Abstract
Several recent reports indicate that mobile elements are frequently found in and flanking many wild-type plant genes. To determine the extent of this association, we performed computer-based systematic searches to identify mobile elements in the genes of two "model" plants, Oryza sativa (domesticated rice) and Arabidopsis thaliana. Whereas 32 common sequences belonging to nine putative mobile element families were found in the noncoding regions of rice genes, none were found in Arabidopsis genes. Five of the nine families (Gaijin, Castaway, Ditto, Wanderer, and Explorer) are first described in this report, while the other four were described previously (Tourist, Stowaway, p-SINE1, and Amy/LTP). Sequence similarity, structural similarity, and documentation of past mobility strongly suggests that many of the rice common sequences are bona fide mobile elements. Members of four of the new rice mobile element families are similar in some respects to members of the previously identified inverted-repeat element families, Tourist and Stowaway. Together these elements are the most prevalent type of transposons found in the rice genes surveyed and form a unique collection of inverted-repeat transposons we refer to as miniature inverted-repeat transposable elements or MITEs. The sequence and structure of MITEs are clearly distinct from short or long interspersed nuclear elements (SINEs or LINEs), the most common transposable elements associated with mammalian nuclear genes. Mobile elements, therefore, are associated with both animal and plant genes, but the identity of these elements is strikingly different.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleman M., Kermicle J. L. Somatic variegation and germinal mutability reflect the position of transposable element Dissociation within the maize R gene. Genetics. 1993 Sep;135(1):189–203. doi: 10.1093/genetics/135.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bugos R. C., Thom M. Glucose transporter cDNAs from sugarcane. Plant Physiol. 1993 Dec;103(4):1469–1470. doi: 10.1104/pp.103.4.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau T. E., Wessler S. R. Mobile inverted-repeat elements of the Tourist family are associated with the genes of many cereal grasses. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1411–1415. doi: 10.1073/pnas.91.4.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau T. E., Wessler S. R. Stowaway: a new family of inverted repeat elements associated with the genes of both monocotyledonous and dicotyledonous plants. Plant Cell. 1994 Jun;6(6):907–916. doi: 10.1105/tpc.6.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau T. E., Wessler S. R. Tourist: a large family of small inverted repeat elements frequently associated with maize genes. Plant Cell. 1992 Oct;4(10):1283–1294. doi: 10.1105/tpc.4.10.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes B., Dekeyser R., Villarroel R., Van den Bulcke M., Bauw G., Van Montagu M., Caplan A. Characterization of a rice gene showing organ-specific expression in response to salt stress and drought. Plant Cell. 1990 Jan;2(1):19–27. doi: 10.1105/tpc.2.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deragon J. M., Landry B. S., Pélissier T., Tutois S., Tourmente S., Picard G. An analysis of retroposition in plants based on a family of SINEs from Brassica napus. J Mol Evol. 1994 Oct;39(4):378–386. doi: 10.1007/BF00160270. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickbush T. H. Transposing without ends: the non-LTR retrotransposable elements. New Biol. 1992 May;4(5):430–440. [PubMed] [Google Scholar]
- Finnegan D. J. Eukaryotic transposable elements and genome evolution. Trends Genet. 1989 Apr;5(4):103–107. doi: 10.1016/0168-9525(89)90039-5. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Dunbar E., Anderson R., Pearce S. R., Hartley R., Kumar A. Ty1-copia group retrotransposons are ubiquitous and heterogeneous in higher plants. Nucleic Acids Res. 1992 Jul 25;20(14):3639–3644. doi: 10.1093/nar/20.14.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Smith D. B., Kumar A. Extreme heterogeneity of Ty1-copia group retrotransposons in plants. Mol Gen Genet. 1992 Jan;231(2):233–242. doi: 10.1007/BF00279796. [DOI] [PubMed] [Google Scholar]
- Flavell R. B. Repetitive DNA and chromosome evolution in plants. Philos Trans R Soc Lond B Biol Sci. 1986 Jan 29;312(1154):227–242. doi: 10.1098/rstb.1986.0004. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Hirochika R. Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn J Genet. 1993 Feb;68(1):35–46. doi: 10.1266/jjg.68.35. [DOI] [PubMed] [Google Scholar]
- Huang N., Reinl S. J., Rodriguez R. L. RAmy2A; a novel alpha-amylase-encoding gene in rice. Gene. 1992 Feb 15;111(2):223–228. doi: 10.1016/0378-1119(92)90690-q. [DOI] [PubMed] [Google Scholar]
- Huang N., Sutliff T. D., Litts J. C., Rodriguez R. L. Classification and characterization of the rice alpha-amylase multigene family. Plant Mol Biol. 1990 May;14(5):655–668. doi: 10.1007/BF00016499. [DOI] [PubMed] [Google Scholar]
- Ishiwatari Y., Honda C., Kawashima I., Nakamura S., Hirano H., Mori S., Fujiwara T., Hayashi H., Chino M. Thioredoxin h is one of the major proteins in rice phloem sap. Planta. 1995;195(3):456–463. doi: 10.1007/BF00202605. [DOI] [PubMed] [Google Scholar]
- Leeton P. R., Smyth D. R. An abundant LINE-like element amplified in the genome of Lilium speciosum. Mol Gen Genet. 1993 Feb;237(1-2):97–104. doi: 10.1007/BF00282789. [DOI] [PubMed] [Google Scholar]
- Matsuoka M., Ichikawa H., Saito A., Tada Y., Fujimura T., Kano-Murakami Y. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 1993 Sep;5(9):1039–1048. doi: 10.1105/tpc.5.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J. F. Evolution and consequences of transposable elements. Curr Opin Genet Dev. 1993 Dec;3(6):855–864. doi: 10.1016/0959-437x(93)90005-a. [DOI] [PubMed] [Google Scholar]
- Mochizuki K., Umeda M., Ohtsubo H., Ohtsubo E. Characterization of a plant SINE, p-SINE1, in rice genomes. Jpn J Genet. 1992 Apr;67(2):155–166. doi: 10.1266/jjg.67.155. [DOI] [PubMed] [Google Scholar]
- Nelson A. J., Doerner P. W., Zhu Q., Lamb C. J. Isolation of a monocot 3-hydroxy-3-methylglutaryl coenzyme A reductase gene that is elicitor-inducible. Plant Mol Biol. 1994 Jun;25(3):401–412. doi: 10.1007/BF00043869. [DOI] [PubMed] [Google Scholar]
- Ronald P. C., Albano B., Tabien R., Abenes L., Wu K. S., McCouch S., Tanksley S. D. Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21. Mol Gen Genet. 1992 Dec;236(1):113–120. doi: 10.1007/BF00279649. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Song J., Koga-Ban Y., Matsui E., Fang F., Higo H., Nagasaki H., Hori M., Miya M., Murayama-Kayano E. Toward cataloguing all rice genes: large-scale sequencing of randomly chosen rice cDNAs from a callus cDNA library. Plant J. 1994 Oct;6(4):615–624. doi: 10.1046/j.1365-313x.1994.6040615.x. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Leclercq L., Göbel E., Saedler H. Cin4, an insert altering the structure of the A1 gene in Zea mays, exhibits properties of nonviral retrotransposons. EMBO J. 1987 Dec 20;6(13):3873–3880. doi: 10.1002/j.1460-2075.1987.tb02727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob E., Choi S. Y., Simmons C., Migliaccio F., Ilag L., Hesse T., Palme K., Söll D. Molecular analysis of three maize 22 kDa auxin-binding protein genes--transient promoter expression and regulatory regions. Plant J. 1993 Sep;4(3):423–432. doi: 10.1046/j.1365-313x.1993.04030423.x. [DOI] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
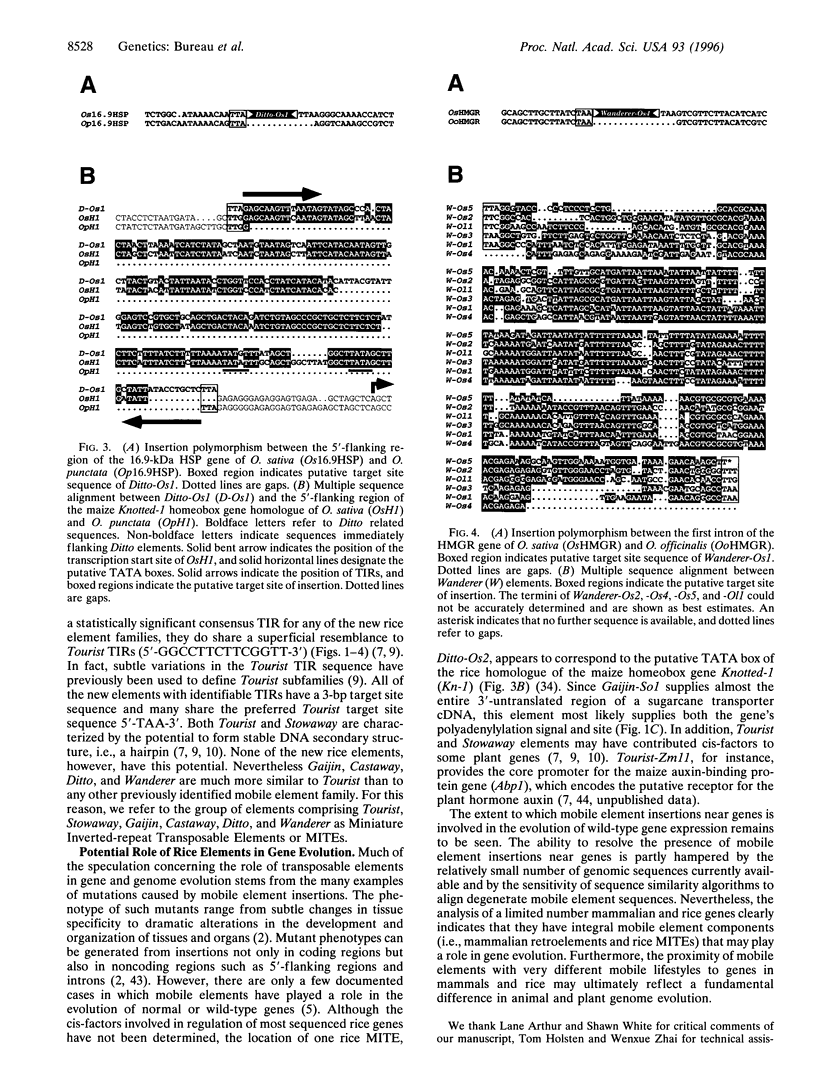
- Tzeng S. S., Yeh K. W., Chen Y. M., Lin C. Y. Two Oryza sativa Genomic DNA Clones Encoding 16.9-Kilodalton Heat-Shock Proteins. Plant Physiol. 1992 Aug;99(4):1723–1725. doi: 10.1104/pp.99.4.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignols F., Lund G., Pammi S., Trémousaygue D., Grellet F., Kader J. C., Puigdomènech P., Delseny M. Characterization of a rice gene coding for a lipid transfer protein. Gene. 1994 May 16;142(2):265–270. doi: 10.1016/0378-1119(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Voytas D. F., Cummings M. P., Koniczny A., Ausubel F. M., Rodermel S. R. copia-like retrotransposons are ubiquitous among plants. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7124–7128. doi: 10.1073/pnas.89.15.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washio K., Ishikawa K. Structure and expression during the germination of rice seeds of the gene for a carboxypeptidase. Plant Mol Biol. 1992 Jul;19(4):631–640. doi: 10.1007/BF00026789. [DOI] [PubMed] [Google Scholar]
- White S. E., Habera L. F., Wessler S. R. Retrotransposons in the flanking regions of normal plant genes: a role for copia-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11792–11796. doi: 10.1073/pnas.91.25.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. C., Végh Z., Wang X. M., Tan C. C., Dudits D. The nucleotide sequences of two rice histone H3 genes. Nucleic Acids Res. 1989 Apr 25;17(8):3297–3297. doi: 10.1093/nar/17.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y., Matsumoto S., Kojima S., Ohshima K., Okada N., Machida Y. Molecular characterization of a short interspersed repetitive element from tobacco that exhibits sequence homology to specific tRNAs. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6562–6566. doi: 10.1073/pnas.90.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Kung S. D., Dube S. K. Nucleotide sequence of rice 4-coumarate:CoA ligase gene, 4-CL.1. Nucleic Acids Res. 1990 Oct 25;18(20):6144–6144. doi: 10.1093/nar/18.20.6144. [DOI] [PMC free article] [PubMed] [Google Scholar]



