMRI) (to measure altered activation and recruitment of brain regions involved in movement).3 Initially, learning-related plasticity involves the strengthening of existing, as well as the formation of new, neural connections that support learned behaviors.3,6 It is followed by pruning, or “focusing,” of neural connections as skill and preferential pathways develop.6,7 Current research focuses on maximizing the functional benefits of poststroke motor rehabilitation by developing interventions to promote motor learning-related neuroplasticity.3 Despite major progress in the understanding of neuroplasticity, very few new treatment interventions have resulted from this research.3 Thus, there is a critical need for the development of novel and more effective approaches for poststroke motor rehabilitation.
Recent advancements in the understanding of the role of brain-derived neurotrophic factor (BDNF) in neuroplasticity may provide important information for the development of new poststroke rehabilitation strategies. Brain-derived neurotrophic factor is a member of the neurotrophin family, a group of proteins involved in neuroprotection, neurogenesis, and neuroplasticity, and has been identified as a key mediator of motor learning and rehabilitation after stroke. New areas of research are beginning to inform the development of rehabilitation strategies that take into account the importance of BDNF for motor recovery after stroke. These areas of research include consideration of aerobic exercise effects on brain function and the incorporation of genetic information to individualize therapy.
Converging evidence suggests that aerobic exercise is a valuable intervention for improving brain function8–12 and that these effects are mediated, in part, by upregulation of BDNF.13,14 Thus, capitalizing on aerobic exercise–induced increases in BDNF could plausibly facilitate motor learning-related neuroplasticity for rehabilitation after stroke. Nevertheless, the basic processes that drive neuroplasticity, such as BDNF signaling, are dependent on the expression of genes. As a result, genetic variation could affect an individual's response to motor rehabilitation training, aerobic exercise training, and overall motor recovery after stroke.15 Thus, the primary aims of the present perspective article are: (1) to discuss evidence that aerobic exercise enhances brain function by increasing BDNF production and consider how these effects may be harnessed to facilitate motor rehabilitation poststroke and (2) to discuss the potential impact of a common variant of the BDNF gene on motor learning, response to motor rehabilitation training, and aerobic exercise effects on the brain poststroke.
Aerobic Exercise to Promote Neuroplasticity for Motor Rehabilitation Poststroke
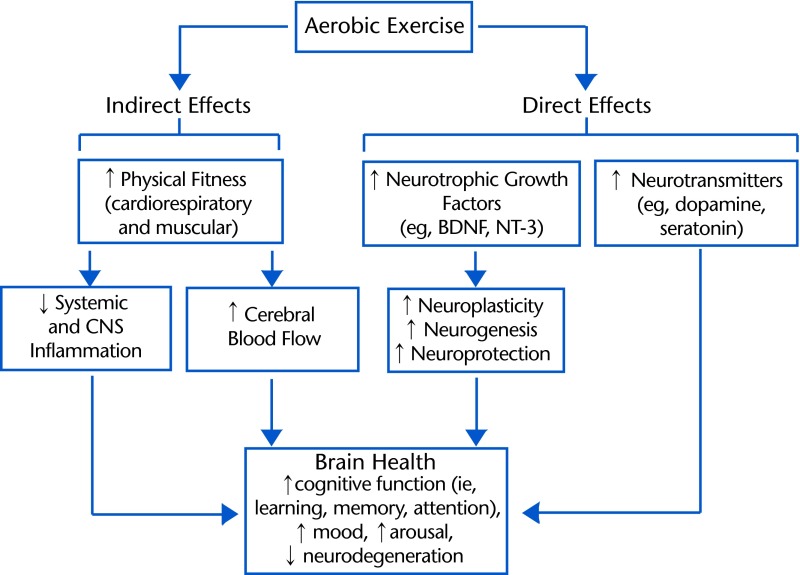
Aerobic exercise affects the brain indirectly through improvements in general health and fitness and through alterations in molecular signaling pathways that act directly on the CNS13,14 (Fig. 1). The primary focus of this article is on the direct pathway of exercise-induced upregulation of BDNF in the CNS.13,14 To begin to consider how exercise-induced increases in BDNF may be a key contributor to the positive effects of aerobic exercise on brain health and function, we address 3 main topics in this article. First, we discuss the involvement of BDNF in facilitating neuroplasticity, motor learning, and poststroke motor rehabilitation. Next, the effects of aerobic exercise on BDNF and its role in mediating exercise-induced increases in brain function are reviewed. Lastly, we consider how the effects of exercise on the brain may best be harnessed to promote neuroplasticity and facilitate motor rehabilitation poststroke. The present article focuses on aerobic exercise, although a growing body of evidence suggests that resistance exercise may have similar or complementary effects.16–18
Figure 1.
Examples of indirect and direct pathways for positive effects of aerobic exercise on the brain.13,14 Indirect effects refer to improvements in general health and reduction of peripheral risk factors that consequently affect brain health. Direct effects refer to aerobic exercise influences on the molecular signaling pathways of the brain itself. The present article focuses on the direct effect of exercise on BDNF production in the brain. BDNF=brain-derived neurotrophic factor, NT-3=neurotrophin-3, CNS=central nervous system.
BDNF Is Involved in Motor Learning and Poststroke Motor Rehabilitation
Brain-derived neurotrophic factor is involved in many facets of brain function, including neuroplastic changes that underlie motor learning. It exerts its effects on neuroplasticity by facilitating long-term potentiation (LTP), a long-lasting increase in the strength of connection between 2 neurons that are repeatedly activated together and by promoting dendritic growth and remodeling.19,20 Unlike other growth factors, BDNF is secreted in the CNS through both a constitutive and an activity-dependent pathway. The activity-dependent secretion is crucial to the role of BDNF in promoting neuroplasticity in circuits activated in response to experience.20
Evidence for the role of BDNF specifically in motor learning can be found in animal work demonstrating that disrupting BDNF synthesis with a pharmacological intervention impaired skilled motor performance and diminished training-induced cortical map plasticity.21 Subsequent application of BDNF by intracortical injection into primary motor cortex (M1) partially restored these functions.21 Similarly, in rat models of focal ischemia, recovery of skilled reaching movements with rehabilitation training was abolished when BDNF was blocked in the CNS.22 In a similar study, response to poststroke rehabilitation training was enhanced when exogenous BDNF was administered through an intravenous bolus in rats.23 Equivalent data in humans showing direct BDNF involvement in motor learning and poststroke rehabilitation are not available due to the invasive nature of intracortical injections and limited capacity to target BDNF application to specific brain regions in humans. Nevertheless, given the strong evidence for BDNF involvement in neuroplasticity within the motor system in animal research, it is plausible that motor rehabilitation strategies that capitalize on the beneficial effects of BDNF in the CNS will be effective for facilitating recovery after stroke.
Aerobic Exercise Effects on Brain Function: BDNF and Cognitive Function
Aerobic exercise may be a particularly effective means to enhance BDNF levels, as it induces a cascade of events that leads to increased BDNF gene expression in multiple regions of the CNS, including the hippocampus, cerebellum, cerebral cortex, and spinal cord.14 Moreover, considerable evidence shows that exercise-induced increases in BDNF benefit cognitive function.11,12,24,25 In rats, completion of a 1-week aerobic exercise program enhanced spatial memory test performance, but these effects were abolished in an experimental group that also received pharmacological blockage of hippocampal BDNF.26 Such a causal effect is more difficult to demonstrate in humans as BDNF cannot be blocked and typically cannot be measured from the CNS in vivo. However, systemic levels of BDNF are increased for approximately 10 to 60 minutes following a bout of aerobic exercise in humans.27 These systemic BDNF measurements are often considered to reflect CNS levels in humans, as BDNF undergoes bidirectional transport across the blood-brain barrier28,29 and is released from the brain into the periphery at rest and during aerobic exercise.30 There also have been reports of increased basal levels of systemic BDNF following several weeks of aerobic exercise training,31–33 but other studies report no effect of aerobic exercise training programs on basal BDNF values.34–36 The return to baseline levels of systemic BDNF levels after 1 hour following aerobic exercise and the lack of training effects on basal systemic levels in some studies are thought to be a result of a subsequent increase in BDNF absorption in the CNS following aerobic exercise–induced increases in production.27
Complementary work demonstrates that aerobic exercise training enhances multiple aspects of cognitive function in individuals who are healthy and across a range of chronic health conditions, including stroke.11,12,24,25 A meta-analysis of 18 aerobic exercise training intervention studies in older adults concluded that the largest effects on cognition occur in the executive control domain, including functions such as planning, scheduling, working memory, and multitasking.10 The majority of studies included in this meta-analysis involved aerobic exercise 3 times per week at a moderate intensity (ie, ∼70% maximum heart rate). Programs that involved aerobic exercise sessions greater than 30 minutes, training periods of more than 6 months, and a combination of aerobic and resistance training had the largest effects.10 Similar positive effects of aerobic exercise training on cognition have been shown in individuals with stroke. Exercise programs combining aerobic and resistance training performed at moderate ratings of perceived exertion on 2 to 3 days per week for 12 weeks11 and 6 months12 improved executive function and memory in individuals with chronic stroke. Combined with animal work,26 the findings of aerobic exercise–induced increases in systemic BDNF27 and cognitive function11,12 in humans are commonly taken as evidence that BDNF contributes, at least in part, to the positive effects of aerobic exercise on cognitive function in humans.27 Nevertheless, a key limitation of current evidence is that relatively few human studies concurrently assess aerobic exercise–induced changes in both BDNF and cognitive function.37–39
Aerobic Exercise Effects on Motor Learning
Aerobic exercise training not only improves poststroke cognitive function but also enhances poststroke mobility, balance, and motor function.11,12,40,41 Increased physical fitness is undoubtedly a large contributor to these improvements in motor function; however, exercise-induced increases in neuroplasticity and motor learning abilities via upregulation of BDNF within the CNS also may contribute to these beneficial effects. Only 1 study has examined the effects of engaging in aerobic exercise over several weeks on motor learning, and it was conducted in individuals with stroke.42 In this study by Quaney et al,42 participation in an 8-week aerobic cycling program (70% maximum heart rate, 45 minutes, 3 times per week) improved within-session performance of a motor sequence task compared with those who participated in an 8-week stretching program. Their study demonstrated that, at least in the short-term, aerobic exercise training improves motor skill acquisition. However, motor performance at a delayed retention test (≥24 hours postpractice) is required to indicate motor learning43 and, unfortunately, was not examined. Nevertheless, Quaney and colleagues' findings indicate that motor learning abilities may be enhanced by aerobic exercise.
Persistence of Aerobic Exercise Effects on the Brain
Another important finding by Quaney et al42 was that the greater within-session performance among aerobic exercisers was not maintained at a follow-up test 8 weeks after exercise training had stopped. This finding raises an important issue concerning the persistence of aerobic exercise–induced increases in brain function that has not been well addressed in the literature. Many randomized clinical trials of aerobic exercise training programs report improvements in performance on cognitive tests performed immediately before and after participation in an aerobic exercise program.10 However, to our knowledge, there is no evidence that the benefits of aerobic exercise on brain function persist at follow-up after aerobic exercise is stopped. Similar to the finding by Quaney et al,42 a recent study of young adults who were healthy showed that improvements in object memory retrieval following a 4-week treadmill training program occurred only when individuals performed an exercise bout on the final testing day.44 A possible explanation for these findings may be found within research investigating the effects of an acute bout of aerobic exercise on cognitive performance in humans. A meta-analysis of 29 studies of young adults who were healthy concluded that information processing and memory are significantly enhanced immediately following a single bout of aerobic exercise.8 Thus, enhanced cognitive function induced by aerobic exercise training programs may simply be due to continuous exposure to acute bouts of aerobic exercise8; when regular training is stopped, these effects are no longer regularly evoked.
Traditionally, enhanced cognitive function following an acute bout of aerobic exercise has been attributed to a temporary increase in arousal and thus is expected to dissipate as arousal levels return to baseline.8 However, the aforementioned meta-analysis determined that the aspects of cognitive function most positively affected by an acute bout of aerobic exercise were short-term and long-term memory.8 These effects were greatest when cycling exercise was used rather than treadmill exercise.8 There also is evidence suggesting that short intervals of high-intensity aerobic exercise (ie, 3 × 3 minutes above ventilatory threshold) may enhance memory more than long-duration, low- to moderate-intensity aerobic exercise (ie, 40 minutes below ventilatory threshold) in young adults.39 Interestingly, if an acute bout of aerobic exercise alters memory processes, it also could affect learning and thus promote relatively permanent changes in motor behavior that persist even after aerobic exercise training has stopped. An important caveat to this idea is that learning and neuroplasticity are dependent on experience. By increasing BDNF production, aerobic exercise may facilitate the neuroplastic processes that underlie learning, such as LTP and dendritic branching, but aerobic exercise alone is not capable of inducing these neuroplastic processes. Thus, for aerobic exercise to have the most meaningful and lasting effects on behavior, it likely needs to be paired closely in time with sufficient and meaningful practice or experience that is consistent with the desired behavioral change. For example, 2 months of a combination of aerobic exercise training and mental training increases cognitive function more than either intervention alone.45
Prescribing Aerobic Exercise to “Prime” Motor Learning and Poststroke Motor Rehabilitation
The basis of engaging in aerobic exercise training in close temporal proximity with behavioral training is that the aerobic exercise will serve to “prime” the CNS for the neuroplastic change that underlies the desired behavior change (ie, learning). With this approach, the positive effects of aerobic exercise on brain function may be more effectively harnessed to facilitate functional improvements in populations with chronic disease, such as stroke. In a recent study, Roig et al46 found that high-intensity interval cycling (3 × 3 minutes, above ventilatory threshold) immediately before or after practice of a motor task enhanced motor performance on retention tests conducted at 1 and 7 days postpractice, demonstrating that a single bout of aerobic exercise enhanced motor learning in young individuals who were healthy. Aerobic exercise immediately before motor task practice was thought to facilitate the detection and encoding of information relevant to the task during the subsequent motor practice, and aerobic exercise immediately after motor task practice was thought to facilitate processes involved in motor memory consolidation.46 As motor learning underlies improvements in motor function evoked by rehabilitation following stroke, these findings suggest that acute bouts of aerobic exercise may have the potential to be used to facilitate response to poststroke motor rehabilitation training (Fig. 2).
Figure 2.
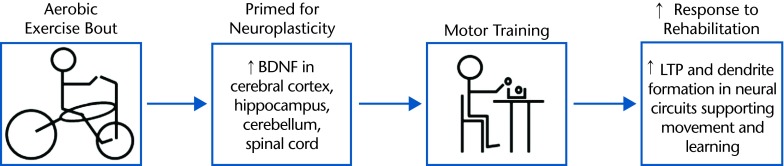
Using aerobic exercise to prime motor rehabilitation poststroke. Performing aerobic exercise immediately before motor rehabilitation training may facilitate improvements in motor function by capitalizing on aerobic exercise–induced increases in the capacity for neuroplasticity. Alternatively, aerobic exercise could be performed immediately after motor training to facilitate motor memory consolidation processes. BDNF=brain-derived neurotrophic factor, LTP=long-term potentiation.
Current guidelines recommend that individuals with stroke engage in a minimum of 20 minutes of moderate intensity aerobic exercise 3 days per week.47 The intensity of the exercise should be greater than 30% of heart rate reserve, the minimal effective training intensity for very unfit individuals,48 but based on individual exercise stress test results and health status.47 Although these recommendations are sufficient to obtain general health benefits for individuals with stroke, there is limited research examining the specific exercise dose necessary to elicit direct effects on brain function and facilitate motor rehabilitation in individuals with stroke. Thus, findings from studies investigating aerobic exercise effects on BDNF production and other cognitive functions currently may be the best source of information when speculating on how to best prescribe aerobic exercise for this purpose.
Based on this literature, to induce large positive effects on cognitive function and increase BDNF levels, exercise training studies should use: (1) aerobic exercise sessions of more than 30 minutes,10 (2) training intensities of approximately 70% heart rate maximum,27 (3) a frequency of 4 days per week,27 and (4) a combination of aerobic and resistance exercises.10 Cycling8 and high-intensity intervals39 may be especially effective for immediate benefits of acute aerobic exercise on cognitive function; however, there also is evidence that just 30 minutes of aerobic exercise at 60% maximum heart rate is effective for increasing BDNF in individuals with chronic disease.27 Lastly, the effects of aerobic exercise training on the brain may be most effectively harnessed if performed at a point close in time to performance of motor rehabilitation training.46 Although further research is needed to determine the precise time course of BDNF effects, it appears that 1 hour postexercise is the most likely window of time in which motor learning will be most facilitated.8,27,46
These findings provide a reference point for prescription of aerobic exercise in future research evaluating the effects of exercise on motor learning and response to rehabilitation poststroke. The idea of priming motor rehabilitation with aerobic exercise is speculative; however, with additional study, researchers may gain further insight into whether—and if so, how—aerobic exercise can be prescribed to facilitate the acquisition and retention of motor skills for rehabilitation.
Genetics Research to Inform Motor Rehabilitation and Aerobic Exercise Prescription Poststroke
The premise for rehabilitation interventions that promote neuroplasticity is that if the CNS can be primed for greater capacity for physiological change, then functional improvements mediated by those physiological changes will be more likely to occur.3 As many of the neuronal processes that drive such changes are dependent on the expression of specific genes, genetic variation may influence the efficacy of rehabilitation strategies that engage these processes.15 For example, although upregulation of BDNF following aerobic exercise may be beneficial for neuroplasticity, a common single nucleotide polymorphism (SNP) on the human BDNF gene could affect the effects of aerobic exercise on the brain.44,49,50 Thus, improved understanding of how genetic variation influences neuroplasticity and motor learning after CNS injury may allow for better individualization of rehabilitation strategies to maximize motor outcome poststroke. In the current section of this article, the effects of the BDNF val66met polymorphism on neuroplasticity, motor learning, and poststroke motor rehabilitation will be discussed. Next, ideas about how knowledge of the effects of this polymorphism could be utilized when prescribing aerobic exercise to prime motor rehabilitation will be considered. Given the role of BDNF in mediating aerobic exercise effects on the brain,26,27 focus is placed on the well-characterized BDNF val66met gene variation throughout this section; however, this is just one of many genetic variants that could potentially affect aerobic exercise effects on the brain and poststroke motor rehabilitation.15
BDNF Gene Val66met Polymorphism Impact on Brain Health and Function
In approximately 30% to 50% of the human population, an SNP exists on the BDNF gene that results in an amino acid change from valine (val) to methionine (met) at position 66 (val66met) of the precursor peptide proBDNF.51 The presence of the met allele results in a 25% reduction in activity-dependent secretion of BDNF in the CNS.49,50 Due to the importance of activity-dependent secretion of BDNF to brain health and function, much research has been dedicated to studying the effects of the BDNF val66met polymorphism on the CNS. In humans, presence of the BDNF val66met polymorphism is associated with abnormalities in brain structure and physiology.52 For example, when compared with those without the polymorphism, val66met carriers demonstrate reduced volume of the prefrontal cortex53 and hippocampus,53,54 reduced hippocampal levels of N-acetyl-aspartate (a marker for neuronal health),49 and abnormal activation of the hippocampus when performing a working memory task during fMRI.49 These changes in the brain coincide with altered cognitive function. For instance, multiple studies have demonstrated that val66met allele carriers demonstrate impaired performance on hippocampal-dependent memory tasks when compared with those without the polymorphism.49,55,56
BDNF Gene Val66met Polymorphism Impact on Motor System
The first study to demonstrate an effect of the BDNF gene val66met polymorphism on activity-dependent plasticity associated with movement was conducted by Kleim and colleagues.57 Following 30 minutes of fast index finger movement training, individuals without the polymorphism demonstrated a greater expansion of motor maps and greater increase in M1 excitability, as measured by transcranial magnetic stimulation, when compared with individuals with the met allele.57 Another study demonstrated similar results utilizing the same simple motor training task and fMRI techniques in young individuals who were healthy.58 Interestingly, it also has been demonstrated that after 1 day of training on a similar index finger motor task, individuals without the polymorphism have greater motor map plasticity compared with those individuals with the polymorphism; however, after 5 and 12 days of training, there was no difference in measures of plasticity between genotypes.59 These results suggest that extensive motor training may overcome deficits in neuroplasticity in met allele carriers. In contrast, 2 other studies showed no effect of BDNF genotype on change in cortical excitability evoked by a single session of fast finger movement tasks in young adults60,61; however, 1 of these studies demonstrated a significant effect of BDNF genotype when a more complex visuomotor task was practiced.61 Additionally, McHughen and Cramer62 found that there was no BDNF val66met polymorphism effect on motor map plasticity evoked by the same fast index finger movement paradigm as described above when performed in older adults who were healthy, suggesting that the BDNF genotype effects may be attenuated with advanced age. However, null effects of BDNF genotype on motor map plasticity within this body of research also may relate to the nature of the motor tasks employed. For example, given the importance of BDNF for motor learning,19–21,63 plasticity may be more dependent on the BDNF val66met polymorphism when induced by tasks that involve learning of a novel motor skill than by tasks that involve simple repetition of a familiar movement.
Despite differences in neuroplasticity, most studies thus far have found no effect of BDNF genotype on motor performance in young healthy individuals.57,60,61 It has been suggested that detecting behavioral effects of the polymorphism may require more sensitive measures of motor performance.57 It is also possible that reduced neuroplasticity may have larger and more detectable behavioral effects when other CNS functions have been compromised, such as after an individual has sustained a stroke. Furthermore, the majority of studies have only tested motor performance during and immediately following motor task training; as a result, they may have missed any longer lasting effects that would be detected with a delayed retention test (ie, true motor learning effects).57,60,61 The only study to use a delayed retention test showed that individuals without the val66met polymorphism demonstrated greater relative retention on a motor learning task compared with those with the polymorphism.58 Thus, altered neuroplasticity as a result of the BDNF val66met polymorphism may manifest behaviorally as deficits in motor learning. Nevertheless, additional research specifically examining motor learning is needed to further elucidate the effects of the BDNF genotype on the motor system.
BDNF Gene Val66met Polymorphism Impact on Recovery Poststroke
Evidence for a BDNF genotype effect on neuroplasticity and motor learning in young individuals who are healthy has led to speculation that the BDNF val66met polymorphism also may influence recovery after stroke.15 Three studies have demonstrated an association between the met allele and poorer recovery relative to those without the polymorphism in the acute and subacute stages following hemorrhagic stroke. However, there are conflicting findings regarding the long-term impact of the polymorphism (ie, >1 month poststroke) and limited evidence to support an impact among individuals with ischemic stroke.64–66 Moreover, these studies have all used global outcome scales that do not differentiate between recovery of cognitive and motor function.64–66 Thus far, only 1 study of the BDNF val66met polymorphism has been conducted in individuals with chronic stroke.67 In that study, reductions in visual memory after subarachnoid hemorrhage were greater in met allele carriers when compared with individuals without the polymorphism; however, this genotype effect was not present in individuals with concurrent cerebral infarctions.67 Thus, more research is needed to understand BDNF genotype effects on different aspects of recovery and long-term outcome, as well as how the type of stroke influences these effects. Additionally, effects of the BDNF gene val66met polymorphism on motor learning could potentially modulate response to motor rehabilitation in the chronic stage of stroke, but this relationship has not yet been investigated.
BDNF Gene Val66met Polymorphism to Inform the Use of Aerobic Exercise for Motor Rehabilitation
Previously, we considered the idea of priming the CNS by prescribing an acute bout of aerobic exercise in concert with motor rehabilitation training. As upregulation of BDNF is thought to partly mediate the benefits of aerobic exercise on brain function,26,27 aerobic exercise effects on motor learning and rehabilitation may be attenuated in individuals with the BDNF gene val66met polymorphism. Supporting this contention is the finding that improvements in object recognition memory following 4 weeks of aerobic exercise are attenuated in individuals with the BDNF val66met polymorphism compared with those in the study without the polymorphism.44
It is possible, then, that any beneficial effects of aerobic exercise on cognitive domains involved in motor learning and rehabilitation also would be reduced in individuals with the BDNF val66met polymorphism. Nevertheless, aerobic exercise may still be beneficial for motor rehabilitation in BDNF val66met carriers but may need to be prescribed in greater amounts or at higher intensity levels than prescribed to those without the polymorphism. As more intensive motor practice can overcome the negative effects of the met allele on motor map plasticity,59 more intensive aerobic exercise also may overcome such negative effects. Alternatively, it could be that rehabilitation strategies that target BDNF are not effective for met carriers, and as a result, other approaches may need to be developed to promote motor recovery in these individuals. Figure 3 illustrates how the BDNF gene val66met polymorphism may influence the effects of aerobic exercise on motor rehabilitation poststroke. Nevertheless, many other factors and molecular pathways, besides BDNF signaling, could influence the effects of aerobic exercise on the brain.68–70 As a result, aerobic exercise may be a uniquely powerful intervention that has positive effects on brain function across many genetic profiles. Regardless, an improved understanding of the role of genetics in motor rehabilitation could potentially enhance the understanding of what effects aerobic exercise may have on specific individuals and, as such, inform how it could be most effectively prescribed.
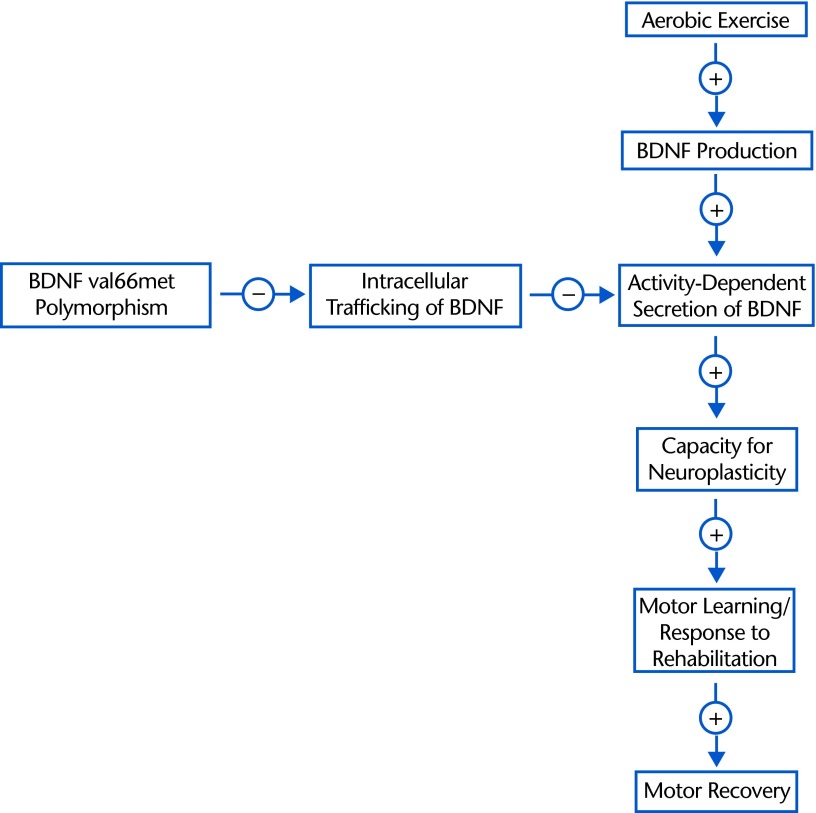
Figure 3.
The potential influence of the brain-derived neurotrophic factor (BDNF) val66met polymorphism on the effects of aerobic exercise on motor recovery poststroke. Aerobic exercise increases the production of BDNF, which then may increase the amount of BDNF available for secretion via its activity-dependent pathway. Increased amounts of BDNF secreted via the activity-dependent pathway could then enhance neuroplasticity, resulting in an increase in response to motor rehabilitation and, ultimately, an increase in motor recovery. However, the BDNF val66met polymorphism impairs the intracellular trafficking of BDNF to the activity-dependent pathway by 25%. As a result, the effect of aerobic exercise on neuroplasticity, response to rehabilitation, and motor recovery may be attenuated in individuals with the BDNF val66met polymorphism compared to those without it. The + and – signs indicate positive and negative effects, respectively.
Conclusions and Clinical Implications
Rehabilitation strategies that promote motor learning-related neuroplasticity hold promise for improving functional outcomes poststroke.3 Aerobic exercise may be a particularly effective means of enhancing the capacity of the motor system for plasticity by upregulation of neurotrophins, such as BDNF.13,14,27 Importantly, aerobic exercise alone does not induce neuroplasticity but rather promotes the development of a neural environment that is supportive of plasticity.71 To capitalize on this effect for motor rehabilitation, aerobic exercise bouts may need to be performed in close temporal proximity to purposeful motor skill practice or experience. This idea is supported by evidence suggesting that an acute bout of aerobic exercise immediately before or after skilled motor practice enhances motor learning in young adults who are healthy.46 Further research is needed to test this idea in individuals with stroke.
Additionally, the basic neuronal processes that mediate aerobic exercise effects on the brain and facilitate motor learning–related neuroplasticity, such as the production and activity-dependent secretion of BDNF, depend on the expression of specific genes.15 For example, the effects of aerobic exercise on motor learning–related neuroplasticity may be attenuated in individuals with a variant of the BDNF gene (val66met) that reduces activity-dependent secretion of BDNF.49,50 Knowledge of this genetic variant could be used to better individualize motor rehabilitation strategies. Although genetics research is a promising avenue for the development of individualized rehabilitation strategies for people with stroke, it is important to note that a number of other factors, including demographic or environmental variables, can modulate the functional effects of genetic variation.24,62,69 Nevertheless, as personalized health care (specifically rehabilitation strategies) becomes more refined, the effects of interventions may be optimized by the incorporation of genetic information. In conclusion, future research into aerobic exercise and genetics may provide exciting new directions for the development of rehabilitation strategies designed to promote optimal neuroplasticity to improve motor recovery after stroke.
Footnotes
All authors provided concept/idea/project design and writing. Dr Boyd provided project management, fund procurement, facilities/equipment, and clerical support. Dr Campbell and Dr Boyd provided consultation (including review of manuscript before submission).
The Natural Sciences and Engineering Research Council of Canada provided support to Mr Mang. Support was provided to Dr Boyd by the Canada Research Chairs and the Michael Smith Foundation for Health Research. Dr Ross is supported by a Canadian Institutes of Health Research/ Drug Safety and Effectiveness Network (CIHR/DSEN) New Investigator Award.
References
- 1. Gresham GE, Duncan PW, Stason WB. Post-Stroke Rehabilitation. Darby, PA: Diane Publishing; 2004 [Google Scholar]
- 2. Jørgensen HS, Nakayama H, Raaschou HO, et al. Outcome and time course of recovery in stroke, part II: time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–412 [DOI] [PubMed] [Google Scholar]
- 3. Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PM&R. 2010;2:S208–S219 [DOI] [PubMed] [Google Scholar]
- 4. Schmidt RA, Lee TD. Motor Control and Learning: A Behavioral Emphasis. Champaign, IL: Human Kinetics Publishers; 2005 [Google Scholar]
- 5. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239 [DOI] [PubMed] [Google Scholar]
- 6. Hosp JA, Luft AR. Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plast. 2011;2011:871296 2011 Oct 26 [Epub ahead of print]. doi: 10.1155/2011/871296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617 [DOI] [PubMed] [Google Scholar]
- 8. Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 2010;1341:12–24 [DOI] [PubMed] [Google Scholar]
- 9. Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–1242 [DOI] [PubMed] [Google Scholar]
- 10. Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults a meta-analytic study. Psychol Sci. 2003;14:125–130 [DOI] [PubMed] [Google Scholar]
- 11. Kluding PM, Tseng BY, Billinger SA. Exercise and executive function in individuals with chronic stroke: a pilot study. J Neurol Phys Ther. 2011;35:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rand D, Eng JJ, Liu-Ambrose T, Tawashy AE. Feasibility of a 6-month exercise and recreation program to improve executive functioning and memory in individuals with chronic stroke. Neurorehabil Neural Repair. 2010;24:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472 [DOI] [PubMed] [Google Scholar]
- 14. Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301 [DOI] [PubMed] [Google Scholar]
- 15. Pearson-Fuhrhop KM, Cramer SC. Genetic influences on neural plasticity. PM&R. 2010;2:S227–S240 [DOI] [PubMed] [Google Scholar]
- 16. Liu-Ambrose T, Donaldson MG. Exercise and cognition in older adults: is there a role for resistance training programmes? Br J Sports Med. 2009;43:25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu-Ambrose T, Nagamatsu LS, Voss MW, et al. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698 [DOI] [PubMed] [Google Scholar]
- 19. Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118 [PubMed] [Google Scholar]
- 20. Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2:24–32 [DOI] [PubMed] [Google Scholar]
- 21. VandenBerg PM, Bruneau RM, Thomas N, Kleim JA. BDNF is required for maintaining motor map integrity in adult cerebral cortex. Soc Neurosci Abstr. 2004;681:5 [Google Scholar]
- 22. Ploughman M, Windle V, MacLellan CL, et al. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke. 2009;40:1490–1495 [DOI] [PubMed] [Google Scholar]
- 23. Schäbitz WR, Berger C, Kollmar R, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997 [DOI] [PubMed] [Google Scholar]
- 24. Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18:82–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laurin D, Verreault R, Lindsay J, et al. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58:498–504 [DOI] [PubMed] [Google Scholar]
- 26. Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590 [DOI] [PubMed] [Google Scholar]
- 27. Knaepen K, Goekint M, Heyman EM, Meeusen R. Neuroplasticity exercise-induced response of peripheral brain-derived neurotrophic factor: a systematic review of experimental studies in human subjects. Sports Med. 2010;40:765–801 [DOI] [PubMed] [Google Scholar]
- 28. Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37:1553–1561 [DOI] [PubMed] [Google Scholar]
- 29. Pan W, Kastin AJ. Polypeptide delivery across the blood-brain barrier. Curr Drug Targets CNS Neurol Disord. 2004;3:131–136 [DOI] [PubMed] [Google Scholar]
- 30. Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94:1062–1069 [DOI] [PubMed] [Google Scholar]
- 31. Zoladz J, Pilc A, Majerczak J, et al. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59:119–132 [PubMed] [Google Scholar]
- 32. Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seifert T, Brassard P, Wissenberg M, et al. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298:R372–R377 [DOI] [PubMed] [Google Scholar]
- 34. Castellano V, White LJ. Serum brain-derived neurotrophic factor response to aerobic exercise in multiple sclerosis. J Neurol Sci. 2008;269:85–91 [DOI] [PubMed] [Google Scholar]
- 35. Schiffer T, Schulte S, Hollmann W, et al. Effects of strength and endurance training on brain-derived neurotrophic factor and insulin-like growth factor 1 in humans. Horm Metab Res. 2009;41:250–254 [DOI] [PubMed] [Google Scholar]
- 36. Schulz KH, Gold SM, Witte J, et al. Impact of aerobic training on immune-endocrine parameters, neurotrophic factors, quality of life and coordinative function in multiple sclerosis. J Neurol Sci. 2004;225:11–18 [DOI] [PubMed] [Google Scholar]
- 37. Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39:728–734 [DOI] [PubMed] [Google Scholar]
- 38. Goekint M, Heyman E, Roelands B, et al. No influence of noradrenaline manipulation on acute exercise-induced increase of brain-derived neurotrophic factor. Med Sci Sports Exerc. 2008;40:1990–1996 [DOI] [PubMed] [Google Scholar]
- 39. Winter B, Breitenstein C, Mooren FC, et al. High impact running improves learning. Neurobiol Learn Mem. 2007;87:597–609 [DOI] [PubMed] [Google Scholar]
- 40. Studenski S, Duncan PW, Perera S, et al. Daily functioning and quality of life in a randomized controlled trial of therapeutic exercise for subacute stroke survivors. Stroke. 2005;36:1764–1770 [DOI] [PubMed] [Google Scholar]
- 41. Ivey FM, Hafer-Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRx. 2006;3:439–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Quaney BM, Boyd LA, McDowd JM, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kantak SS, Winstein CJ. Learning-performance distinction and memory processes for motor skills: a focused review and perspective. Behav Brain Res. 2012;228:219–231 [DOI] [PubMed] [Google Scholar]
- 44. Hopkins ME, Davis FC, Vantieghem MR, et al. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience. 2012;215:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fabre C, Chamari K, Mucci P, et al. Improvement of cognitive function by mental and/or individualized aerobic training in healthy elderly subjects. Int J Sports Med. 2002;23:415–421 [DOI] [PubMed] [Google Scholar]
- 46. Roig M, Skriver K, Lundbye-Jensen J, et al. A single bout of exercise improves motor memory. PLoS One. 2012;7:e44594 2012 Sep 4 [Epub ahead of print]. doi: 10.1371/journal.pone.0044594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mackay-Lyons MS, Macko R, Eng J, et al. Aerobic exercise recommendations to optimize best practices in care after stroke. 2013. Available at: http://strokebestpractices.ca/wp-content/uploads/2013/07/AEROBICS-FINAL-July-2013.pdf Accessed May 1, 2013 [DOI] [PMC free article] [PubMed]
- 48. Swain DP, Franklin BA. VO(2) reserve and the minimal intensity for improving cardiorespiratory fitness. Med Sci Sports Exerc. 2002;34:152–157 [DOI] [PubMed] [Google Scholar]
- 49. Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269 [DOI] [PubMed] [Google Scholar]
- 50. Chen ZY, Patel PD, Sant G, et al. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 2004;24:4401–4411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 2004;126:122–123 [DOI] [PubMed] [Google Scholar]
- 52. Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn Affect Behav Neurosci. 2006;6:79–85 [DOI] [PubMed] [Google Scholar]
- 53. Pezawas L, Verchinski BA, Mattay VS, et al. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 2004;24:10099–10102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bueller JA, Aftab M, Sen S, et al. BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry. 2006;59:812–815 [DOI] [PubMed] [Google Scholar]
- 55. Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ho BC, Milev P, O'Leary DS, et al. Cognitive and magnetic resonance imaging brain morphometric correlates of brain-derived neurotrophic factor Val66Met gene polymorphism in patients with schizophrenia and healthy volunteers. Arch Gen Psychiatry. 2006;63:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–737 [DOI] [PubMed] [Google Scholar]
- 58. McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20:1254–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McHughen SA, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66met BDNF polymorphism on short-term plasticity. Exp Brain Res. 2011;213:415–422 [DOI] [PubMed] [Google Scholar]
- 60. Li Voti P, Conte A, Suppa A, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212:91–99 [DOI] [PubMed] [Google Scholar]
- 61. Cirillo J, Hughes J, Ridding M, et al. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. Eur J Neurosci. 2012;36:2640–2649 [DOI] [PubMed] [Google Scholar]
- 62. McHughen SA, Cramer SC. The BDNF val(66)met polymorphism is not related to motor function or short-term cortical plasticity in elderly subjects. Brain Res. 2013;1495:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104 [DOI] [PubMed] [Google Scholar]
- 64. Siironen J, Juvela S, Kanarek K, et al. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2007;38:2858–2860 [DOI] [PubMed] [Google Scholar]
- 65. Cramer SC, Procaccio V; GAIN Americas; GAIN International Study Investigators Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012;19:718–724 [DOI] [PubMed] [Google Scholar]
- 66. Mirowska-Guzel D, Gromadzka G, Czlonkowski A, Czlonkowska A. BDNF −270 C>T polymorphisms might be associated with stroke type and BDNF −196 G>A corresponds to early neurological deficit in hemorrhagic stroke. J Neuroimmunol. 2012;249:71–75 [DOI] [PubMed] [Google Scholar]
- 67. Vilkki J, Lappalainen J, Juvela S, et al. Relationship of the Met allele of the brain-derived neurotrophic factor Val66Met polymorphism to memory after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2008;63:198–203 [DOI] [PubMed] [Google Scholar]
- 68. Tong L, Shen H, Perreau VM, et al. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Dis. 2001;8:1046–1056 [DOI] [PubMed] [Google Scholar]
- 69. Nagel IE, Chicherio C, Li SC, et al. Human aging magnifies genetic effects on executive functioning and working memory. Front Hum Neurosci. 2008;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Erickson KI, Kim JS, Suever BL, et al. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci. 2008;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6 [DOI] [PubMed] [Google Scholar]